Abstract
Aberrant functioning of serine proteases in inflammatory and carcinogenic processes within the gastrointestinal tract (GIT) has prompted scientists to investigate the potential of serine protease inhibitors, both natural and synthetic, as modulators of their proteolytic activities. Protease inhibitors of the Bowman-Birk type, a major protease inhibitor family in legume seeds, which inhibit potently and specifically trypsin- and chymotrypsin-like proteases, are currently being investigated as colorectal chemopreventive agents. Physiologically relevant amounts of Bowman-Birk inhibitors (BBI) can reach the large intestine in active form due to their extraordinary resistance to extreme conditions within the GIT. Studies in animal models have proven that dietary BBI from several legume sources, including soybean, pea, lentil and chickpea, can prevent or suppress carcinogenic and inflammatory processes within the GIT. Although the therapeutic targets and the action mechanism of BBI have not yet been elucidated, the emerging evidence suggests that BBI exert their preventive properties via protease inhibition; in this sense, serine proteases should be considered as primary targets in early stages of carcinogenesis. The validation of candidate serine proteases as therapeutic targets together with the identification, within the wide array of natural BBI variants, of the most potent and specific protease inhibitors, are necessary to better understand the potential of this protein family as colorectal chemopreventive agents.
Keywords: Bowman-Birk inhibitors, Cell proliferation, Chemoprevention, Colorectal cancer, Legumes, Serine proteases
Core tip: Bowman-Birk inhibitors (BBI) from legumes, such as soybean, pea, lentil and chickpea, are a class of naturally-occurring serine protease inhibitors with potential anti-inflammatory and chemopreventive properties within the gastrointestinal tract (GIT). BBI are extensively disulphide-linked within proteins and have been demonstrated to be structurally and functionally resistant to the challenges of the GIT in vivo. Recent data suggest that trypsin- and chymotrypsin-like proteases involved in early stages of carcinogenesis should be primary targets in investigating the potential of BBI as colorectal chemopreventive agents; so far, the therapeutic targets as well as action mechanism of BBI remain unknown.
INTRODUCTION
Proteases are hydrolytic enzymes acting on peptide bonds, in a process termed proteolysis. The serine proteases constitute one of the largest families of proteolytic enzymes and are well recognized for their pivotal roles in a wide range of physiological processes as diverse as digestion, blood coagulation, fibrinolysis, immune responses, cell cycle progression and apoptosis. Their proteolytic activities are tightly controlled by an array of regulatory mechanisms, such as gene expression, substrate recognition, activation of inactive protease precursors (zymogens) by specific and limited proteolysis, localization of both enzyme and substrate, cofactor binding, post-translational modifications and interaction with other proteins and/or protease inhibitors than can form tight complexes with target enzymes[1]. Aberrant functioning of certain serine proteases underlies pathological disorders such as cancer, angiogenesis, rheumatoid arthritis, neurodegenerative and cardiovascular diseases[2-4]. Understanding the fundamental role played by serine proteases and their cognate inhibitors in pathological disorders offers challenging opportunities for preventive and/or therapeutic intervention[5]. Naturally-occurring plant protease inhibitors are being investigated for their potential in the prevention and/or treatment of a diverse set of human pathologies, including cancer, neurodegenerative and cardiovascular diseases, muscle atrophy and inflammatory processes[6,7]. In this context, the United States Food and Drug Administration (FDA) granted a protein extract of soybean (Glycine max) enriched in Bowman-Birk inhibitors (BBI), namely Bowman-Birk inhibitor concentrate (BBIC), as investigational new drug. Up to six clinical trials has been accomplished in patients with benign prostatic hyperplasia[8], oral leukoplakia[9-12] and ulcerative colitis (UC)[13]. The inherent ability of BBI to inhibit serine proteases has been related to their potential health benefits; however, the mechanism/s of action and the identity of their therapeutic targets remain unknown[14]. Herein, we report recent evidences regarding the contribution of BBI from legumes as colorectal chemopreventive agents.
BOWMAN-BIRK FAMILY IN LEGUMES
Legumes seeds, compared to other vegetative organs and botanical families, are particularly rich in protease inhibitors of the Bowman-Birk family. BBI from legumes, such as soybean, pea (Pisum sativum), lentil (Lens culinaris), field bean (Vicia faba) or chickpea (Cicer arietinum), are canonical serine protease inhibitors of molecular weight in the range 7-9 kDa and usually contain two protease inhibitory domains, located in the external loops of the so-called “bow tie” motif, centred around residues 16 and 43[15]; each inhibitory domain is located within a nonapeptide region joined via a disulphide bond between flanking cysteine residues[16]. The inhibitory domains of BBI are very exposed and easily accessible to proteolytic enzymes (Figure 1A). This structural arrangement allows the interaction of BBI with two enzyme molecules, not necessarily identical, simultaneously and independently, without any significant conformational adjustment[17]; the resulting non-covalent complex renders the protease target inactive. Molecular recognition of serine proteases is governed by the P1 residue[18]; this residue inserts into the S1 cavity of the cognate enzyme upon protease-inhibitor formation[19]. In legume seeds, the target enzyme for the N-terminal inhibitory domain is trypsin with BBI having a positively charged residue, either Arg or Lys, at the P1 position; the presence of Ala determines inhibition for elastase, as reported in wild soybean (Glycine soja)[20] and grass pea (Lathyrus sativus)[21]. Greater variation exists at the P1 position for the C-terminal inhibitory domain, with Arg, Phe, Tyr or Leu at this position, with predictions of either trypsin or chymotrypsin inhibition. The high affinity of chymotrypsin-like proteases for aromatic residues within substrates (Tyr or Phe at position P1) is well documented, as is reflected in their inhibitors, with Tyr showing the strongest binding[22,23]. A limited number of amino acids located within the inhibitory domains of BBI seem to be responsible for their primary functional and biological properties. By using synthetic cyclic peptides mimicking the inhibitory domains of BBI, the significance of additional residues adjacent to the reactive site peptide bond (P1-P1’) on potency and resistance to hydrolysis against specific target proteases has been revealed[24,25]. BBI from legumes exert extremely potent inhibitory activity against both trypsin and chymotrypsin enzymes, with Ki values within the nanomolar range reported for different legume species, such as soybean[26], pea[27,28], lentil[29,30] and lupin (Lupinus albus) BBI[31].
Figure 1.
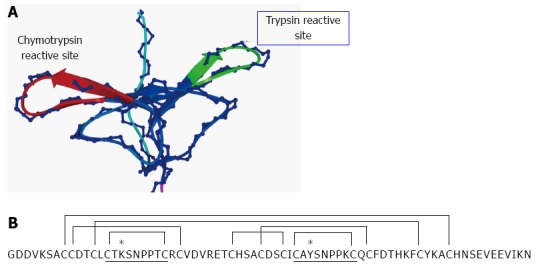
Bowman-Birk family in legumes. A: Homology model of TI1, a major pea Bowman-Birk inhibitor (BBI) isoinhibitor, showing the trypsin and chymotrypsin inhibitory domains[33]; B: Amino acid sequence deduced from the TI1 gene from the pea cultivar Birte. The sequence of both inhibitory domains are underlined and the positions of the seven disulphide bonds are indicated by connecting lines[39]. K and Y at position P1(*) determines specificity for trypsin and chymotrypsin, respectively.
