Abstract
AIM: To calculate the proportion of potentially preventable hospitalizations due to peptic ulcer disease (PUD), erosive gastritis (EG) or duodenitis (ED).
METHODS: Retrospective cohort study using ICD-10 codes to identify all patients with upper gastrointestinal hemorrhage secondary to endoscopically proven PUD, EG or ED during the period from March 2007 to October 2010 in three major metropolitan hospitals in Melbourne, Australia. Patients were divided into “high risk” (those who would benefit from gastroprotection) and “not high risk” groups as defined by established guidelines. Mean Rockall score, transfusion requirement, length of stay, rebleeding rates, need for surgery and in-hospital mortality was compared between “high risk” and “not high risk” groups. Within the “high risk” group, those on gastroprotection and those with no gastroprotection were also compared.
RESULTS: Five hundred and seven patients were included for analysis of which 174 were classified as high risk. Median values of complete Rockall Score (5 vs 4, P = 0.002) and length of stay (5 d vs 4 d, P = 0.04) were higher in the high risk group but in-hospital mortality was lower (0.6% vs 3.9%, P = 0.03). 130 out of the 174 patients in the high risk group were not taking recommended gastroprotective therapy prior to hospitalization. Past history of PUD (OR = 3.7, P = 0.006) and clopidogrel use (OR = 3.2, P = 0.007) significantly predicted prescription of gastroprotective therapy. Using proton pump inhibitor protection rates of 50%-85% from published studies, an estimation of 13% to 22% of the total number of the hospitalizations due to PUD or EG/ED related bleeding may have been preventable.
CONCLUSION: Up to one fifth of all hospitalizations for bleeding secondary to PUD or EG/ED are potentially preventable.
Keywords: Peptic ulcer, Gastrointestinal hemorrhage, Prevention, Non-steroidal anti-inflammatory drug, Proton pump inhibitor, Gastroprotection
Core tip: Gastroprotective therapies reduce the risk of bleeding from peptic ulcer disease. For certain high risk groups, the risk reduction is significant: in the order of 50%-85%. Despite this, gastroprotection is still underutilised in this setting. It is unclear what proportion of hospitalizations that occur due to peptic ulcer disease bleeding is preventable. This original research finds that adherence to gastroprotective therapies in high risk populations is poor, and that up to one fifth of all hospitalizations due to peptic ulcer disease related bleeding are potentially preventable.
INTRODUCTION
Upper gastrointestinal hemorrhage (UGIH) is a major cause of morbidity and mortality worldwide, accounting for about 30 to 160 hospitalizations per 100000 population per year and a mortality of 6 per 100000 per year[1-6]. About half of these episodes are related to peptic ulcer disease (PUD). The annual cost to the United States from these hospitalizations is estimated at between one and two billion dollars[7].
The predominant risk factors for peptic ulcer disease are Helicobacter pylori (H. pylori) infection, antiplatelet and non-steroidal anti-inflammatory drug (NSAID) use[8]. The epidemiology has shifted towards the latter in the past few decades as a result of reducing prevalence of H. pylori as well as increasing eradication rates and a later age of presentation[9]. Patient groups at highest risk of bleeding include those with advanced age, prior history of PUD, use of NSAIDs at high dose for prolonged periods (> 2 wk), or concurrent antiplatelet, anticoagulant or corticosteroid therapy[10-12].
The risk of bleeding from PUD is reduced by the use of gastroprotective therapies such as proton pump inhibitors (PPIs), high dose H2 receptor antagonists (H2RA) or misoprostol[10,11]. PPI therapy in groups at high risk reduces the risk of bleeding by 50%-85%[13-20] and is more effective than H2RA[21]. A large randomised controlled trial[15] showed a UGIH hazard ratio of 0.13 (P = 0.001) in those patients taking a dual antiplatelet therapy with a concurrent PPI compared to placebo. Another randomised controlled trial comparing clopidogrel alone with concurrent PPI demonstrated a similar risk reduction in UGIH[14]. Endoscopic findings of gastric ulcers or duodenal ulcers have also been shown to be reduced with concurrent PPI use for clopidogrel alone (HR = 0.10)[22] and aspirin alone (RR = 70%)[16]. Observational studies have demonstrated risk reductions of between around 40%-70% for aspirin[13,23], 50%-80% for clopidogrel[18,23], 85% for dual antiplatelet therapy[13] and 50%-87% for those on NSAIDs[13,17,20,23]. Despite this evidence, gastroprotection in patients at high risk for PUD bleeding is still underutilised, with adherence by clinicians and patients ranging from 15%-40%[19,24-28].
