Abstract
Accumulated evidences have demonstrated that signal transducer and activator of transcription 3 (STAT3) is a critical link between inflammation and cancer. Multiple studies have indicated that persistent activation of STAT3 in epithelial/tumor cells in inflammation-associated colorectal cancer (CRC) is associated with sphingosine-1-phosphate (S1P) receptor signaling. In inflammatory response whereby interleukin (IL)-6 production is abundant, STAT3-mediated pathways were found to promote the activation of sphingosine kinases (SphK1 and SphK2) leading to the production of S1P. Reciprocally, S1P encourages the activation of STAT3 through a positive autocrine-loop signaling. The crosstalk between IL-6, STAT3 and sphingolipid regulated pathways may play an essential role in tumorigenesis and tumor progression in inflamed intestines. Therapeutics targeting both STAT3 and sphingolipid are therefore likely to contribute novel and more effective therapeutic strategies against inflammation-associated CRC.
Keywords: Sphingosine-1-phosphate, Signal transducer and activator of transcription 3, Interleukin-6, Inflammation, Colorectal cancer, Tumorigenesis, Tumor progression, Inflammatory bowel disease
Core tip: Patients with inflammatory bowel diseases have a predisposition for the development of colorectal cancer (CRC). We summarize current literature on the interleukin (IL)-6/signal transducer and activator of transcription 3 (STAT3) inflammatory pathway and its association with CRC. Recent papers within the last couple of years have demonstrated the crosstalk between IL-6, STAT3 and sphingosine-1-phosphate pathways in inflammation associated tumorigenesis in intestine. This signaling cascade in tumor cells appears to be an essential pathway for CRC tumor progression. Current therapies exploiting sphingolipid signaling have provided an attractive strategy against inflammation-associated CRC.
COLORECTAL CANCER AND INFLAMMATION
Colorectal cancer (CRC) is a third leading cause of cancer death in United States[1]. Patients with inflammatory bowel disease (IBD) of which two major known types are ulcerative colitis (UC) and Crohn’s disease (CD) have a predisposition for the development of CRC[2-5]. IBD-associated CRC occurs frequently in patients at younger age compared to sporadic CRC[4,6,7]. Among IBD patients, IBD-associated CRC accounts for a high mortality rate (10%-15%) with a 5-year survival of approximately 50%[6]. The association between inflammation and cancer is well recognized but the mechanistic link of these events is still under investigation[4]. Although the pathogenesis of IBD itself is unknown, cumulative data strongly support the hypothesis that IBD is due to a genetic predisposition that leads to a dysregulation of mucosal immune reactions to enteric microbes or environmental antigens[8-10].
In almost every IBD intestines, there is an overproduction of proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and interferon-gamma (IFN-γ)[11]. These factors have been known to stimulate the activation of intracellular STAT proteins, which are phosphorylated, then dimerized, and translocated to nucleus for transactivation a number of genes[12]. IL-6 has emerged as a key cytokine mediating IBD pathogenesis since serum level of IL-6 was shown to significantly elevate in patients with IBD[13-15] and local production of IL-6 was highly correlated with degree of intestinal inflammation[16]. During chronic inflammation, IL-6 functions are believed to recruit B and T cells to the inflamed site[17,18] by activation of JAK kinase pathway which phosphorylates signal transducer and activator of transcription (STAT) proteins, mainly STAT3[19].
Persistent activation of STAT3 has been observed in multiple human malignancies including various stages of colorectal cancer[20-23]. Several studies have shown that high expression of STAT3 alters the cell cycle[24,25] or inhibits apoptosis by up regulating anti-apoptotic signaling[26,27] in inflammation-associated CRC or and in other human cancers[28]. Moreover, STAT3 activation in tumor is often associated with poor prognosis of various human malignancies[29-32] suggesting that STAT3 promotes tumor progression or metastasis.