Protease inhibitors of the Bowman-Birk family show considerable variation between and within legume species where seed and vegetative isoforms may be distinguished[32,33]. The expression of distinct genes, together with the post-translational modifications of primary gene products, which mainly occurs during seeds desiccation, is responsible for the wide array of isoinhibitors reported for different legume species. As an example, protease inhibitors from winter pea seeds (cv. Frilene) comprise up to six isoforms (PSTI I, II, III, IVa, IVb and V) which all belong to the Bowman-Birk family[34]. An amino acid sequence alignment of major BBI isoinhibitors from soybean and those from other representative legume species shows that there is a high sequence homology among BBI isoforms (Table 1). For the N-terminal inhibitory domain, there is a consensus amino acid sequence, P3-P6’: CTP1SXPPQC, where P1 is the position providing specificity for trypsin inhibition, and X (at P2’ position) can be any amino acid but with predicted significant effect on inhibitory potency and hydrolysis rates. Within the C-terminal inhibitory domain, amino acid sequence variation profoundly affects inhibitory potency against trypsin- or chymotrypsin-like enzymes[33] (Table 1).
Table 1.
Amino acid sequence alignment of Bowman-Birk inhibitor-like proteins from several legumes species
| Species | Entry name (accession number) | Amino acid sequence |
| Dolichos biflorus | IBB_DOLBI (Q9S9E3) | EPSESSKPCCDQCTCTKSIPPQCRCTDVRLNSCHSACSSCVCTFSIPAQCVCVDMKDFCYAPCKSSHDD |
| Glycine max | IBB1_SOYBN (P01055) | DDESSKPCCDQCACTKSNPPQCRCSDMRLNSCHSACKSCICALSYPAQCFCVDITDFCYEPCKPSEDDKEN |
| IBBD2_SOYBN (P1064) | DDEYSKPCCDLCMCTRSMPPQCSCEDIRLNSCHSDCKSCMCTRSQPGQCRCLDTNDFCYKPCKSRDD | |
| IBBC2_SOYBN (P01063) | DDESSKPCCDLCMCTASMPPQCHCADIRLNSCHSACDRCACTRSMPGQCRCLDTTDFCYKPCKSSDEDDD | |
| Lens culinaris | IBB_LENCU (Q8W4Y8) | GDDVKSACCDTCLCTRSQPPTCRCVDVRESCHSACDKCVCAYSNPPQCQCYDTHKFCYKACHNSEIEE |
| Lupinus albus | IBB1_LUPAL (P85172) | SLASKPCCDSCLCTRSIPPQCRCTDIGETCHSACKSCICTRSFPPQCRCSDITHFCYKPCTSS |
| Phaseolus vulgaris | IBB2_PHAVU (P01060) | EPSESSEPCCDICVCTASIPPICQCTDVRLNSCHSACKSCMCTRSMPGKCRCLDTTDYCYKSCKSSGEDDD |
| IBB3_PHAVU (P81484) | ASXSSKPCCBHCACTKSIPPQCRCSBLRLNSCHSECKGCICTFSIPAQCICTDTNNFCYEPCKSSHGPBBNN | |
| Pisum sativum | IBBA_PEA (Q41065) | GDDVKSACCDTCLCTKSNPPTCRCVDVRETCHSACDSCICAYSNPPKCQCFDTHKFCYKACHNSEVEEVIKN |
| IBB2_PEA (Q41066) | GDDVKSACCDTCLCTKSDPPTCRCVDVGETCHSACDSCICALSYPPQCQCFDTHKFCYKACHNSEVEE VIKN | |
| IBBB_PEA (P56679) | GDDVKSACCDTCLCTKSNPPTCRCVDVGETCHSACLSCICAYSNPPKCQCFDTQKFCYKACHNSELEEVIKN | |
| Vicia faba | IBB_VICFA (P24661) | GDDVKSACCDTCLCTKSEPPTCRCVDVGERCHSACNSCVCRYSNPPKCQCFDTHKFCYKSCHN |
| Vigna unguiculata | IBB_VIGUN (P17734) | ZASZSSKPCCRZCACTKSIPPZCRCSZVRLNSCHSACKSCACTFSIPAZCFCGBIBBFCYKPCKSSHSBBBBWN |
The primary accession numbers below are reported in UniProtKB database. P1-P1’ are the reactive peptide bond sites, in bold text. Either K or R at P1 position determines specificity for trypsin, whereas L, Y or F determines specificity against chymotrypsin; A determines specificity for elastase.
BBI from legume seeds contain high levels of cysteine residues involved in a conserved network of disulphide bridges (Figure 1B). Circular dichroism spectroscopy and fluorescence studies have revealed that the cysteine residues, involved in seven intramolecular disulphide bridges, provide extreme stability to high temperatures and resistance to proteolysis and help to maintain the structural and functional features of the inhibitory domains[35,36]. Mutational studies of the disulphide bonds in the N-terminal inhibitory domain of soybean BBI and the consequences of such mutations on inhibitory activity against serine proteases have been reported[37]. In particular, the mutations induced a dramatic effect on trypsin inhibition, with Ki values diminishing more than one order of magnitude in most of the mutants, compared with native BBI. A significant contribution of disulphide bonds in the anti-trypsin domain of BBI from horsegram (Dolichos biflorus) to thermal stability and control of the inhibitory activities, towards both trypsin and chymotrypsin, has been recently reported[38].
SURVIVAL OF BBI TO GUT DIGESTION
In order to exert any physiological effect in large intestine, physiologically relevant amounts of dietary BBI must survive, after food processing and further passage through the GIT, in biologically active form(s)[39]. In vitro and in vivo studies have demonstrated that BBI are functionally and structurally resistant to the extreme conditions within the GIT. Under acidic conditions, BBI are highly resistant to severe heat treatment, retaining their ability to inhibit serine proteases[40]. In processed legumes or their derived products, a high percentage of the trypsin inhibitory activity is associated to heat-stable BBI. In addition, soybean BBI have demonstrated to be remarkably resistant to the action of proteolytic enzymes under simulated gastric and intestinal digestion[41]. Soybean BBI is active at low pH in the presence of pepsin with no significant loss of protease inhibitory activity[42]. The structural rigidity provided by the disulphide bridge network play an essential role in maintaining both correct folding and functional structure of BBI[38,43,44]. Reduction of disulphide bridges and subsequent alkylation of the cysteinyl sulfhydryl groups abolishes almost completely both trypsin and chymotrypsin inhibitory activity of soybean BBI due to conformational changes and/or unfolding[26]; these structural changes increase the vulnerability of BBIs to digestive proteases and decrease thermal stability. The survival of functional BBI in the small intestine of animal models (rodent and pig) has been clearly demonstrated. Hajós et al[45] reported the presence of immunological reactive forms (5% of total ingested) of soybean BBI in the small intestine of rats; similar results were shown for cowpea (Vigna unguiculata) BBI in rat feeding trials[46]. Due to methodological difficulties, the protease inhibitory activities of BBI were not evaluated in these experiments. In pigs, generally held as a suitable model for human digestive physiology[47], it has been demonstrated that significant amounts of ingested chickpea BBI (5%-8%) can survive the extreme conditions within the GIT; chromatographic, electrophoretic and enzymatic data obtained from ileal samples revealed that both trypsin and chymotrypsin inhibitory activities were associated to a protein core comprising the two binding loops. Although processing at both N- and C-terminal ends of BBI during passage within the GIT was revealed, the network of disulphide bridges seems to exert a protective effect, avoiding an extensive proteolysis. By using mixed faecal samples from pigs, fermentation assays for a period of 24 h demonstrated that soybean BBI remained active and their ability to inhibit trypsin and chymotrypsin were not significantly diminished by the enzymatic and/or metabolic activity of faecal microbiota[48]. All of these results make protease inhibitors of the Bowman-Birk family attractive for further pharmacological and pre-clinical studies in order to assess their potential as colorectal chemopreventive agents.