Patients who receive NSAIDs experience more PUD-related complications resulting in hospitalizations in the absence of gastroprotection[19,29]. However, it is unclear what proportion of PUD-related hospitalizations is potentially preventable with adequate gastroprotection. The aim of the present study was, therefore, to assess the proportion of patients hospitalised for PUD, erosive gastritis (EG) or duodenitis (ED) who met eligibility criteria for gastroprotection and to elicit rates of gastroprotection in these patients. Such data were used to calculate the proportion of potentially preventable hospitalizations due to PUD or EG/ED.
MATERIALS AND METHODS
Study design
All patients admitted with gastrointestinal bleeding at three major metropolitan hospitals in Melbourne, Australia - Box Hill Hospital and Maroondah Hospital between March 2007 and October 2009, and Austin Hospital between March 2008 and October 2010 - were identified. ICD-10 codes were used for gastric/duodenal/peptic ulcers, gastritis, duodenitis, haematemesis, melaena and unspecified gastrointestinal hemorrhage to ensure completeness of capture. Those who underwent upper gastrointestinal endoscopy with or without intervention during the index hospitalization were then selected using procedure codes. All case histories were reviewed, and those in whom PUD or EG/ED were deemed to be the cause of the UGIH were selected for analysis. Patients with gastritis or duodenitis that was not felt to be the cause of the UGIH by the endoscopist, as well as bleeding felt to be due to portal hypertensive gastropathy or duodenopathy, were excluded. Demographic, clinical (including co-morbid illnesses and medications), laboratory and endoscopic data were recorded.
The selected patient population was divided into “high risk” and “not high risk” groups according to their risk of developing complications from PUD without gastroprotective therapy. Patients at high risk were defined according to guidelines published by the American College of Cardiology Foundation, the American College of Gastroenterology, the American Heart Association and Gastroenterological Society of Australia[10-12] (Table 1).
Table 1.
“High risk” group selection
| Non-selective NSAID users | COX-2 inhibitor users | Anti-platelet users | |
| Past history of PUD | Y | Y | Y |
| Concurrent antiplatelet or anticoagulant therapy | Y | Y | Y |
| Concurrent corticosteroid therapy | Y | ||
| Age ≥ 60 yr | Y | ||
| Both: Age ≥ 60 yr and concurrent corticosteroids | Y | Y | |
| High dose, duration > 2 wk | Y |
Patients were considered as high risk according to guidelines [use of non-steroidal anti-inflammatory drug (NSAIDs), cyclooxygenase-2 (COX-2) inhibitor and/or anti-platelet agents in addition to features as listed in the first column; Y: High risk group; PUD: Peptic ulcer disease.
This study was approved by the Ethics Committees for Austin Health (reference H2011/04239) and Eastern Health (reference LR92/1112).
Statistical analysis
Once identified, patients at high risk were evaluated for a history of use of gastroprotective therapies. Patients not taking gastroprotective therapies prior to admission were then assessed for the potentially preventable nature of their hospitalization, assuming an efficacy of 50%-85% from previous prospective studies. Patients at high risk and those not at high risk were compared for clinical endpoints, including Rockall Score, blood transfusion requirement, length of hospital stay, in-hospital rebleeding, mortality and need for surgery. Logistic regression was performed to assess the impact of each indication for gastroprotective therapy on observed incidence of gastroprotection in patients deemed to be at high risk of bleeding secondary to PUD or EG/ED, with seven independent variables incorporated into the model: age ≥ 60 years, past history of PUD, and intake of NSAIDs, aspirin, corticosteroids, clopidogrel, or anticoagulants.
Statistical analyses were performed using Microsoft Excel (Microsoft 2010), GraphPad Prism v5.04 (Graphpad software, 2010) and SPSS v20 (IBM Corporation 2011). The Mann Whitney test was used for comparison of non-parametric data, and χ2 and Fisher’s exact test were used for comparison of 2 × 2 contingency tables. A P value of less than 0.05 was considered statistically significant.