Recent genome-wide analysis of CD has identified the Stat3 gene as one of the susceptibility loci for the development of inflammatory bowel disease[33]. A study of human ulcerative colitis associated cancer has shown that dysplastic cells exhibited significantly higher expression of IL-6 and phosphorylated STAT3 (p-STAT3)[34] suggesting that the signaling cascade involving IL-6 and STAT3 is one of the important pathways in inflammation associated-CRC development. The potential pro-tumor role of IL-6/STAT3 pathway in inflamed intestine is also supported by studies using experimental models. Bollrath et al[35] have shown that mice carrying genetic modification in IL-6 cytokine family receptor β subunit, gp130 (gp130Y757F), exhibiting hyperactivation of Jak1/2-STAT3 signaling cascade upon IL-6 stimulation, had increased multiplicity of frequency and size of tumor in intestine when mice were treated with a carcinogen, azoxymethane (AOM) followed by dextran sodium sulfate (DSS) to induce inflammation and tumorigenesis in intestine. The development of tumors in AOM/DSS model was significantly suppressed when either IL-6 or epithelial stat3 was inactivated[35,36]. These experiments suggest that IL-6 plays a crucial role in inflammation-induced tumorigenesis in intestine and the activities of IL-6 is potentially mediated by STAT3[37-40]. We and others have recently demonstrated that IL-6/STAT3 linked to the sphingosine-1-phosphate (S1P) signaling in CRC tumor progression[41-43].
S1P/S1P KINASES IN INFLAMMATION ASSOCIATED HUMAN CANCER
S1P is a signaling sphingolipid characterized by a presence of a particular aliphatic amino alcohol (sphingosine)[44]. S1P is activated from sphingosine, a derivative of ceramide, by two known sphingosine kinases, SphK1 and SphK2, that have a broad but overlapping tissue distribution[45-47]. Numerous studies have shown that S1P signaling orchestrates many important physiological and pathophysiological processes including cell proliferation, migration and immune regulations[44,48-50]. As an inflammatory mediator, S1P has a pleotrophic effects including modulation of macrophage and neutrophil anaphylatoxin C5a signaling pathway[51,52] and stimulation lymphocyte trafficking into inflamed area[53]. The chemotatic ability of S1P to specific cell migration was reported to be dependent on the S1P receptors found on the cell membrane[54].
The uniqueness of S1P as an inflammatory mediator is ability to function either inside or outside of the cell. Intracellularly, S1P has been shown to bind to histone deacetylases regulating epigenic gene expression[55]. It can form complex with TNF-α receptor and TRAF2 in activation of NF-κB signaling[56]. S1P can also be transported across the plasma membrane as part of the “inside-out” signaling mediated by inflammation regulator ATP-binding cassette (ABC) family-member receptors including ABCC1, ABCG2 and sphingolipid transporter spinster homolog 2 (SPNS2)[57-61]. In addition, S1P has also been shown to regulate cell growth in a variety of cells by increase expression of anti-apoptotic Bcl2 and MCL1 while down regulate the pro-apoptotic proteins BAD and BAX[62-64].
Consistent to the potential tumor promoting function of S1P, increased expression of SphK1 has been found in multiple types of human cancers including stomach, lung, brain, colon and kidney[65]. Elevated expression of SphK1 has been correlated with increased tumor grade and mortality in several human malignancies including astrocytoma, gastric cancer and non-Hodgkin’s lymphoma[66-68]. In colon cancer, over-expression of SphK1 exhibits increased cell viability and enhances invasiveness associated with an upregulation of metalloproteinases 2/9 and urokinase plasminogen activator[69]. Interestingly, CRC cells resistant to cetuximab, a monoclonal antibody against epidermal growth factor (EGF) receptor prescribed for treatment of metastatic colon cancer exhibit an over expression of SphK1 suggesting a cross signaling between SphK1 and EGF receptor[70]. Further analysis of human colon cancer demonstrates a strong correlation between upregulation of SphK1 and focal adhesion kinases in cancer cells indicating that SphK1 activity is linked to an increase of tumor cell attachment and migration, initial steps in malignant transition[71].
The significance of S1P signaling in gastrointestinal tract tumor was demonstrated by Kohno et al[72] in 2006 who initially showed that the expression of SphK1 was required for intestinal adenoma development in Apc Min/+ mice. Further studies indicate that SphK1 was upregulated in colonic adenocarcinoma of rats induced by AOM[73]. Similarly, inactivation of SphK1 results in a significant delay in tumor progression in AOM/DSS induced mouse model of colitis-associated CRC[74]. Collectively, these studies suggest that S1P-SphK1 axis may also play a role CRC tumorigenesis. Interestingly, a study by Liang et al[42] observed that AOM/DSS treatment of SphK2-/- mice did not caused a reduction in tumor formation as expected but rather caused an increase in colitis associated tumorigenesis. This observation at first seems counter intuitive as both SphK1 and SphK2 phosphorylate sphingosine to S1P and thus, inactivation of SphK2 would result in a decrease of the overall S1P pool. In fact, the group reported that deletion of SphK2 caused a compensatory effect that resulted in an increase of SphK1 expression hence overall increase in S1P[42]. The study demonstrates that SphK1 and SphK2 are functionally compensated in the major positive feedback loop of inflammation-induced tumorigenesis in colon[42].