COLORECTAL CHEMOPREVENTIVE PROPERTIES OF BBI
Colorectal carcinogenesis is one of the major causes of cancer-related mortality worldwide in both men and women, with over 1.2 million new cases diagnosed globally[49]. In recent years, substantial evidence has pointed to the link between dietary patterns and lifestyle in primary prevention and control of colorectal cancer (CRC). The anti-cancer effects of legumes have been explored extensively; although the evidence is still limited, several studies have claimed that a high intake of legumes may decrease risk of CRC. In a case-control study, Aune et al[50] reported that the level of legume intake necessary for being protective against different types of cancer, including CRC, is easily achievable by populations by including two small portions or 100 g of legumes per week. A meta-analysis of three cohort studies and eleven case control studies suggested an inverse association between legume intake levels and CRC risk[51]. It has been hypothesized that the direct contact of potential cancer preventive constituents of legumes with organs of the digestive system may be responsible of such beneficial effects. In particular, several studies suggest that dietary BBI from different legumes sources are effective at preventing or suppressing radiation- and chemical carcinogen-induced transformation in vitro, as well as carcinogenic and associated inflammatory processes within the mammalian GIT[14,39] (Table 2). The anti-carcinogenic properties of soybean BBI has been extensively investigated, both in purified form and as a BBIC. Soybean BBI exerted a protective role in dimethylhydrazine (DMH)-treated rats when ingested at low concentrations (10 mg/100 g diet), decreasing the frequency and incidence rates of colorectal tumours; no adverse effects on animal growth and organ/tissue morphology were observed[52]. These results are consistent to those reported previously on the suppression of colon carcinogenesis in DMH-treated mice when BBIC was administrated[53]. Autoclaved BBIC, in which the protease inhibitory activity was abolished, had no significant suppressive effects on DMH-induced colon carcinogenesis in rats, suggesting that the inherent ability of BBI to inhibit serine proteases is required for their reported chemopreventive properties[52].
Table 2.
Preclinical studies showing colorectal chemopreventive properties of Bowman-Birk inhibitor-like proteins from several legume species
| Species (common name) | Model system (carcinogen) | Effect and/or mechanisms of action | Ref. |
| Glycine max (soy) | Rodent colon carcinogenesis (DMH) | Soybean BBI is effective at concentrations as low as 10 mg/100 g diet in reducing the incidence and frequency of colorectal tumors. Its ability to inhibit serine proteases is required for their chemopreventive properties. No adverse effects are observed in treated animals | [52,53] |
| Mouse colorectal carcinogenesis (DMH) | Soybean BBI, when simultaneously treated with DMH, prevent the development of neoplastic lesions and protect against the onset of severe inflammatory processes | [79] | |
| Mouse colon inflammation (DSS) | A soybean Bomwan-Birk inhibitor concentrate reduces colon inflammation in mice with induced ulcerative colitis. Lower mortality rates and delayed onset of mortality are observed | [58] | |
| Colon cancer cell proliferation | The antiproliferative properties of BBI isoinhibitors, IBB1 and IBBD2, reveal that both trypsin- and chymotrypsin–like proteases involved in carcinogenesis should be considered as potential targets | ||
| [26] | |||
| Lens culinaris (lentil) | Colon cancer cell proliferation | Lentil BBI is able to inhibit the growth of HT29 colon cancer cells at concentrations as low as 19 μmol/L, in a concentration-dependent manner; by contrast, colonic fibroblast CCD-18Co cells are unaffected | [30] |
| Pisum sativum (pea) | Colon cancer cell proliferation | TI1B, a major pea protease inhibitor, affect in a dose-dependent manner the growth of HT29 colon cancer cells whereas an inactive mutant did not show any significant effect | [55] |
| Vicia faba (field bean) | Mouse stomach carcinogenesis (benzopyrene) | BBI proves to be biologically active, under acidic conditions, in suppressing benzopyrene-induced forestomach carcinogenesis in mice following oral treatment; the oncopreventive properties are related to its protease inhibitory activity | [93] |
DMH: Dimethylhydrazine; DSS: Dextran sulphate sodium; BBI: Bowman-Birk inhibitor.
Immortalized human epithelial cell lines are well-established models to investigate the action mechanism/s by which certain bioactive compounds might exert a chemoprotective effect in early stages of colorectal carcinogenesis. Recent studies have demonstrated a significant concentration- and time-dependent decrease in the proliferation of colorectal human adenocarcinoma cells (HT29, Caco2, LoVo), following treatment with BBI variants from pea[54], lentil[30] and soybean[26]. The BBI concentration that reduced cell viability by 50% (IC50), as compared with untreated controls, ranged from 32 to 73 μmol/L. Neither protein affected the growth of non-malignant colonic fibroblastic CCD18-Co cells. By using reducing and alkylating agents, the inhibitory activity of soybean BBI against serine proteases was abolished; inactive BBI was unable to inhibit cell proliferation of HT29 colon cancer cells suggesting that, in order to exert anti-proliferative effect on colon cancer cells, BBI need to be in active form(s)[26]. Nevertheless, when severe disruptive treatments are used, the native conformation of BBI is irreversibly affected, alongside significant reduction in both trypsin and chymotrypsin inhibitory activities, making the relationship between protease inhibitory activities, protein structure and health beneficial effects unclear. To answer this crucial question, a comparative study with rTI1, a major BBI from pea seeds expressed heterologously in Pichia pastoris, and a related synthetic mutant derivative lacking trypsin and chymotrypsin inhibitory activity was carried out[55]. rTI1 inhibited both trypsin and chymotrypsin, with Ki values at nanomolar concentrations, whereas the mutant protein was inactive against both enzymes. The proliferation of HT29 colon adenocarcinoma cells was significantly affected by rTI1 in a dose-dependent manner; in contrast, the inactive derivative did not show any suppressive effect on cell growth. Although the molecular mechanism(s) of such anti-proliferative activity remains unknown, the reported data indicate that cellular serine proteases should be considered as BBI primary targets in early stages of colorectal carcinogenesis.
The scission of soybean BBI with cyanogen bromide followed by pepsin treatment results in two active fragments, one having trypsin inhibitory activity and the other having chymotrypsin inhibitory activity. In early studies, Yavelow et al[56] using these two active fragments concluded that the chymotrypsin inhibitory domain of soybean BBI was responsible for suppression of radiation-induced transformation in vitro whereas the BBI fragment having only ability to inhibit trypsin-like proteases was ineffective. These observations led to the hypothesis that chymotrypsin-like proteases are potential therapeutic targets of BBI in clinical research; however, the enzymatically modified soybean BBI may have been impaired in the inhibition of several molecular targets compared with the native protein. Later on, it was demonstrated that a major soybean BBI isoform, IBBD2, which inhibits trypsin-like proteases only, exerts anti-proliferative effect against HT29 colon cancer cells in a dose-dependent manner[26] (Figure 2). These studies revealed that both trypsin- and chymotrypsin-like proteases involved in the early stages of carcinogenesis should be considered as potential targets of BBI. No data regarding the effectiveness of BBI variants having elastase inhibitory activity[20,21] on colon cancer cell proliferation has been reported so far.
Figure 2.