RESULTS
In total, 2406 episodes were identified across the three hospitals. As shown in Figure 1, after careful review of patient histories, 1899 patients were excluded from analysis. Most of these episodes were excluded either due to the indication for gastroscopy being something other than suspected UGIH (e.g., lower gastrointestinal bleeding or abdominal pain/dyspepsia) or because the UGIH was deemed by the endoscopist to be unrelated to PUD or EG/ED. In a few cases, the case notes were unavailable. Thus, 507 patient episodes were selected for final analysis. The baseline characteristics of the selected patients are shown in Table 2. No significant differences were noted between the three hospitals.
Figure 1.
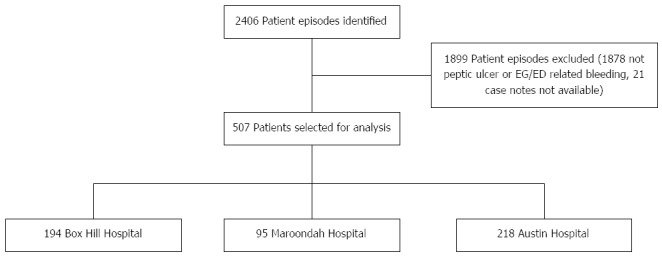
Patient episode selection. EG: Erosive gastritis; ED: Erosive duodenitis.
Table 2.
Baseline patient characteristics n (%)
| Characteristics | Total | Patients |
| (n = 507) | ||
| Demographic | Age > 65 yr | 335 (66) |
| Male | 313 (62) | |
| Medication use | Aspirin | 239 (47) |
| NSAIDS | 124 (24) | |
| Anticoagulant | 71 (14) | |
| Clopidogrel | 70 (14) | |
| Corticosteroids | 46 (9) | |
| Comorbidity | Ischaemic heart disease | 150 (30) |
| Diabetes | 114 (22) | |
| Atrial fibrillation | 72 (14) | |
| Renal failure | 66 (13) | |
| Cerebrovascular disease | 58 (11) | |
| Malignancy | 51 (10) | |
| Chronic obstructive pulmonary disease | 40 (8) | |
| Cirrhosis | 20 (4) | |
| Haematological malignancy | 14 (3) | |
| Aortic stenosis | 9 (2) |
NSAIDS: Non-steroidal anti-inflammatory drug.
One hundred and seventy-four patients were classified as high risk and hence met criteria for gastroprotection. A significant proportion (47%) was on aspirin at the time of the UGIH. Smaller proportions were taking NSAIDs (24%), clopidogrel (14%), corticosteroids (9%) and anticoagulants (14%). Patients at high risk were identified by dividing the 507 patients according to a number of mutually exclusive criteria as shown in Figure 2.
Figure 2.
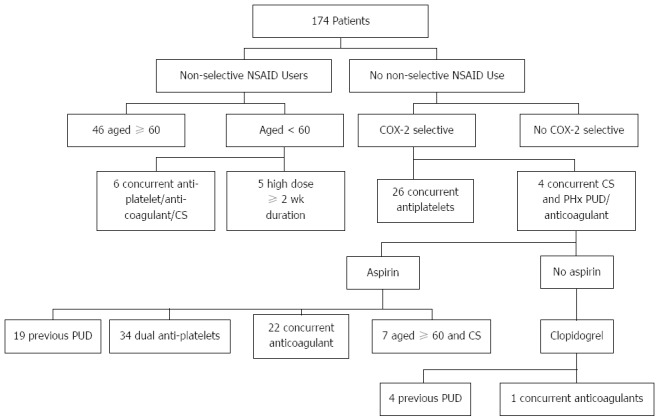
High risk patient selection according to mutually exclusive groups. NSAID: Non-steroidal anti-inflammatory drug; PUD: Peptic ulcer disease; COX-2: Cyclooxygenase-2.
As shown in Table 3, the median complete Rockall Score was higher (P = 0.002) in patients considered at high risk compared with those not considered at high risk for clinically relevant end-points. Length of stay was higher (P = 0.043) in the high risk group, but in-hospital mortality was lower (P = 0.03). There was no difference in mean blood transfusion quantity, length of stay, rebleeding or need for surgery.
