Abstract
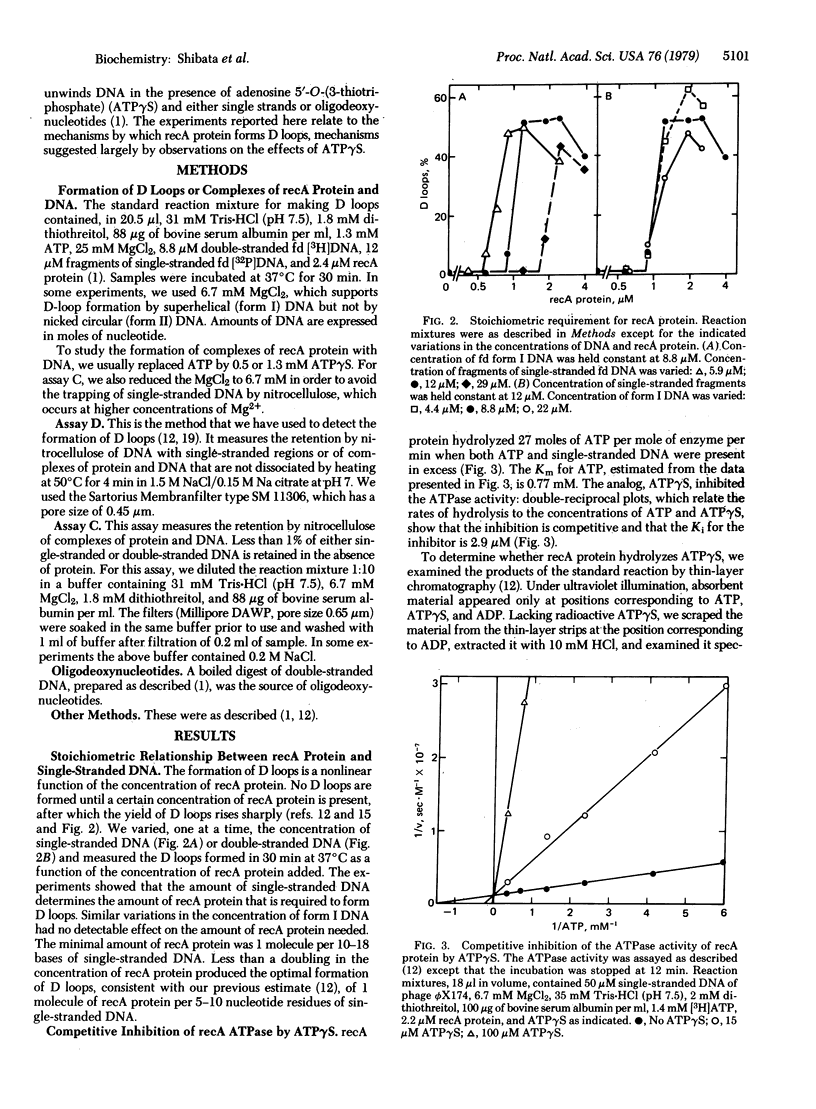
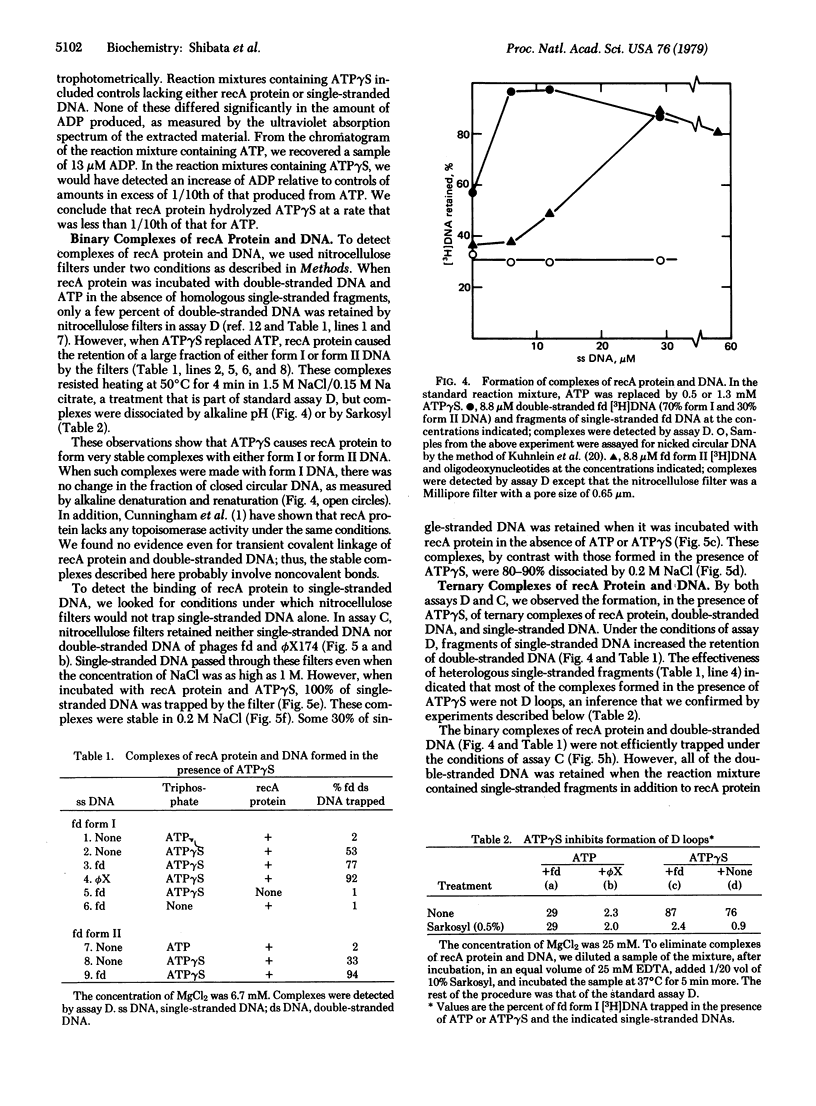
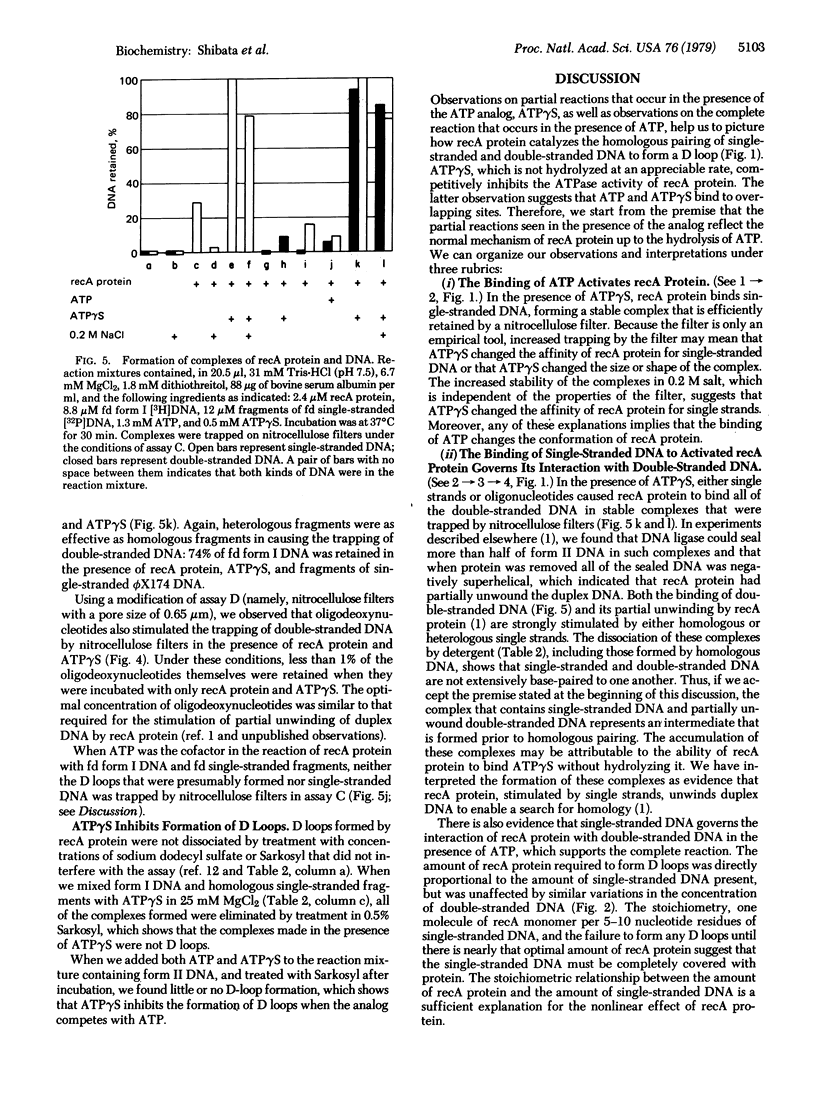
recA protein, which is essential for general genetic recombination in Escherichia coli, promotes the homologous pairing of single-stranded DNA with double-stranded DNA to form a D loop. The amount of recA protein required for the reaction was directly proportional to the amount of single stranded DNA and was unaffected by similar variations in the amount of double-stranded DNA. The ATP analog, adenosine 5'-O-(3-thiotriphosphate) (ATP gamma S), which was not rapidly hydrolyzed by recA protein, blocked the formation of D loops but promoted the formation of stable complexes of recA protein and single-stranded DNA. These complexes, in turn, bound homologous or heterologous double-stranded DNA and partially unwound it. Because ATP gamma S competitively inhibited the ATPase activity of recA protein (Km/Ki approximately 300), we infer that ATP gamma S binds at a site that overlaps the site for ATP and that the functional complexes formed in the presence of the analog probably