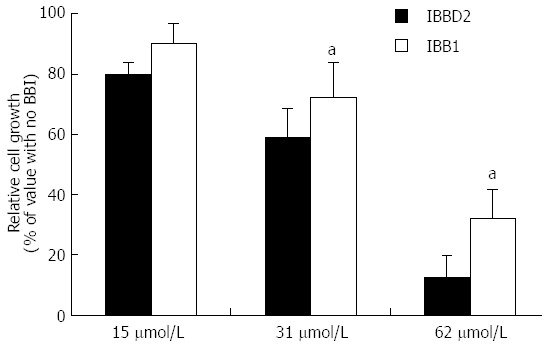
Effect of the major soybean isoinhibitors, IBBD2 and IBB1, on the in vitro growth of HT29 human colorectal adenocarcinoma cells. Growth media were supplemented with protein in the concentration range 0-62 μmol/L and cells harvested after a period of 96 h. Values are means, with standard deviations represented by vertical bars of at least three independent experiments, each having four technical replicates. aP < 0.05 vs IBBD2 group (Bonferroni´s test)[26].
A growing body of evidence suggests that dietary BBI may exert anti-inflammatory properties within the GIT. Soybean BBI and BBIC appears to exert a potent suppressive effect on colon and anal gland inflammation of carcinogen-treated rodents[57], or when assessed in the dextran sulfate sodium (DSS) model of UC[58]. Histological studies and mortality rates show that the DSS treatment induced a severe inflammatory condition in mice that was reduced in extent and severity by soybean BBIC. These preclinical studies suggest that soybean BBI might exert beneficial effects in inflammatory processes. In order to evaluate safety and efficacy of soybean BBI in patients with active UC, a randomized double-blind placebo-controlled trial was performed[13]. A daily dose of 800 chymotrypsin inhibitor units was administrated in patients receiving 12 wk of therapy. The BBIC treatment exerted a potential benefit over placebo in terms of clinical response and induction of remission in patients with active UC, as assessed by the Sutherland Disease Activity Index (an index that consist of four major criteria as follows: stool frequency, rectal bleeding, mucosal appearance, and physician rating of disease activity)[59]. After BBIC treatment, no adverse side-effects or apparent toxicity in UC patients were observed. Approximately 50% of patients responded clinically and 36% showed remission of disease; in contrast, only 29% and 7.1% on the placebo group achieved a partial response or remission, respectively.
Although not fully understood, several mechanisms of action have been proposed to explain the anti-inflammatory properties of BBI within the GIT. Soybean BBI or BBIC has been shown to decrease the production and release of superoxide anion radicals and hydrogen peroxide, mediators of acute and chronic inflammation, in purified human polymorphonuclear leukocytes[60] and in differentiated HL60 cells[61]. The decrease in superoxide radical levels might reduce levels of oxidative damage to DNA, lipid peroxidation of cellular membranes and incidence of malignant transformation. Although BBI do not function as free radical-scavenging agents, they prevent the release of oxygen free radicals from cells, which would be expected to contribute to their reported anti-inflammatory properties[62]. Serine proteases are key components of the inflammatory response, as they can trigger hypersensitivity and may cause severe proteolytic damage to the extracellular matrix[63,64]. Dysregulation of the epithelial barrier function play a central role in either the etiology or the pathology of intestinal inflammation. Currently, there is a strong interest in identifying candidate serine proteases involved in pathogenesis and in designing selective protease inhibitors to regulate their proteolytic activities[39]. In this sense, the use of anti-tryptase therapy on human inflammatory bowel disease and experimental colitis has been demonstrated[65]. In intestinal lesions and faecal samples from UC patients, a significant increase of serine protease activity when compared to healthy controls was observed[66,67]; these proteolytic activities can be completely abolished by soybean BBI[68].
SERINE PROTEASES AS POTENTIAL TARGETS OF BBI IN COLORECTAL CHEMOPREVENTION
The homeostatic control between proteolytic enzymes and their cognate inhibitors plays a fundamental role in a number of physiological as well as pathological processes, where their activities become dysregulated. Serine proteases are involved in crucial biological functions associated to tumor development, such as cell growth (dys)regulation, cell invasion, angiogenesis and inflammatory processes. Some of these serine proteases have been proposed as candidate cancer biomarkers[69-71] (Table 3). An understanding of the role played by certain serine proteases in pathological processes may suggest modes of therapeutic intervention[1,72]. In eukaryotes, the ubiquitin-proteasome pathway regulates many fundamental cellular processes such as protein quality control, cell cycle, signal transduction and DNA repair[73]. The 20/26S proteasome complex is the most downstream element of the ubiquitin-proteasome pathway. Inhibition of proteasome activity leads to accumulation of poly-ubiquitinylated and misfolded proteins, endoplastic reticulum stress, reduction in cell proliferation rates and induction of apoptosis through multiple mechanisms[74,75]. The proteasome complex is currently considered an important intracellular target for the treatment of cancer; proteasome inhibition results in cellular homeostasis disruption and in the induction of apoptosis. Until now, only a few studies have demonstrated the use of BBIs as potential inhibitors of proteasomal activities. BBI from soybean inhibits the chymotrypsin-like proteasomal activity of MCF7 breast cancer cells in vitro and in vivo[76]. The proteasomal inhibition results in the accumulation of ubiquitinated proteins and proteasome substrates, p21Cip1/WAFF1 and p27Kip1, and the consequent down-regulation of cyclin D1 and cyclin E that modulate the G1/S phase progression, suggesting that BBI might induce cell-cycle arrest. Soybean BBI decrease proteasomal function and results in up-regulation of MAP kinase phosphatase-1 (MKP-1), which in turn suppresses phosphorylation coupled to extracellular signal-related kinase activity in MCF7 treated cells. The inhibition of proteasomal chymotrypsin-like activity in vivo reveals that MCF7 cellular membranes are very permeable to soybean BBI facilitating the inhibition of intracellular target proteases. Soybean BBI has been demonstrated to be taken up by intestinal epithelia cells in a time-dependent manner, being the bulk of the internalised protease inhibitor present in the cytosol in active form[77]. It has been also reported that soybean BBI is internalised into NIH/3T3 mouse embryo fibroblastic cells and is localized in the nucleus[41]. More recently, confocal microscopy studies have demonstrated that black-eyed pea BBI crosses the membrane of breast MCF7 cancer cells, likely via endocytosis, and co-localizes with the proteasome in cytoplasm and mainly in nucleus, inhibiting the chymotrypsin-, trypsin- and caspase-like activities of the 20S proteasome[78]. Further studies to determine the correct localization of BBI in colon cancer cells will be relevant in order to identify serine proteases as potential therapeutic targets. Either soybean or perennial horsegram BBIs, when administrated at a dose of 30 mg/kg during 12 wk, exerted a protective role in the development of pre-neoplastic lesions induced by intraperitoneal injections of DMH in mice; such effect seems to be associated to the inhibition of both the lysosomal and proteasome-dependent proteolytic pathways[79]. Although soybean BBI has been demonstrated to inhibit the proteosomal activity of MCF7 breast cancer cells[76] and U2OS osteosarcoma cells[80], the proteosomal inhibition in colon cancer cells need to be unambiguously demonstrated. Another potential therapeutic target of BBI is matriptase (also known as MT-SP1 or epithin), an epithelial-specific member of the type II transmembrane serine protease family, which is a key activator of multiple signaling pathways associated with cell proliferation and modification of the extracellular matrix. Matriptase is recognized as a cancer-associated trypsin-like protease, being over-expressed in malignant prostate, ovarian, uterine and colon tumors[81,82]. This serine protease contributes to the epithelial integrity and upstream activation of cellular regulatory proteins, including urokinase-plasminogen activator, hepatocyte-growth factor/scatter factor and protease-activated receptor, being also involved in cancer invasion and metastasis[83,84]. Although the ability of naturally-occurring protease inhibitors, including soybean BBI, lima bean trypsin inhibitor and sunflower trypsin inhibitor (SFTI-1), to inhibit a secreted form of recombinant MT-SP1 has been demonstrated[85,86], the clinical relevance of such inhibition has not been yet elucidated.
Table 3.