Table 3.
Comparison of outcomes during hospitalization for patients classified as “high risk” with those “not high risk” according to concurrent medication use (see text) n (%)
| High Risk | Not High Risk | P value | |
| (n = 174) | (n = 333) | ||
| Median Rockall Score (IQR) | |||
| Pre-endoscopic | 3 (2-4) | 3 (1-4) | < 0.0011 |
| Complete | 5 (4-6) | 4 (3-6) | 0.0021 |
| Median packed red cells units transfused (IQR) | 3 (1-4) | 2 (0-4) | 0.1941 |
| Median length of stay in days (IQR) | 5 (3-10) | 4 (3-7) | 0.0431 |
| Rebleeding | 13 (7.5) | 42 (12.6) | 0.0772 |
| Need for surgery | 4 (2.3) | 17 (5.1) | 0.1322 |
| In-hospital mortality | 1 (0.6)3 | 13 (3.9)4 | 0.0302 |
Mann-Whitney test;
χ2 test;
Cause of death: uncontrolled bleeding (1);
Cause of death: Decompensated heart failure (1), acute myocardial infarction (2), advanced malignancy (5), decompensated cirrhosis (1), hospital acquired pneumonia (1), uncontrolled bleeding (1), uncertain from case notes (2). IQR: Interquartile range.
Ninety-three (18%) patients were on gastroprotective therapy prior to hospitalization, which comprised PPI in 79 and HR2A in 14. Of the 333 patients in the “not high risk” group, 49 (15%) were on gastroprotective therapy prior to hospitalization (36 PPI; 13 H2RA). Of the 174 high risk patients, 44 (25%) were on gastroprotective therapy prior to hospitalization (43 PPI; 1 H2RA). Those in the high risk group were more likely to be prescribed gastroprotection prior to hospitalization compared to those in the low risk group (RR = 1.72, P = 0.004).
One hundred and thirty of the total number of hospitalizations (507) were deemed at high risk for bleeding, but were not on appropriate gastroprotective therapies at the time of admission. If it is assumed that protection offered by PPI therapies is 50%-85% from studies (as above), then between 65 and 110 (13%-22%) of the total number of patient hospitalizations due to bleeding from PUD or EG/ED were potentially preventable.
There was no significant difference in any of the clinical outcomes during hospitalization for patients at high risk who received gastroprotection when compared with those patients who did not receive gastroprotection (Table 4).
Table 4.
Comparison of outcomes during hospitalization in high risk patients according to the use of gastroprotection vs no gastroprotection n (%)
| Gastroprotection (n = 44) | No gastroprotection (n = 130) | P value | |
| Median Rockall Score (IQR) | |||
| Pre-endoscopic | 3 (3-4) | 3 (2-4) | 0.351 |
| Complete | 5 (4-6) | 5 (3-6) | 0.581 |
| Median packed red cells units transfused (IQR) | 3 (2-4) | 3 (1-4) | 0.811 |
| Median length of stay in d (IQR) | 5 (3-11) | 5 (3-8) | 0.821 |
| Rebleeding | 5 (11.4) | 8 (6.2) | 0.312 |
| Need for surgery | 1 (2.3) | 3 (2.3) | 1.02 |
| In-hospital mortality | 0 (0) | 1 (0.8)3 | 1.02 |
There were no significant differences for any variables between the 2 groups.
Mann-Whitney;
Fisher’s Exact test;
Cause of death: uncontrolled bleeding (1). IQR: Interquartile range.
On logistic regression analysis, a past history of PUD [OR = 3.7 (1.5-9.2), P = 0.006] and concurrent clopidogrel therapy [OR = 3.2 (1.4-7.4), P = 0.007] significantly predicted prescription of gastroprotective therapy, but not age or aspirin, NSAID, corticosteroid or anticoagulant intake.
DISCUSSION
Peptic ulcer disease contributes to a significant healthcare burden worldwide. Hospitalizations for UGIH due to PUD and EG/ED account for significant morbidity, mortality and healthcare expense. While gastroprotective therapies have been demonstrated to reduce these hospitalizations, the potential impact for their use has not been previously quantified in actual practice. Indeed, the current study has identified the failure to institute such preventive therapy maybe primarily responsible for up to a fifth of all hospitalizations for bleeding secondary to PUD or EG/ED.