represent partial steps in the overall reaction. If the complexes formed in the presence of ATP gamma S reflect natural intermediates in the formation of D loops, recA protein must promote homologous pairing either by moving juxtaposed single-stranded and double-stranded DNA relative to one another or by forming and dissociating complexes reiteratively until a homologous match occurs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie K. L., Wiegand R. C., Radding C. M. Uptake of homologous single-stranded fragments by superhelical DNA. II. Characterization of the reaction. J Mol Biol. 1977 Nov;116(4):783–803. doi: 10.1016/0022-2836(77)90271-6. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Shibata T., DasGupta C., Radding C. M. Single strands induce recA protein to unwind duplex DNA for homologous pairing. Nature. 1979 Sep 20;281(5728):191–195. doi: 10.1038/281191a0. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Fox M. S. Some features of genetic recombination in procaryotes. Annu Rev Genet. 1978;12:47–68. doi: 10.1146/annurev.ge.12.120178.000403. [DOI] [PubMed] [Google Scholar]
- Holloman W. K., Radding C. M. Recombination promoted by superhelical DNA and the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3910–3914. doi: 10.1073/pnas.73.11.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Ikeda H. On the role of recA gene product in genetic recombination: an analysis by in vitro packaging of recombinant DNA molecules formed in the absence of protein synthesis. Mol Gen Genet. 1978 Oct 25;166(1):25–29. doi: 10.1007/BF00379725. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Estein W. Isolation and characterization of specialized transducing bacteriophages for the recA gene of Escherichia coli. Virology. 1977 Mar;77(1):306–318. doi: 10.1016/0042-6822(77)90427-5. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Genetic recombination: strand transfer and mismatch repair. Annu Rev Biochem. 1978;47:847–880. doi: 10.1146/annurev.bi.47.070178.004215. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Physical map of the recA gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3144–3148. doi: 10.1073/pnas.76.7.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]