Serine proteases as potential therapeutic targets of Bowman-Birk inhibitor-like proteins in pathological processes
| Serine protease | Function | Pathological processes | Evidence of interaction with BBI-like proteins |
| Proteasome | Control of the turn-over of regulatory proteins involved in critical cellular processes including cell cycle progression, cell development and differentiation, apoptosis, angiogenesis and signaling pathways | Cancer, inflammatory processes, autoimmune diseases and aging | Soybean BBI specifically and potently inhibits the proteasomal chymotrypsin-like activity in vitro and in vivo in MCF7 cancer breast cells[76] |
| Mice treated simultaneously with BBI and DMH show a significant decrease in the chymotrypsin- and trypsin-like proteasomal activity in comparison with those treated with DMH only[79] | |||
| Soybean BBI suppress proteasomal chymotrypsin-like activity in U2OS human osteosarcoma cells in vitro[80] | |||
| Matriptase | Differentiation and function of epithelial tissues | Activator of critical molecules associated with tumor invasion and metastasis | SFTI-1, a cyclic peptide from sunflower having similar features to the trypsin inhibitory binding domain of BBI, is a very potent inhibitor (Ki: 0.92 nmol/L)[85] |
| BBI from soybean and lima bean have been reported to inhibit matriptase activity in vitro[86] | |||
| Chymase | Key mediator in inflammatory cell signaling pathways | Inflammatory processes, allergic reactions and pulmonary fibrosis | Soybean BBI strongly inhibits chymase from rat mast cells (Ki: 13.2 nmol/L)[94] |
| Soybean BBI is a highly effective inhibitor of human mast cell chymase, being not effective against human tryptase[88] | |||
| Cathepsin G | Degradation of extracellular matrix components, regulates inflammatory response and promotes apoptosis | Inflammatory processes, cancer and aging | Soybean BBI inhibits strongly cathepsin G (Ki: 1.2 nmol/L)[86] |
| Duodenase | Morphogenesis and tissue repair; inflammatory and mitogenic role; participation in activation cascade of digestive proteases | Inflammatory processes | Duodenase interacts specifically with the chymotrypsin inhibitory domain of soybean BBI (Ki: 4 nmol/L)[87] |
| Elastase | Degradation of extracellular matrix components | Pulmonary emphysema, cystic fibrosis, infections, inflammation and atherosclerosis | Soybean BBI inhibits hydrolysis of extracellular matrix components by leukocyte enzymes[95] |
| Soybean BBI inhibit human leukocyte elastase (Ki: 2.3 nmol/L)[96] |
BBI: Bowman-Birk inhibitor; DMH: Dimethylhydrazine; Ki: Constant of inhibition; SFTI-1: Sunflower trypsin inhibitor.
Serine proteases are extensively involved in immunological responses and pro-inflammatory actions; therefore, there is a growing interest towards determining the therapeutic potential of serine protease inhibitors in treatment of inflammatory diseases by modifying various inflammatory pathways[4]. The inhibition of serine proteases involved in inflammatory processes, such as cathepsin G[87,88], elastase and mast cell chymase[89] by soybean BBI has been reported (Table 3). Secreted chymases promote inflammation[90], matrix destruction, tissue remodelling as well as the regulation of collagenase[91] and interleukin 1β (IL-1β)[92]. Nevertheless, a clinical correlation between the inhibition of these serine proteases and the anti-inflammatory properties associated with soybean BBI and homologous proteins is still far of being elucidated.
CONCLUSION
In vitro and in vivo studies have demonstrated that soybean BBI and homologous proteins can exert a protective and/or suppressive effect on cancer development and inflammatory processes within the GIT; so far, the therapeutic targets and the action mechanism of BBI as colorectal chemopreventive agents remain unknown. Recent investigations suggest that cellular serine proteases should be considered as potential targets of BBI in further investigations of their chemopreventive properties. The validation of serine proteases as clinical targets together with the identification and elucidation of the molecular basis for variation in the biological activity of natural BBI variants and/or design of selective potent inhibitors against their putative protease targets will contribute to the assessment of BBI as colorectal chemopreventive agents for preventive and/or therapeutic medicine[39].
Footnotes
Supported by ERDF-co-financed grant from the Spanish CICYT, No. AGL2011-26353 to Clemente A; Clemente A involved in COST Action FA1005 INFOGEST on Food Digestion
P- Reviewer: Chen JL, Serafino A S- Editor: Gou SX L- Editor: A E- Editor: Ma S
References
- 1.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 2.Soreide K, Janssen EA, Körner H, Baak JP. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–156. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]
- 3.Sharony R, Yu PJ, Park J, Galloway AC, Mignatti P, Pintucci G. Protein targets of inflammatory serine proteases and cardiovascular disease. J Inflamm (Lond) 2010;7:45. doi: 10.1186/1476-9255-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safavi F, Rostami A. Role of serine proteases in inflammation: Bowman-Birk protease inhibitor (BBI) as a potential therapy for autoimmune diseases. Exp Mol Pathol. 2012;93:428–433. doi: 10.1016/j.yexmp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Deu E, Verdoes M, Bogyo M. New approaches for dissecting protease functions to improve probe development and drug discovery. Nat Struct Mol Biol. 2012;19:9–16. doi: 10.1038/nsmb.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemente A, Domoney C, Therapeutic properties of legume protease inhibitors from the Bowman birk class. In: Govil JN, Singh VK, Sharma RK, editors. Recent Progress in Medicinal Plants. Houston: Studium Press; 2007. pp. 345–365. [Google Scholar]
- 7.Oliva ML, Ferreira Rda S, Ferreira JG, de Paula CA, Salas CE, Sampaio MU. Structural and functional properties of Kunitz proteinase inhibitors from Leguminosae: a mini review. Curr Protein Pept Sci. 2011;12:348–357. doi: 10.2174/138920311796391061. [DOI] [PubMed] [Google Scholar]
- 8.Malkowicz SB, McKenna WG, Vaughn DJ, Wan XS, Propert KJ, Rockwell K, Marks SH, Wein AJ, Kennedy AR. Effects of Bowman-Birk inhibitor concentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate. 2001;48:16–28. doi: 10.1002/pros.1077. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong WB, Kennedy AR, Wan XS, Atiba J, McLaren CE, Meyskens FL. Single-dose administration of Bowman-Birk inhibitor concentrate in patients with oral leukoplakia. Cancer Epidemiol Biomarkers Prev. 2000;9:43–47. [PubMed] [Google Scholar]
- 10.Armstrong WB, Kennedy AR, Wan XS, Taylor TH, Nguyen QA, Jensen J, Thompson W, Lagerberg W, Meyskens FL. Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial. Clin Cancer Res. 2000;6:4684–4691. [PubMed] [Google Scholar]