In this retrospective cohort study of three major metropolitan hospitals, a third of patients hospitalised with UGIH due to PUD or EG/ED were deemed at high risk for bleeding secondary to PUD according to published guidelines, and hence met criteria for gastroprotective therapy. Although there was a higher rate of gastroprotective therapy use in the high risk group, only around a quarter of these patients were taking these therapies prior to admission. These results are consistent with previous studies[24-28] that suggest very low rates of gastroprotective agent prescriptions for those on NSAIDS. The alarming implication of this is the number of patients admitted to hospital with potentially preventable bleeding.
The effectiveness of gastroprotection has been demonstrated in numerous studies, which reduces the likelihood of UGIH by 50%-85% in high risk patients. Assuming this figure, about one in five patient hospitalizations with bleeding secondary to PUD or EG/ED may have been prevented with attention to gastroprotection by the physician prescribing the agent or agents associated with gastrointestinal bleeding. As an analogy, the benefit of statins in secondary prevention of hospitalizations for ischaemic heart disease is about 30%[30], and of antiplatelet agents in secondary prevention after ischaemic stroke about 22%[31]; both of which are well recognised and practised.
An improvement in practice such that gastroprotection is optimised in those patients at highest risk is prudent. Continuing education of health care providers regarding PUD prophylaxis is required, as well as reinforcement with electronic reminder systems. One study found that a combination of physician education and a computer alert at time of physician prescription led to a 41% increase in the use of gastroprotection amongst NSAID users[32]. Similar systems could be developed for patients deemed to be at high risk for UGIH.
However, by itself, such measures may not result in optimal outcomes. Although the proportion of patients on antiplatelet agents or aspirin with an inappropriate indication for these medications is beyond the scope of this study, an approach to rational prescribing by physicians from a variety of disciplines may be as relevant as appropriate gastroprotective therapy. In addition, these medications are often purchased over-the counter and self-initiated by patients. Appropriate H. pylori testing and eradication in certain groups prior to commencing ulcerogenic medications has also been shown to be of benefit. Hence, in order to maximise the use of gastroprotective therapies, a multi-modality approach may be required, whereby an information campaign for physicians, pharmacists and the wider public is conducted. Electronic reminder systems at the point of sale may be an approach worth considering.
Of note, most of the hospitalizations from UGIH in this study occurred in patients not considered high risk as per current expert consensus guidelines. This is not an unexpected finding given the baseline population in the “not high risk” group is much larger than the far more specifically defined “high risk” group. Clearly, the focus of guidelines on higher risk patients is due to the greater potential for risk reduction, leading to more targeted recommendations to maximise the benefit from gastroprotection whilst minimising costs in the real world setting.
Patients deemed at high risk for PUD bleeding were noted to have longer inpatient stays and higher pre-endoscopic and complete Rockall Scores than patients not deemed at high risk. This is not surprising given the overlapping nature of co-morbidities requiring antiplatelet therapy and those comprising the Rockall Score. However, the finding of a higher mortality in patients not at high risk of bleeding (13 of 333) compared with those at high risk (1 of 174) was somewhat unexpected. This can be explained by noting that most of the deaths in the lower risk group were in patients with advanced co-morbid illnesses but without specific indications for gastroprotective therapy (Table 3).
Furthermore, patients who received gastroprotection prior to admission did not have significantly different outcomes of hospitalization than those who did not receive gastroprotection. Though this finding seems to be counter-intuitive, one may hypothesise that once bleeding has occurred despite gastroprotective therapy, the primary determinants of outcome are underlying co-morbid illnesses and ulcer risk stigmata rather than the previous use of gastroprotection per se.
A past history of PUD and concurrent clopidogrel therapy significantly predicted use of gastroprotective therapy. This may reflect possible changes from aspirin to clopidogrel in patients who have had a UGIH in the past whilst on aspirin.
Rockall score was used rather than Blatchford score for a number of reasons. The Rockall score is the most well validated scoring system for UGIH[33]. The Blatchford score’s high sensitivity and poor specificity in identifying high risk groups has led to its use in identifying patients who can be triaged for early discharge or outpatient endoscopy[34]. In our study, all patients included in the analysis were hospitalised and had undergone endoscopy which forms part of the Rockall score.