The potential connection between the two pathways, IL-6/STAT3 and S1P-SphK1, in tumor biology was not illuminated until Lee et al[41] who demonstrated that in many tumors (lymphoma, breast, prostate and adenocarcinoma) with persistently activation of STAT3 correlated with an elevation of S1P receptor, S1PR1. Enhanced S1PR1 expression, which activated STAT3 and upregulated IL-6 expression, accelerated tumor growth and metastasis[41]. The study demonstrates that SphK1/S1P/S1PR1 axis plays an essential role in regulating the production of IL-6 and persistent activation of STAT3 that linked to inflammation and tumor progression. Using a conditional genetic targeting approach, we reported that mice with Stat3 inactivation specifically in hematopoietic cells developed chronic inflammation in large intestine that led to tumorigenesis in the flamed colon[75]. In this model, termed Stat3-IKO, persistent activation of STAT3 in colonic epithelial cells was found to be associated with a marked elevation of IL-6 expression consistent to the observation in human IBD[15,40,75]. The over expression of IL-6 and persistent activation of STAT3 in epithelial cells were associated with a significant elevation of S1PR1 and SphK2 expression in epithelial cells in inflamed colon 43 consistent with the other reports[76-78]. Interestingly, inactivation of epithelial Stat3 in Stat3-IKO mice did not inhibit inflammation-associated epithelial over proliferation but significantly delayed tumor progression to invasive stages[43].
The delayed tumor progression was associated with a significantly decreased expression of S1PR1 and SphK2 in colonic epithelial/tumor cells[43]. We observed that the decreased expression of S1PR1 and SphK2 in this model is associated with marked increase of CD8+ T cell and regulatory T lymphocytes (Treg) populations in colon suggesting that epithelial STAT3-S1P pathway may regulate tumor progression through regulating the recruitment of specific populations of immune cells consistent with previous studies[41,79]. Based on previous studies including ours, a positive feedback pathway linking inflammation and tumor progression via the IL-6/STAT3/S1PR1 is proposed as illustrated in Figure 1. Proinflammatory cytokines including IL-6 produced by infiltrated inflammatory cells initiate the activation of STAT3 in epithelial cell. In addition, to promote pathways that regulate cell proliferation and survival, epithelial STAT3 triggers the activation of SphK-S1P-S1PR pathway that further stimulates the activation of epithelial STAT3 through (1) enhancing the infiltration of inflammatory cells; and (2) promoting the positive feedback loop via S1PR-STAT3 expressed on epithelial cells. This vicious cycle maintains the persistent activation of STAT3 in epithelial cells and consequently leads to malignant transformation in these cells.
Figure 1.
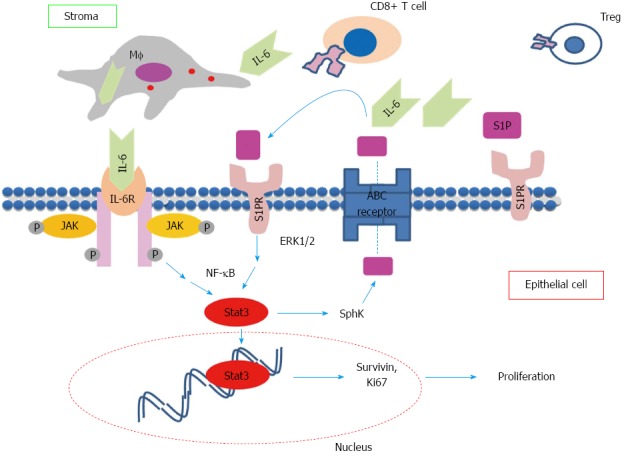
Hypothetical positive feedback pathway necessary for tumor progression. In an inflamed region, pro inflammatory interleukin (IL)-6 produced by macrophages and other immune cells activated epithelial signal transducer and activator of transcription 3 via IL6R signaling. Signal transducer and activator of transcription 3 (STAT3) mediates cell proliferation and up regulation of S1PR’s. Signaling from S1PR maintains persistently activated STAT3 and up regulation of S1P kinases, which contributes to the overall increase in S1P pool. S1P auto-regulates epithelial cell growth and modifies immune cell recruitment including CD8+ T and Treg cells. S1P: Sphingosine-1-P.