- 11.Meyskens FL, Taylor T, Armstrong W, Kong L, Gu M, Gonzalez R, Villa M, Wong V, Garcia A, Perloff M, et al. Phase IIb randomized clinical chemoprevention trial of a soybean-derived compound (Bowman-Birk inhibitor concentrate) for oral leukoplakia. Cancer Prev Res. 2010;3:CN02–05. [Google Scholar]
- 12.Armstrong WB, Taylor TH, Kennedy AR, Melrose RJ, Messadi DV, Gu M, Le AD, Perloff M, Civantos F, Goodwin WJ, et al. Bowman birk inhibitor concentrate and oral leukoplakia: a randomized phase IIb trial. Cancer Prev Res (Phila) 2013;6:410–418. doi: 10.1158/1940-6207.CAPR-13-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Deren JJ, Katz S, Lewis JD, Kennedy AR, Ware JH. Bowman-Birk inhibitor concentrate: a novel therapeutic agent for patients with active ulcerative colitis. Dig Dis Sci. 2008;53:175–180. doi: 10.1007/s10620-007-9840-2. [DOI] [PubMed] [Google Scholar]
- 14.Clemente A, Marín-Manzano MC, Arques MC, Domoney C. Bowman-Birk inhibitors from legumes: utilisation in disease prevention and therapy. In: Hernández-Ledesma B, Hsieh CC, editors. Bioactive food peptides in health and disease. Rijeka (Croatia): InTech-Open Access Publisher; 2013. pp. 23–44. [Google Scholar]
- 15.Chen P, Rose J, Love R, Wei CH, Wang BC. Reactive sites of an anticarcinogenic Bowman-Birk proteinase inhibitor are similar to other trypsin inhibitors. J Biol Chem. 1992;267:1990–1994. doi: 10.2210/pdb1pi2/pdb. [DOI] [PubMed] [Google Scholar]
- 16.Bode W, Huber R. Natural protein proteinase inhibitors and their interaction with proteinases. Eur J Biochem. 1992;204:433–451. doi: 10.1111/j.1432-1033.1992.tb16654.x. [DOI] [PubMed] [Google Scholar]
- 17.Li de la Sierra I, Quillien L, Flecker P, Gueguen J, Brunie S. Dimeric crystal structure of a Bowman-Birk protease inhibitor from pea seeds. J Mol Biol. 1999;285:1195–1207. doi: 10.1006/jmbi.1998.2351. [DOI] [PubMed] [Google Scholar]
- 18.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. 1967. Biochem Biophys Res Commun. 2012;425:497–502. doi: 10.1016/j.bbrc.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Koepke J, Ermler U, Warkentin E, Wenzl G, Flecker P. Crystal structure of cancer chemopreventive Bowman-Birk inhibitor in ternary complex with bovine trypsin at 2.3 A resolution. Structural basis of Janus-faced serine protease inhibitor specificity. J Mol Biol. 2000;298:477–491. doi: 10.1006/jmbi.2000.3677. [DOI] [PubMed] [Google Scholar]
- 20.Deshimaru M, Hanamoto R, Kusano C, Yoshimi S, Terada S. Purification and characterization of proteinase inhibitors from wild soja (Glycine soja) seeds. Biosci Biotechnol Biochem. 2002;66:1897–1903. doi: 10.1271/bbb.66.1897. [DOI] [PubMed] [Google Scholar]
- 21.De Paola D, Blanco E, Pierri CL, Sonnante G. Isolation and characterization of novel variants of BBI coding genes from the legume Lathyrus sativus. Plant Physiol Biochem. 2012;57:45–53. doi: 10.1016/j.plaphy.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu W, Apostol I, Qasim MA, Warne N, Wynn R, Zhang WL, Anderson S, Chiang YW, Ogin E, Rothberg I, et al. Binding of amino acid side-chains to S1 cavities of serine proteinases. J Mol Biol. 1997;266:441–461. doi: 10.1006/jmbi.1996.0781. [DOI] [PubMed] [Google Scholar]
- 24.Gariani T, Leatherbarrow RJ. Stability of protease inhibitors based on the Bowman-Birk reactive site loop to hydrolysis by proteases. J Pept Res. 1997;49:467–475. doi: 10.1111/j.1399-3011.1997.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 25.Gariani T, McBride JD, Leatherbarrow RJ. The role of the P2’ position of Bowman-Birk proteinase inhibitor in the inhibition of trypsin. Studies on P2’ variation in cyclic peptides encompassing the reactive site loop. Biochim Biophys Acta. 1999;1431:232–237. doi: 10.1016/s0167-4838(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 26.Clemente A, Moreno FJ, Marín-Manzano MC, Jiménez E, Domoney C. The cytotoxic effect of Bowman-Birk isoinhibitors, IBB1 and IBBD2, from soybean (Glycine max) on HT29 human colorectal cancer cells is related to their intrinsic ability to inhibit serine proteases. Mol Nutr Food Res. 2010;54:396–405. doi: 10.1002/mnfr.200900122. [DOI] [PubMed] [Google Scholar]
- 27.Ferrasson E, Quillien L, Gueguen J. Proteinase inhibitors from pea seeds: purification and characterization. J Agric Food Chem. 1997;45:127–131. [Google Scholar]
- 28.Clemente A, MacKenzie DA, Jeenes DJ, Domoney C. The effect of variation within inhibitory domains on the activity of pea protease inhibitors from the Bowman-Birk class. Protein Expr Purif. 2004;36:106–114. doi: 10.1016/j.pep.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Ragg EM, Galbusera V, Scarafoni A, Negri A, Tedeschi G, Consonni A, Sessa F, Duranti M. Inhibitory properties and solution structure of a potent Bowman-Birk protease inhibitor from lentil (Lens culinaris, L) seeds. FEBS J. 2006;273:4024–4039. doi: 10.1111/j.1742-4658.2006.05406.x. [DOI] [PubMed] [Google Scholar]
- 30.Caccialupi P, Ceci LR, Siciliano RA, Pignone D, Clemente A, Sonnante G. Bowman-Birk inhibitors in lentil: heterologous expression, functional characterization and anti-proliferative properties in human colon cancer cells. Food Chem. 2010;120:1058–1066. [Google Scholar]
- 31.Scarafoni A, Consonni A, Galbusera V, Negri A, Tedeschi G, Rasmussen P, Magni C, Duranti M. Identification and characterization of a Bowman-Birk inhibitor active towards trypsin but not chymotrypsin in Lupinus albus seeds. Phytochemistry. 2008;69:1820–1825. doi: 10.1016/j.phytochem.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Domoney C, Welham T, Ellis N, Mozzanega P, Turner L. Three classes of proteinase inhibitor gene have distinct but overlapping patterns of expression in Pisum sativum plants. Plant Mol Biol. 2002;48:319–329. doi: 10.1023/a:1013379430582. [DOI] [PubMed] [Google Scholar]
- 33.Clemente A, Domoney C. Biological significance of polymorphism in legume protease inhibitors from the Bowman-Birk family. Curr Protein Pept Sci. 2006;7:201–216. doi: 10.2174/138920306777452349. [DOI] [PubMed] [Google Scholar]
- 34.Quillien L, Ferrasson E, Molle D, Gueguen J. Trypsin inhibitor polymorphism: multigene family expression and posttranslational modification. J Protein Chem. 1997;16:195–203. doi: 10.1023/a:1026326808553. [DOI] [PubMed] [Google Scholar]
- 35.Ramasarma PR, Rao AG, Rao DR. Role of disulfide linkages in structure and activity of proteinase inhibitor from horsegram (Dolichos biflorus) Biochim Biophys Acta. 1995;1248:35–42. doi: 10.1016/0167-4838(95)00004-e. [DOI] [PubMed] [Google Scholar]