There are some limitations to this retrospective analysis. The case records may have contained incomplete recording of medications on arrival. However, this applies to both the intake of aspirin and NSAIDs as well as gastroprotective therapies, such that any bias would be bi-directional and hence may have only a slight net bias in either direction. Furthermore, documentation of the presence or absence of previous H. pylori eradication therapy may have been incomplete, hence not enabling adequate analysis for impact of this factor to overall risk of bleeding secondary to PUD or EG/ED.
It is important to consider the potential morbidity from long-term PPI therapy in advocating increased prescription of these treatments. These include iron deficiency, B12 deficiency, hypomagnesaemia, rebound hypersecretion, osteoporosis, interactions with other medications, increased rates of Clostridium difficile infection and ventilator acquired pneumonia[35].
The observed in vitro interaction between omeprazole and clopidogrel with inhibitor effect on the enzyme CYP 2C19, with potential reduction in efficacy of clopidogrel, remains an unresolved issue. Reassuringly, a large recent prospective randomised controlled trial demonstrated no increase in cardiovascular events with this combination[15].
A full cost-effectiveness analysis of PPI therapy as gastroprotective therapy is beyond the scope of this study, but has been addressed in previous studies. The number needed to prevent one episode of UGIH over six months with a PPI has been estimated at 382 (low dose aspirin), 230 (clopidogrel), 97 (dual antiplatelet agents) and 265 (NSAIDs)[13]. The COGENT trial[15] separately estimated the number-needed-to-treat for dual antiplatelet agent users to be 98. Studies using a Markov model comparing lifelong low dose aspirin alone with aspirin and a PPI estimated a per life-year saved incremental cost-effectiveness ratio (ICER) of $10433 to $51505[36] and $19000[37]. A nested case control study estimated an ICER of €4907 per NSAID ulcer complication prevented with PPIs[38]. Variation in healthcare costs around the world, both for gastroprotective therapies and hospitalization, need to be considered in calculating a relative cost-effectiveness analysis for individual countries. In addition, alternative measures such as physician and community education on appropriate use of ulcerogenic therapies as outlined previously need to be considered.
In conclusion, gastroprotective therapies are still underutilised in patients at high risk of hospitalization secondary to UGIH. Up to one fifth of all hospitalizations due to PUD or EG/ED related bleeding may be preventable by optimal use of gastroprotection. Measures to educate health professionals and reminder systems at the point of sale of antiplatelet agents and NSAIDs are required to minimise gastrointestinal complications.
COMMENTS
Background
The risk of bleeding from peptic ulcer disease (PUD) is reduced by the use of gastroprotective therapies. However, it is unknown what proportion of PUD-related hospitalizations is potentially preventable with adequate gastroprotection.
Research frontiers
Gastroprotective agents have shown efficacy in reducing the risk of bleeding from peptic ulcer disease. Recent research has focussed on the costs of gastroprotective therapies both in terms of monetary expense and potential adverse effects. Given this, more information about the potential benefits of gastroprotective therapies (including reduction in hospitalization) is required).
Applications
This study finds that adherence to gastroprotective therapies in high risk populations is poor, and that up to one fifth of all hospitalizations due to peptic ulcer disease related bleeding are potentially preventable.
Terminology
Gastroprotective agents/therapies: proton pump inhibitors or high dose H2 receptor antagonists.
Peer review
The paper reports a retrospective analysis. By placing data on low-risk patients the authors could, for comparison, highlight the use of gastroprotection in patients at low risk, that constitute the majority of bleeding (2/3) and discuss the use of proton-pump inhibitor on the basis of the prevalence estimates obtained.