THERAPIES TARGETING INFLAMMATION AND S1P/SPHK1 PATHWAY IN COLORECTAL CANCER
In light of mouse models of inflammation associated colorectal cancer linking persistent activation of STAT3 with S1P signaling pathway, many distinctive features seen in mice are observed in human CRC. Therefore, strategies targeting these pathways may improve the current treatment of the disease. Indeed, multiple strategies have been proposed and currently in clinical trials.
It has been long recognized that the balance between sphingosine and ceramide known as the sphingosine rheostat determines the fate of the cell as increase of ceramide mediates apoptosis while increase of sphingosine/S1P induces cell mitogenesis[80,81], as illustrated in Figure 2. A therapeutic strategy for the treatment of CRC is to shift the balance of the sphingosine rheostat in favor of the increase of ceramide. Inhibitors for ceramidase, an enzyme that cleaves ceramide to sphingosine, such as, B13, have been shown to induce apoptosis in metastatic human colon cancer[82]. Direct application of ceramide derivatives including ceramide LCL-30 and sphingosine analogue, (2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane (Enigmol), have also been used to inhibit CRC development in mice[83-85]. Another interesting approach is to target S1P lyase, an enzyme that cleaves S1P to hexadecenal and phosphothanolamine. S1P lysase isolated from the prokaryote Symbiobacterium thermophilum called StSPL was shown to inhibit MCF-7 human breast cancer cell and HCT-116 colon carcinoma cell growth by decreasing the S1P pool[86]. Monoclonal antibody against human S1P, iSONEP, manufactured by Lpath Inc., provides a direct method to immuno-deplete S1P and is in lined for clinical testing[87].
Figure 2.
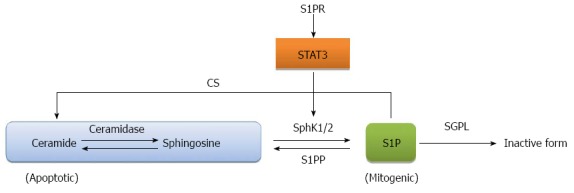
Regulation of sphingosine 1 phosphate. Ceramide and sphingosine 1 phosphate (S1P) are balanced by regulatory enzymes: sphingosine 1 phosphate kinases (SphK1 and 2), S1P phosphatase (S1PP), S1P lyases (SGPL) and ceramide synthase (CS). Activation of signal transducer and activator of transcription 3 (STAT3) from S1PR signaling shifts the balance toward more S1P production.
Several inhibitors targeting the SphK have been developed in the treatment of different cancers including CRC[70,88,89]. Of note worth mentioning is methylated sphingosine derivative N,N-dimethylsphingosine (DMS)[88]. DMS was originally identified as an inhibitor of protein kinase C that regulates cell growth and induces apoptosis in number of cancer cells[88,90]. It was later shown to inhibit SphK’s[91]. Colon cancer cells that are resistant to cetuximab due to over expression of SphK 1[70] responded well to cetuximab in conjunction with DMS or with SphK1 siRNA inhibitor[70]. Another inhibitor of SphK1 is dihydrosphingosine (DHS)[92]. Interestingly, an increase of intracellular DHS was observed in human PC-3 and LNCaP prostate cancer cells treated with vitamin E γ-tocotrienol[93]. The elevation of DHS was associated with an increase of apoptosis, necrosis and autophagy in these cells[93]. Similarly, γ-tocotrienol treatment of human colon carcinoma SW620 and HCT-8 cells also caused similar paraptosis-like cell death[94]. These and other SphK1 inhibitors such as, F-12509a, B-5354c, and SKI-I-V[89] have been shown to prevent the development of CRC in AOM/DSS model[95].
Prevention therapy that target S1P mediated inflammation is being developed. For instance, several drugs that target S1P receptor, such as, ONO-4641 and KRP-203 have been shown to reduce inflammation in mouse models of colitis[96,97]. While these drugs have yet to be tested on patients with colitis-associated CRC, the pharmacokinetic of ONO-4641 has been tested using experimental models[98,99]. The most promising drug in clinical testing for inflammation associated with CRC is FTY720, fingolimod. This drug is particularly important since it blocks S1P pathway at multiple levels. Activated intracellularly by SphK2, phosphorylated FTY720 is believed to bind to G coupled-protein S1P receptor and prevents the receptor from recycling to the membrane thereby rendering cells unresponsive to S1P[100]. FTY720 blocks T and B lymphocyte egression from secondary lymphoid tissue to the blood[101]. Additionally, the anti-inflammatory effect of FTY720 is demonstrated in a colitis mouse model induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) in which FTY720 appears to block Th1 mediated colitis[102]. Interestingly, FTY720 treatment of these mice resulted in up-regulation of FoxP3+ T cells consistent with our hypothesis that the S1P/S1PR1 pathway modulates the activities of FoxP3+ Treg cells[102]. In inflammation associated CRC, FTY720 inhibits tumor angiogenesis by blocking S1P-mediated Ca+2 mobilization important for vascular endothelial cell migration[100]. Furthermore, the FTY720 was recently shown to decrease SphK1 and S1PR1 expression and eliminate the NF-κB/IL6/STAT3 amplification cascade critical for the development of CRC[42].