- 36.Singh RR, Appu Rao AG. Reductive unfolding and oxidative refolding of a Bowman-Birk inhibitor from horsegram seeds (Dolichos biflorus): evidence for “hyperreactive” disulfide bonds and rate-limiting nature of disulfide isomerization in folding. Biochim Biophys Acta. 2002;1597:280–291. doi: 10.1016/s0167-4838(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 37.Philipp S, Kim YM, Dürr I, Wenzl G, Vogt M, Flecker P. Mutational analysis of disulfide bonds in the trypsin-reactive subdomain of a Bowman-Birk-type inhibitor of trypsin and chymotrypsin--cooperative versus autonomous refolding of subdomains. Eur J Biochem. 1998;251:854–862. doi: 10.1046/j.1432-1327.1998.2510854.x. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Gowda LR. The contribution of two disulfide bonds in the trypsin binding domain of horsegram (Dolichos biflorus) Bowman-Birk inhibitor to thermal stability and functionality. Arch Biochem Biophys. 2013;537:49–61. doi: 10.1016/j.abb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Clemente A, Sonnante G, Domoney C. Bowman-Birk inhibitors from legumes and human gastrointestinal health: current status and perspectives. Curr Protein Pept Sci. 2011;12:358–373. doi: 10.2174/138920311796391133. [DOI] [PubMed] [Google Scholar]
- 40.Muricken DG, Gowda LR. Functional expression of horsegram (Dolichos biflorus) Bowman-Birk inhibitor and its self-association. Biochim Biophys Acta. 2010;1804:1413–1423. doi: 10.1016/j.bbapap.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Jeong HJ, Lumen BO. In vitro digestibility of the cancer-preventive soy peptides lunasin and BBI. J Agric Food Chem. 2007;55:10703–10706. doi: 10.1021/jf072107c. [DOI] [PubMed] [Google Scholar]
- 42.Weder JK. Inhibition of human proteinases by grain legumes. Adv Exp Med Biol. 1986;199:239–279. doi: 10.1007/978-1-4757-0022-0_16. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi MV, Laurence JS, Siahaan TJ. The role of thiols and disulfides on protein stability. Curr Protein Pept Sci. 2009;10:614–625. doi: 10.2174/138920309789630534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateman KS, James MN. Plant protein proteinase inhibitors: structure and mechanism of inhibition. Curr Protein Pept Sci. 2011;12:340–347. doi: 10.2174/138920311796391124. [DOI] [PubMed] [Google Scholar]
- 45.Hajós G, Gelencsér E, Pusztai A, Grant G, Sakhri M, Bardocz S. Biological effects and survival of trypsin inhibitors and the agglutinin from soybean in the small intestine of the rat. J Agric Food Chem. 1995;43:165–170. [Google Scholar]
- 46.Pusztai A, Grant G, Brown DJ, Stewart JC, Bardocz S, Ewen SW, Gatehouse AM, Hilder V. Nutritional evaluation of the trypsin (EC 3.4.21.4) inhibitor from cowpea (Vigna unguiculata Walp.) Br J Nutr. 1992;68:783–791. doi: 10.1079/bjn19920133. [DOI] [PubMed] [Google Scholar]
- 47.Clemente A, Jiménez E, Marín-Manzano MC, Rubio LA. Active Bowman-Birk inhibitors survive gastrointestinal digestion at the terminal ileum of pigs fed chickpea-based diets. J Sci Food Agric. 2008;88:523–531. [Google Scholar]
- 48.Marín-Manzano MC, Ruiz R, Jiménez E, Rubio LA, Clemente A. Anti-carcinogenic soyabean Bowman-Birk inhibitors survive faecal fermentation in their active form and do not affect the microbiota composition in vitro. Br J Nutr. 2009;101:967–971. doi: 10.1017/s0007114508057590. [DOI] [PubMed] [Google Scholar]
- 49.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 50.Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Legume intake and the risk of cancer: a multisite case-control study in Uruguay. Cancer Causes Control. 2009;20:1605–1615. doi: 10.1007/s10552-009-9406-z. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Wang Z, Fu L, Chen Y, Fang J. Legume consumption and colorectal adenoma risk: a meta-analysis of observational studies. PLoS One. 2013;8:e67335. doi: 10.1371/journal.pone.0067335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kennedy AR, Billings PC, Wan XS, Newberne PM. Effects of Bowman-Birk inhibitor on rat colon carcinogenesis. Nutr Cancer. 2002;43:174–186. doi: 10.1207/S15327914NC432_8. [DOI] [PubMed] [Google Scholar]
- 53.St Clair WH, Billings PC, Carew JA, Keller-McGandy C, Newberne P, Kennedy AR. Suppression of dimethylhydrazine-induced carcinogenesis in mice by dietary addition of the Bowman-Birk protease inhibitor. Cancer Res. 1990;50:580–586. [PubMed] [Google Scholar]
- 54.Clemente A, Gee JM, Johnson IT, Mackenzie DA, Domoney C. Pea (Pisum sativum L.) protease inhibitors from the Bowman-Birk class influence the growth of human colorectal adenocarcinoma HT29 cells in vitro. J Agric Food Chem. 2005;53:8979–8986. doi: 10.1021/jf051528w. [DOI] [PubMed] [Google Scholar]
- 55.Clemente A, Carmen Marín-Manzano M, Jiménez E, Carmen Arques M, Domoney C. The anti-proliferative effect of TI1B, a major Bowman-Birk isoinhibitor from pea (Pisum sativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br J Nutr. 2012;108 Suppl 1:S135–S144. doi: 10.1017/S000711451200075X. [DOI] [PubMed] [Google Scholar]
- 56.Yavelow J, Collins M, Birk Y, Troll W, Kennedy AR. Nanomolar concentrations of Bowman-Birk soybean protease inhibitor suppress x-ray-induced transformation in vitro. Proc Natl Acad Sci USA. 1985;82:5395–5399. doi: 10.1073/pnas.82.16.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Billings PC, Newberne PM, Kennedy AR. Protease inhibitor suppression of colon and anal gland carcinogenesis induced by dimethylhydrazine. Carcinogenesis. 1990;11:1083–1086. doi: 10.1093/carcin/11.7.1083. [DOI] [PubMed] [Google Scholar]
- 58.Ware JH, Wan XS, Newberne P, Kennedy AR. Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig Dis Sci. 1999;44:986–990. doi: 10.1023/a:1026616832119. [DOI] [PubMed] [Google Scholar]
- 59.Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 60.Frenkel K, Chrzan K, Ryan CA, Wiesner R, Troll W. Chymotrypsin-specific protease inhibitors decrease H2O2 formation by activated human polymorphonuclear leukocytes. Carcinogenesis. 1987;8:1207–1212. doi: 10.1093/carcin/8.9.1207. [DOI] [PubMed] [Google Scholar]
- 61.Ware JH, Wan XS, Kennedy AR. Bowman-Birk inhibitor suppresses production of superoxide anion radicals in differentiated HL-60 cells. Nutr Cancer. 1999;33:174–177. doi: 10.1207/S15327914NC330209. [DOI] [PubMed] [Google Scholar]
- 62.Baturay NZ, Roque H. In vitro reduction of peroxidation in UVC-irradiated cell cultures by concurrent exposure with Bowman-Birk protease inhibitor. Teratog Carcinog Mutagen. 1991;11:195–202. doi: 10.1002/tcm.1770110404. [DOI] [PubMed] [Google Scholar]
- 63.Shimoda N, Fukazawa N, Nonomura K, Fairchild RL. Cathepsin G is required for sustained inflammation and tissue injury after reperfusion of ischemic kidneys. Am J Pathol. 2007;170:930–940. doi: 10.2353/ajpath.2007.060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Annaházi A, Gecse K, Dabek M, Ait-Belgnaoui A, Rosztóczy A, Róka R, Molnár T, Theodorou V, Wittmann T, Bueno L, et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain. 2009;144:209–217. doi: 10.1016/j.pain.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida N, Isozaki Y, Takagi T, Takenaka S, Uchikawa R, Arizono N, Yoshikawa T, Okanoue T. Anti-tryptase therapy in inflammatory bowel disease. Aliment Pharmacol Ther Symp Ser. 2006;2:249–255. [Google Scholar]