Footnotes
P- Reviewer: Tosetti C S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of nonsteroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol. 2002;97:2540–2549. doi: 10.1111/j.1572-0241.2002.06037.x. [DOI] [PubMed] [Google Scholar]
- 2.Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633–1641. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 3.Post PN, Kuipers EJ, Meijer GA. Declining incidence of peptic ulcer but not of its complications: a nation-wide study in The Netherlands. Aliment Pharmacol Ther. 2006;23:1587–1593. doi: 10.1111/j.1365-2036.2006.02918.x. [DOI] [PubMed] [Google Scholar]
- 4.Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, Gisbert JP, Bujanda L, Castro M, Muñoz M, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33:585–591. doi: 10.1111/j.1365-2036.2010.04563.x. [DOI] [PubMed] [Google Scholar]
- 5.Feinstein LB, Holman RC, Yorita Christensen KL, Steiner CA, Swerdlow DL. Trends in hospitalizations for peptic ulcer disease, United States, 1998-2005. Emerg Infect Dis. 2010;16:1410–1418. doi: 10.3201/eid1609.091126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkun A, Sabbah S, Enns R, Armstrong D, Gregor J, Fedorak RN, Rahme E, Toubouti Y, Martel M, Chiba N, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004;99:1238–1246. doi: 10.1111/j.1572-0241.2004.30272.x. [DOI] [PubMed] [Google Scholar]
- 7.Viviane A, Alan BN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11:1–3. doi: 10.1111/j.1524-4733.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Padol IT, Hunt RH. Peptic ulcer disease today. Nat Clin Pract Gastroenterol Hepatol. 2006;3:80–89. doi: 10.1038/ncpgasthep0393. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM, Harrington RA, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502–1517. doi: 10.1016/j.jacc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, Furberg CD, Johnson DA, Kahi CJ, Laine L, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;56:2051–2066. doi: 10.1016/j.jacc.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Yeomans N, Bertouch J, Stiel D. NSAIDs and the Gastrointestinal Tract. 2nd ed. Melbourne: Digestive Health Foundation; 2008. [Google Scholar]
- 13.Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71–79. doi: 10.1053/j.gastro.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Hsu PI, Lai KH, Liu CP. Esomeprazole with clopidogrel reduces peptic ulcer recurrence, compared with clopidogrel alone, in patients with atherosclerosis. Gastroenterology. 2011;140:791–798. doi: 10.1053/j.gastro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, Shook TL, Lapuerta P, Goldsmith MA, Laine L, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 16.Yeomans N, Lanas A, Labenz J, van Zanten SV, van Rensburg C, Rácz I, Tchernev K, Karamanolis D, Roda E, Hawkey C, et al. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am J Gastroenterol. 2008;103:2465–2473. doi: 10.1111/j.1572-0241.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 17.Ray WA, Chung CP, Stein CM, Smalley WE, Hall K, Arbogast PG, Griffin MR. Risk of peptic ulcer hospitalizations in users of NSAIDs with gastroprotective cotherapy versus coxibs. Gastroenterology. 2007;133:790–798. doi: 10.1053/j.gastro.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, Daugherty JR, Kaltenbach LA, Stein CM. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. 2010;152:337–345. doi: 10.1059/0003-4819-152-6-201003160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koncz TA, Lister SP, Makinson GT. Gastroprotection in patients prescribed non-selective NSAIDs, and the risk of related hospitalization. Curr Med Res Opin. 2008;24:3405–3412. doi: 10.1185/03007990802547121. [DOI] [PubMed] [Google Scholar]
- 20.Höer A, Gothe H, Schiffhorst G, Sterzel A, Grass U, Häussler B. Comparison of the effects of diclofenac or other non-steroidal anti-inflammatory drugs (NSAIDs) and diclofenac or other NSAIDs in combination with proton pump inhibitors (PPI) on hospitalisation due to peptic ulcer disease. Pharmacoepidemiol Drug Saf. 2007;16:854–858. doi: 10.1002/pds.1387. [DOI] [PubMed] [Google Scholar]