Additional strategies targeting IL-6/STAT3 in the treatment of colitis-associated CRC are also being developed. Tofacitinib, which targets the Janus kinases, the major enzyme phosphorylating STAT3, was reported to have a better clinical remission rates in UC patients compared to those on other anti-inflammatory drugs, mesalamine, or glucocorticoids immunosuppressant, or anti-TNF therapy[103]. Furthermore, chemotherapeutic agents, camptothecin (CPT) and oxaliplatin (OXP) were shown to enhance apoptosis by inhibiting IL-6 activation of STAT3 in human colon cancer cells HCT116 and HT29[104]. Other STAT3 targeting drugs worth mentioning are trichostatin A (TSA), a histone deacetylase inhibitor, but has shown to inhibit Jak2/STAT3 activation in CRC cells[105,106] and 2 β-cyclodextrin inclusion compounds of auraptene and 4’-geranyloxyferulic acid used as part of the diet in reducing inflammation[107]. Small interfering RNA (siRNA) targeting STAT3 has shown to inhibit colon cancer cell invasion ability[108] and sensitize the cells to chemoradiotherapy[109]. Collectively, these drugs and other inhibitor of S1P/SphK inhibitors offering promising therapeutic potential in CRC treatment.
CONCLUSION
Persistent activation of STAT3 in epithelial/tumor cells has been linked to multiple human malignancies including inflammation-associated colorectal cancer. Studies have shown that in patients with inflammatory bowel diseases, IL-6 may play an crucial role in controlling inflammation in intestine. IL-6 may stimulate the activation of STAT3 in colonic epithelial cells in inflamed intestine that consequently promotes the activation of S1P pathway. STAT3 promotes the activation of S1P-SphK-S1PR axis that reciprocally facilitates the maintenance of STAT3 activation in epithelial cells through a positive-feedback loop. Multiple studies have demonstrated that the cross talk of STAT3 and S1P-SphK-S1PR pathways may play an essential role in inflammation-induced tumorigenesis and tumor progression in intestine. Several classes of drugs have developed to specifically target these pathways and have shown promising results in mouse models of CRC and in some clinical trials. Thus, the development of sphogolipid-centric therapeutics in conjunction with anti-inflammatory drug would likely to add novel and more effective therapeutic strategies against colorectal cancer especially those cancers associated with inflammation.
Footnotes
P- Reviewer: Daniel F, Ghobadloo SM, Hee Y, Koss K, Kisiel JB, Wang DS, Yang CH S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–16. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 3.Chawla A, Judge TA, Lichtenstein GR. Evaluation of polypoid lesions in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:525–34, ix. doi: 10.1016/s1052-5157(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azer SA. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol. 2013;25:271–281. doi: 10.1097/MEG.0b013e32835b5803. [DOI] [PubMed] [Google Scholar]
- 6.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205–211. doi: 10.1097/01.MIB.0000217770.21261.ce. [DOI] [PubMed] [Google Scholar]
- 8.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 9.Dubinsky MC, Taylor K, Targan SR, Rotter JI. Immunogenetic phenotypes in inflammatory bowel disease. World J Gastroenterol. 2006;12:3645–3650. doi: 10.3748/wjg.v12.i23.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, Domschke W. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 13.Holub MC, Makó E, Dévay T, Dank M, Szalai C, Fenyvesi A, Falus A. Increased interleukin-6 levels, interleukin-6 receptor and gp130 expression in peripheral lymphocytes of patients with inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;228:47–50. [PubMed] [Google Scholar]
- 14.Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104:1285–1292. doi: 10.1016/0016-5085(93)90336-b. [DOI] [PubMed] [Google Scholar]
- 15.Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 16.Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66–74. doi: 10.1007/s005350050218. [DOI] [PubMed] [Google Scholar]
- 17.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 18.Xing Z, Braciak T, Jordana M, Croitoru K, Graham FL, Gauldie J. Adenovirus-mediated cytokine gene transfer at tissue sites. Overexpression of IL-6 induces lymphocytic hyperplasia in the lung. J Immunol. 1994;153:4059–4069. [PubMed] [Google Scholar]