- 66.Róka R, Rosztóczy A, Leveque M, Izbéki F, Nagy F, Molnár T, Lonovics J, Garcia-Villar R, Fioramonti J, Wittmann T, et al. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:550–555. doi: 10.1016/j.cgh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 68.Hawkins JV, Emmel EL, Feuer JJ, Nedelman MA, Harvey CJ, Klein HJ, Rozmiarek H, Kennedy AR, Lichtenstein GR, Billings PC. Protease activity in a hapten-induced model of ulcerative colitis in rats. Dig Dis Sci. 1997;42:1969–1980. doi: 10.1023/a:1018887832465. [DOI] [PubMed] [Google Scholar]
- 69.Weldon S, McNally P, McElvaney NG, Elborn JS, McAuley DF, Wartelle J, Belaaouaj A, Levine RL, Taggart CC. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J Immunol. 2009;183:8148–8156. doi: 10.4049/jimmunol.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue Y, Yokobori T, Yokoe T, Toiyama Y, Miki C, Mimori K, Mori M, Kusunoki M. Clinical significance of human kallikrein7 gene expression in colorectal cancer. Ann Surg Oncol. 2010;17:3037–3042. doi: 10.1245/s10434-010-1132-y. [DOI] [PubMed] [Google Scholar]
- 71.Petraki C, Dubinski W, Scorilas A, Saleh C, Pasic MD, Komborozos V, Khalil B, Gabril MY, Streutker C, Diamandis EP, et al. Evaluation and prognostic significance of human tissue kallikrein-related peptidase 6 (KLK6) in colorectal cancer. Pathol Res Pract. 2012;208:104–108. doi: 10.1016/j.prp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Scott CJ, Taggart CC. Biologic protease inhibitors as novel therapeutic agents. Biochimie. 2010;92:1681–1688. doi: 10.1016/j.biochi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Latonen L, Moore HM, Bai B, Jäämaa S, Laiho M. Proteasome inhibitors induce nucleolar aggregation of proteasome target proteins and polyadenylated RNA by altering ubiquitin availability. Oncogene. 2011;30:790–805. doi: 10.1038/onc.2010.469. [DOI] [PubMed] [Google Scholar]
- 74.Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 75.Wu WK, Cho CH, Lee CW, Wu K, Fan D, Yu J, Sung JJ. Proteasome inhibition: a new therapeutic strategy to cancer treatment. Cancer Lett. 2010;293:15–22. doi: 10.1016/j.canlet.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Chen YW, Huang SC, Lin-Shiau SY, Lin JK. Bowman-Birk inhibitor abates proteasome function and suppresses the proliferation of MCF7 breast cancer cells through accumulation of MAP kinase phosphatase-1. Carcinogenesis. 2005;26:1296–1306. doi: 10.1093/carcin/bgi062. [DOI] [PubMed] [Google Scholar]
- 77.Billings PC, Brandon DL, Habres JM. Internalisation of the Bowman-Birk protease inhibitor by intestinal epithelial cells. Eur J Cancer. 1991;27:903–908. doi: 10.1016/0277-5379(91)90144-3. [DOI] [PubMed] [Google Scholar]
- 78.Souza Lda C, Camargo R, Demasi M, Santana JM, de Sá CM, de Freitas SM. Effects of an anticarcinogenic Bowman-Birk protease inhibitor on purified 20S proteasome and MCF-7 breast cancer cells. PLoS One. 2014;9:e86600. doi: 10.1371/journal.pone.0086600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Paula Carli A, de Abreu Vieira PM, Silva KT, de Sá Cota RG, Carneiro CM, Castro-Borges W, de Andrade MH. Bowman-Birk inhibitors, proteasome peptidase activities and colorectal pre neoplasias induced by 1,2-dimethylhydrazine in Swiss mice. Food Chem Toxicol. 2012;50:1405–1412. doi: 10.1016/j.fct.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 80.Saito T, Sato H, Virgona N, Hagiwara H, Kashiwagi K, Suzuki K, Asano R, Yano T. Negative growth control of osteosarcoma cell by Bowman-Birk protease inhibitor from soybean; involvement of connexin 43. Cancer Lett. 2007;253:249–257. doi: 10.1016/j.canlet.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 81.Bhatt AS, Takeuchi T, Ylstra B, Ginzinger D, Albertson D, Shuman MA, Craik CS. Quantitation of membrane type serine protease 1 (MT-SP1) in transformed and normal cells. Biol Chem. 2003;384:257–266. doi: 10.1515/BC.2003.029. [DOI] [PubMed] [Google Scholar]
- 82.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 84.List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol. 2009;175:1453–1463. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long YQ, Lee SL, Lin CY, Enyedy IJ, Wang S, Li P, Dickson RB, Roller PP. Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett. 2001;11:2515–2519. doi: 10.1016/s0960-894x(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 86.Yamasaki Y, Satomi S, Murai N, Tsuzuki S, Fushiki T. Inhibition of membrane-type serine protease 1/matriptase by natural and synthetic protease inhibitors. J Nutr Sci Vitaminol (Tokyo) 2003;49:27–32. doi: 10.3177/jnsv.49.27. [DOI] [PubMed] [Google Scholar]
- 87.Larionova NI, Gladysheva IP, Tikhonova TV, Kazanskaia NF. [Inhibition of cathepsin G and elastase from human granulocytes by multiple forms of the Bowman-Birk type of soy inhibitor] Biokhimiia. 1993;58:1437–1444. [PubMed] [Google Scholar]
- 88.Gladysheva IP, Zamolodchikova TS, Sokolova EA, Larionova NI. Interaction between duodenase, a proteinase with dual specificity, and soybean inhibitors of Bowman-Birk and Kunitz type. Biochemistry (Mosc) 1999;64:1244–1249. [PubMed] [Google Scholar]
- 89.Ware JH, Wan XS, Rubin H, Schechter NM, Kennedy AR. Soybean Bowman-Birk protease inhibitor is a highly effective inhibitor of human mast cell chymase. Arch Biochem Biophys. 1997;344:133–138. doi: 10.1006/abbi.1997.0182. [DOI] [PubMed] [Google Scholar]
- 90.Tani K, Ogushi F, Kido H, Kawano T, Kunori Y, Kamimura T, Cui P, Sone S. Chymase is a potent chemoattractant for human monocytes and neutrophils. J Leukoc Biol. 2000;67:585–589. doi: 10.1002/jlb.67.4.585. [DOI] [PubMed] [Google Scholar]
- 91.Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134–18140. [PubMed] [Google Scholar]
- 92.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fernandes AO, Banerji AP. Inhibition of benzopyrene-induced forestomach tumors by field bean protease inhibitor(s) Carcinogenesis. 1995;16:1843–1846. doi: 10.1093/carcin/16.8.1843. [DOI] [PubMed] [Google Scholar]
- 94.Fukusen N, Kato Y, Kido H, Katunuma N. Kinetic studies on the inhibitions of mast cell chymase by natural serine protease inhibitors: indications for potential biological functions of these inhibitors. Biochem Med Metab Biol. 1987;38:165–169. doi: 10.1016/0885-4505(87)90076-4. [DOI] [PubMed] [Google Scholar]
- 95.Tikhonova TV, Gladysheva IP, Larionova NI. Retardation by the soybean Bowman-Birk inhibitor of elastin hydrolysis catalyzed by leukocyte proteinases. FEBS Lett. 1995;362:225–228. doi: 10.1016/0014-5793(95)00202-k. [DOI] [PubMed] [Google Scholar]
- 96.Larionova NI, Gladysheva IP, Gladyshev DP. Human leukocyte elastase inhibition by Bowman-Birk soybean inhibitor. Discrimination of the inhibition mechanisms. FEBS Lett. 1997;404:245–248. doi: 10.1016/s0014-5793(97)00089-6. [DOI] [PubMed] [Google Scholar]