- 21.Ng FH, Wong SY, Lam KF, Chu WM, Chan P, Ling YH, Kng C, Yuen WC, Lau YK, Kwan A, et al. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology. 2010;138:82–88. doi: 10.1053/j.gastro.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, Lau GK, Wong WM, Yuen MF, Chan AO, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 23.Lanas A, García-Rodríguez LA, Arroyo MT, Bujanda L, Gomollón F, Forné M, Aleman S, Nicolas D, Feu F, González-Pérez A, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507–515. doi: 10.1111/j.1572-0241.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.van Soest EM, Sturkenboom MC, Dieleman JP, Verhamme KM, Siersema PD, Kuipers EJ. Adherence to gastroprotection and the risk of NSAID-related upper gastrointestinal ulcers and haemorrhage. Aliment Pharmacol Ther. 2007;26:265–275. doi: 10.1111/j.1365-2036.2007.03358.x. [DOI] [PubMed] [Google Scholar]
- 25.Valkhoff VE, van Soest EM, Sturkenboom MC, Kuipers EJ. Time-trends in gastroprotection with nonsteroidal anti-inflammatory drugs (NSAIDs) Aliment Pharmacol Ther. 2010;31:1218–1228. doi: 10.1111/j.1365-2036.2010.04281.x. [DOI] [PubMed] [Google Scholar]
- 26.Sturkenboom MC, Burke TA, Dieleman JP, Tangelder MJ, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology (Oxford) 2003;42 Suppl 3:iii23–iii31. doi: 10.1093/rheumatology/keg495. [DOI] [PubMed] [Google Scholar]
- 27.Thiéfin G, Schwalm MS. Underutilization of gastroprotective drugs in patients receiving non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2011;43:209–214. doi: 10.1016/j.dld.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Van der Linden MW, Gaugris S, Kuipers EJ, Van den Bemt BJ, van Herk-Sukel MP, Herings RM. Gastroprotection among new chronic users of non-steroidal anti-inflammatory drugs: a study of utilization and adherence in The Netherlands. Curr Med Res Opin. 2009;25:195–204. doi: 10.1185/03007990802632915. [DOI] [PubMed] [Google Scholar]
- 29.Abraham NS, Hartman C, Hasche J. Reduced hospitalization cost for upper gastrointestinal events that occur among elderly veterans who are gastroprotected. Clin Gastroenterol Hepatol. 2010;8:350–356; quiz e45. doi: 10.1016/j.cgh.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Penning-van Beest FJ, Termorshuizen F, Goettsch WG, Klungel OH, Kastelein JJ, Herings RM. Adherence to evidence-based statin guidelines reduces the risk of hospitalizations for acute myocardial infarction by 40%: a cohort study. Eur Heart J. 2007;28:154–159. doi: 10.1093/eurheartj/ehl391. [DOI] [PubMed] [Google Scholar]
- 31.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Coté GA, Rice JP, Bulsiewicz W, Norvell JP, Christensen K, Bobb A, Postelnick M, Howden CW. Use of physician education and computer alert to improve targeted use of gastroprotection among NSAID users. Am J Gastroenterol. 2008;103:1097–1103. doi: 10.1111/j.1572-0241.2008.01907.x. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson RJ, Hurlstone DP. Usefulness of prognostic indices in upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:233–242. doi: 10.1016/j.bpg.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Le Jeune IR, Gordon AL, Farrugia D, Manwani R, Guha IN, James MW. Safe discharge of patients with low-risk upper gastrointestinal bleeding (UGIB): can the use of Glasgow-Blatchford Bleeding Score be extended? Acute Med. 2011;10:176–181. [PubMed] [Google Scholar]
- 35.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected] Am J Gastroenterol. 2009;104 Suppl 2:S27–S32. doi: 10.1038/ajg.2009.49. [DOI] [PubMed] [Google Scholar]
- 36.Saini SD, Schoenfeld P, Fendrick AM, Scheiman J. Cost-effectiveness of proton pump inhibitor cotherapy in patients taking long-term, low-dose aspirin for secondary cardiovascular prevention. Arch Intern Med. 2008;168:1684–1690; discussion 1691. doi: 10.1001/archinte.168.15.1684. [DOI] [PubMed] [Google Scholar]
- 37.Saini SD, Fendrick AM, Scheiman JM. Cost-effectiveness analysis: cardiovascular benefits of proton pump inhibitor co-therapy in patients using aspirin for secondary prevention. Aliment Pharmacol Ther. 2011;34:243–251. doi: 10.1111/j.1365-2036.2011.04707.x. [DOI] [PubMed] [Google Scholar]
- 38.Vonkeman HE, Braakman-Jansen LM, Klok RM, Postma MJ, Brouwers JR, van de Laar MA. Incremental cost effectiveness of proton pump inhibitors for the prevention of non-steroidal anti-inflammatory drug ulcers: a pharmacoeconomic analysis linked to a case-control study. Arthritis Res Ther. 2008;10:R144. doi: 10.1186/ar2577. [DOI] [PMC free article] [PubMed] [Google Scholar]