- 19.Hemmann U, Gerhartz C, Heesel B, Sasse J, Kurapkat G, Grötzinger J, Wollmer A, Zhong Z, Darnell JE, Graeve L, et al. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II. Src homology SH2 domains define the specificity of stat factor activation. J Biol Chem. 1996;271:12999–13007. doi: 10.1074/jbc.271.22.12999. [DOI] [PubMed] [Google Scholar]
- 20.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang M, Page C, Reynolds RK, Lin J. Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000;79:67–73. doi: 10.1006/gyno.2000.5931. [DOI] [PubMed] [Google Scholar]
- 22.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 23.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 24.Lin Q, Lai R, Chirieac LR, Li C, Thomazy VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K, et al. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol. 2005;167:969–980. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu LZ, Wang HY, Yang SP, Yuan ZP, Xu FY, Sun C, Shi RH. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J Gastroenterol. 2013;19:2638–2649. doi: 10.3748/wjg.v19.i17.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 27.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 30.Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M. Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: a study of incidence and its association with pathological features and clinical outcome. J Urol. 2002;168:762–765. [PubMed] [Google Scholar]
- 31.Kawada M, Seno H, Uenoyama Y, Sawabu T, Kanda N, Fukui H, Shimahara Y, Chiba T. Signal transducers and activators of transcription 3 activation is involved in nuclear accumulation of beta-catenin in colorectal cancer. Cancer Res. 2006;66:2913–2917. doi: 10.1158/0008-5472.CAN-05-3460. [DOI] [PubMed] [Google Scholar]
- 32.Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452–1462. doi: 10.1158/1078-0432.CCR-10-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 35.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 38.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuyama K, Sata M, Rose-John S. Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev. 2006;17:451–461. doi: 10.1016/j.cytogfr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6: STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen AV, Wu YY, Liu Q, Wang D, Nguyen S, Loh R, Pang J, Friedman K, Orlofsky A, Augenlicht L, et al. STAT3 in epithelial cells regulates inflammation and tumor progression to malignant state in colon. Neoplasia. 2013;15:998–1008. doi: 10.1593/neo.13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyne S, Pyne NJ. Translational aspects of sphingosine 1-phosphate biology. Trends Mol Med. 2011;17:463–472. doi: 10.1016/j.molmed.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- 48.Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92:707–715. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwalm S, Pfeilschifter J, Huwiler A. Sphingosine-1-phosphate: a Janus-faced mediator of fibrotic diseases. Biochim Biophys Acta. 2013;1831:239–250. doi: 10.1016/j.bbalip.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Kee TH, Vit P, Melendez AJ. Sphingosine kinase signalling in immune cells. Clin Exp Pharmacol Physiol. 2005;32:153–161. doi: 10.1111/j.1440-1681.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J Biol Chem. 2004;279:44802–44811. doi: 10.1074/jbc.M403977200. [DOI] [PubMed] [Google Scholar]
- 53.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 54.Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 55.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Tang C. Regulation of ABCA1 functions by signaling pathways. Biochim Biophys Acta. 2012;1821:522–529. doi: 10.1016/j.bbalip.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin K, Liao DF, Tang CK. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med. 2010;16:438–449. doi: 10.2119/molmed.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758–1766. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avery K, Avery S, Shepherd J, Heath PR, Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17:1195–1205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 63.Rodgers A, Mormeneo D, Long JS, Delgado A, Pyne NJ, Pyne S. Sphingosine 1-phosphate regulation of extracellular signal-regulated kinase-1/2 in embryonic stem cells. Stem Cells Dev. 2009;18:1319–1330. doi: 10.1089/scd.2009.0023. [DOI] [PubMed] [Google Scholar]
- 64.Watterson K, Sankala H, Milstien S, Spiegel S. Pleiotropic actions of sphingosine-1-phosphate. Prog Lipid Res. 2003;42:344–357. doi: 10.1016/s0163-7827(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 65.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Guan HY, Gong LY, Song LB, Zhang N, Wu J, Yuan J, Zheng YJ, Huang ZS, Li M. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res. 2008;14:6996–7003. doi: 10.1158/1078-0432.CCR-08-0754. [DOI] [PubMed] [Google Scholar]
- 67.Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- 68.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 69.Liu SQ, Huang JA, Qin MB, Su YJ, Lai MY, Jiang HX, Tang GD. Sphingosine kinase 1 enhances colon cancer cell proliferation and invasion by upregulating the production of MMP-2/9 and uPA via MAPK pathways. Int J Colorectal Dis. 2012;27:1569–1578. doi: 10.1007/s00384-012-1510-y. [DOI] [PubMed] [Google Scholar]
- 70.Rosa R, Marciano R, Malapelle U, Formisano L, Nappi L, D’Amato C, D’Amato V, Damiano V, Marfè G, Del Vecchio S, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19:138–147. doi: 10.1158/1078-0432.CCR-12-1050. [DOI] [PubMed] [Google Scholar]
- 71.Liu SQ, Su YJ, Qin MB, Mao YB, Huang JA, Tang GD. Sphingosine kinase 1 promotes tumor progression and confers malignancy phenotypes of colon cancer by regulating the focal adhesion kinase pathway and adhesion molecules. Int J Oncol. 2013;42:617–626. doi: 10.3892/ijo.2012.1733. [DOI] [PubMed] [Google Scholar]
- 72.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 74.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, et al. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol. 2010;176:952–967. doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun DF, Gao ZH, Liu HP, Yuan Y, Qu XJ. Sphingosine 1-phosphate antagonizes the effect of all-trans retinoic acid (ATRA) in a human colon cancer cell line by modulation of RARβ expression. Cancer Lett. 2012;319:182–189. doi: 10.1016/j.canlet.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Xiao M, Liu Y, Zou F. Sensitization of human colon cancer cells to sodium butyrate-induced apoptosis by modulation of sphingosine kinase 2 and protein kinase D. Exp Cell Res. 2012;318:43–52. doi: 10.1016/j.yexcr.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, Okano Y, Murate T, Hara A, Ueda H, Nozawa Y, et al. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garris CS, Wu L, Acharya S, Arac A, Blaho VA, Huang Y, Moon BS, Axtell RC, Ho PP, Steinberg GK, et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat Immunol. 2013;14:1166–1172. doi: 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beckham TH, Cheng JC, Marrison ST, Norris JS, Liu X. Interdiction of sphingolipid metabolism to improve standard cancer therapies. Adv Cancer Res. 2013;117:1–36. doi: 10.1016/B978-0-12-394274-6.00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saddoughi SA, Ogretmen B. Diverse functions of ceramide in cancer cell death and proliferation. Adv Cancer Res. 2013;117:37–58. doi: 10.1016/B978-0-12-394274-6.00002-9. [DOI] [PubMed] [Google Scholar]
- 82.Selzner M, Bielawska A, Morse MA, Rüdiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- 83.Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, Graf R, Clavien PA. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006;5:1520–1529. doi: 10.1158/1535-7163.MCT-05-0513. [DOI] [PubMed] [Google Scholar]
- 84.Dahm F, Bielawska A, Nocito A, Georgiev P, Szulc ZM, Bielawski J, Jochum W, Dindo D, Hannun YA, Clavien PA. Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br J Cancer. 2008;98:98–105. doi: 10.1038/sj.bjc.6604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Symolon H, Bushnev A, Peng Q, Ramaraju H, Mays SG, Allegood JC, Pruett ST, Sullards MC, Dillehay DL, Liotta DC, et al. Enigmol: a novel sphingolipid analogue with anticancer activity against cancer cell lines and in vivo models for intestinal and prostate cancer. Mol Cancer Ther. 2011;10:648–657. doi: 10.1158/1535-7163.MCT-10-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huwiler A, Bourquin F, Kotelevets N, Pastukhov O, Capitani G, Grütter MG, Zangemeister-Wittke U. A prokaryotic S1P lyase degrades extracellular S1P in vitro and in vivo: implication for treating hyperproliferative disorders. PLoS One. 2011;6:e22436. doi: 10.1371/journal.pone.0022436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stoller GL, Lapierre-Holme F, Peterkin J, Garland W, Sabbadini R. iSONEP, an antisphingosine-1-phsphate (anti-S1P) monoclonal antibody for investigation in exudative AMD: results from a Phase 1 prospective open-label dose-escalating multi-center study. Invest. Ophthalmol Vis Sci. 2010;51:1253. [Google Scholar]
- 88.Endo K, Igarashi Y, Nisar M, Zhou QH, Hakomori S. Cell membrane signaling as target in cancer therapy: inhibitory effect of N,N-dimethyl and N,N,N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res. 1991;51:1613–1618. [PubMed] [Google Scholar]
- 89.Alshaker H, Sauer L, Monteil D, Ottaviani S, Srivats S, Böhler T, Pchejetski D. Therapeutic potential of targeting SK1 in human cancers. Adv Cancer Res. 2013;117:143–200. doi: 10.1016/B978-0-12-394274-6.00006-6. [DOI] [PubMed] [Google Scholar]
- 90.Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, Igarashi Y. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer. 1996;66:358–366. doi: 10.1002/(SICI)1097-0215(19960503)66:3<358::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 91.Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 92.Pfaff M, Powaga N, Akinci S, Schütz W, Banno Y, Wiegand S, Kummer W, Wess J, Haberberger RV. Activation of the SPHK/S1P signalling pathway is coupled to muscarinic receptor-dependent regulation of peripheral airways. Respir Res. 2005;6:48. doi: 10.1186/1465-9921-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, Jiang Z, Li G. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer. 2012;130:685–693. doi: 10.1002/ijc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang JS, Li DM, Ma Y, He N, Gu Q, Wang FS, Jiang SQ, Chen BQ, Liu JR. γ-Tocotrienol induces paraptosis-like cell death in human colon carcinoma SW620 cells. PLoS One. 2013;8:e57779. doi: 10.1371/journal.pone.0057779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanada Y, Mizushima T, Kai Y, Nishimura J, Hagiya H, Kurata H, Mizuno H, Uejima E, Ito T. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One. 2011;6:e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, Kinoshita M, Yasue T, Sawa Y, Ito T. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 98.Ohno T, Hasegawa C, Nakade S, Kitagawa J, Honda N, Ogawa M. The prediction of human response to ONO-4641, a sphingosine 1-phosphate receptor modulator, from preclinical data based on pharmacokinetic-pharmacodynamic modeling. Biopharm Drug Dispos. 2010;31:396–406. doi: 10.1002/bdd.719. [DOI] [PubMed] [Google Scholar]
- 99.Komiya T, Sato K, Shioya H, Inagaki Y, Hagiya H, Kozaki R, Imai M, Takada Y, Maeda T, Kurata H, et al. Efficacy and immunomodulatory actions of ONO-4641, a novel selective agonist for sphingosine 1-phosphate receptors 1 and 5, in preclinical models of multiple sclerosis. Clin Exp Immunol. 2013;171:54–62. doi: 10.1111/j.1365-2249.2012.04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaMontagne K, Littlewood-Evans A, Schnell C, O’Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau G, et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 101.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 102.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 103.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 104.Cross-Knorr S, Lu S, Perez K, Guevara S, Brilliant K, Pisano C, Quesenberry PJ, Resnick MB, Chatterjee D. RKIP phosphorylation and STAT3 activation is inhibited by oxaliplatin and camptothecin and are associated with poor prognosis in stage II colon cancer patients. BMC Cancer. 2013;13:463. doi: 10.1186/1471-2407-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiong H, Du W, Zhang YJ, Hong J, Su WY, Tang JT, Wang YC, Lu R, Fang JY. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol Carcinog. 2012;51:174–184. doi: 10.1002/mc.20777. [DOI] [PubMed] [Google Scholar]
- 106.Humphreys KJ, Cobiac L, Le Leu RK, Van der Hoek MB, Michael MZ. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol Carcinog. 2013;52:459–474. doi: 10.1002/mc.21879. [DOI] [PubMed] [Google Scholar]
- 107.Tanaka T, de Azevedo MB, Durán N, Alderete JB, Epifano F, Genovese S, Tanaka M, Tanaka T, Curini M. Colorectal cancer chemoprevention by 2 beta-cyclodextrin inclusion compounds of auraptene and 4’-geranyloxyferulic acid. Int J Cancer. 2010;126:830–840. doi: 10.1002/ijc.24833. [DOI] [PubMed] [Google Scholar]
- 108.Fan Y, Zhang YL, Wu Y, Zhang W, Wang YH, Cheng ZM, Li H. Inhibition of signal transducer and activator of transcription 3 expression by RNA interference suppresses invasion through inducing anoikis in human colon cancer cells. World J Gastroenterol. 2008;14:428–434. doi: 10.3748/wjg.14.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz J, et al. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134:997–1007. doi: 10.1002/ijc.28429. [DOI] [PMC free article] [PubMed] [Google Scholar]


