Abstract
AIM: To investigate possible predictors for failed self-expandable metallic stent (SEMS) therapy in consecutive patients with benign esophageal perforation-rupture (EPR).
METHODS: All patients between 2003-2013 treated for EPR at the Karolinska University Hospital, a tertiary referral center, were studied with regard to initial management with SEMS. Patients with malignancy as an underlying cause and those with anastomotic leakages were excluded. Sealing of the perforation with a covered SEMS was the primary strategy whenever feasible. Stent therapy failure was defined as a radical change of treatment strategy due to uncontrolled mediastinitis, which in this setting consisted of emergency esophagectomy with end-esophagostomy or death as a consequence of the perforation and subsequent uncontrolled sepsis. Patient and lesion characteristics were analyzed and are presented as median and interquartile range. Possible predictors for failed stent therapy were analyzed with uni-variate logistic regression, while variables with P < 0.2 were further analyzed with multi-variate logistic regression.
RESULTS: Of the total number of 48 patients presenting with EPR, 40 patients (83.3%) were treated with SEMS at the time of admission, with an intention to heal the perforation. Twenty-three patients had Boerhaave’s syndrome (58%), 16 had an iatrogenic perforation (40%) and 1 had external trauma to the esophagus (3%). The total in-hospital mortality, including the cases that had other initial treatments (n = 8), was 10.4% and 7.5% among those who were subjected to the SEMS-based strategy. In 33 of the 40 patients (82.5%) who were treated with stent, the EPR healed without further change in treatment strategy. Patients classified as treatment success received a SEMS at a median time of 1 (1-1) d after the actual EPR, compared to 3 (1-10) d among those where the initial treatment failed, P = 0.039 in uni-variate analysis and P = 0.052 in multi-variate analysis. No other significant factors emerged, indicating an increased risk for failure. Six of 7 patients, where stent treatment of the defect failed, underwent an emergency esophagectomy with end esophagostomy and one patient died.
CONCLUSION: SEMS as an upfront therapeutic strategy seems to be a successful concept, when applied to an unselected group of patients with EPR.
Keywords: Esophageal perforation, Stents, Esophagectomy, Morbidity, Mortality, Mediastinitis
Core tip: It is unclear to which extent esophageal stenting can heal esophageal perforation-rupture in unselected patients. In this single institution study 83.3% of all benign esophageal perforations/ruptures of mixed etiology, excluding anastomotic leakages, were treated with stent with an intention to heal the perforation, as first-line treatment during a 10-year period. Eighty-two point five percent recovered after stenting and no further intervention was required. Time between perforation and placement of stent emerged as potential risk factor for failure of stenting. The high rate of stenting as primary treatment may have contributed to the relatively low overall in-hospital mortality of 10.4%.
INTRODUCTION
Esophageal perforation-rupture (EPR) is a rare condition[1,2] associated with high morbidity and mortality[3]. The transmural disruption of the esophageal wall and the subsequent contamination of the mediastinum by gastric and oral secretions rapidly progresses to mediastinitis, sepsis, organ failure and, if not adequately treated, to fatal outcome[4,5]. Despite advancements in diagnostics, surgical techniques, endoscopy and critical care; both diagnosis and treatment of EPR still present challenges[6]. A recent systematic review and meta-analysis covering 75 studies found that mortality after EPR remained high (17%-21% in Europe), regardless of the treatment strategy practiced[3]. The dominating prognostic factor for the subsequent course of patients with EPR is the time interval between the perforation and initiation of treatment. A delay of diagnosis more than 24 h after onset of symptoms leads to a two-fold increase in mortality according to several studies[4,7,8]. The traditional treatment of choice has been surgery, although the surgical strategy has varied substantially between institutions and over time[3,6,7]. With the development and refinement of covered self-expanding metal stent (SEMS), which have been frequently tested[9-13] to cover the EPR, promising results have been presented in several studies[9-11,14-17]. However, most of the literature regarding stent treatment of EPR contains small, heterogeneous and mostly strongly selected case series presenting results after stenting of EPR with a success rate exceeding 60%[9,10,18-21]. Nevertheless, in complex situations like these, one single therapeutic principle cannot be the solution to all cases. Therefore, it is important to develop therapeutic strategies in order to achieve the best clinical outcome. A clinical research approach, with the potential to add significant information to the clinical management of patients with EPR, would be to e.g., apply SEMS to virtually all patients with EPR and then carefully follow these patients in order to define when and how failures for this therapeutic concept emerge. At our institution, we have prospectively tried to treat all patients admitted with EPR with SEMS, after which we carefully monitor them and reassess the clinical course with readiness to change strategy. Our hope was that this study would allow us to identify factors associated with poor outcome related to SEMS treatment.
MATERIALS AND METHODS
Patient inclusion
This cohort study includes all patients who were admitted for either spontaneous (ICD-10 code K22.3) or iatrogenic and traumatic (ICD-10 code S27.8, T28.1, T28.6) esophageal perforation-rupture at the Karolinska University Hospital between 1st of March 2003 and 1st of March 2013. Patients with malignant diseases in the esophagus or surrounding organs, leading to perforation or anastomotic leakages after surgery, were excluded. Patients were identified and studied through the hospital databases Vis-portalen, Take Care and Orbit.
Management strategies
The diagnosis of esophageal perforation was established on the basis of clinical history, pulmonary X-ray and computed tomography with or without administration of oral contrast medium. Many times, the first endoscopy was performed at the time of stenting in cases with spontaneous rupture. Sealing of the perforation with a covered SEMS was the primary strategy whenever feasible, except for perforations of the cervical esophagus where stents were judged to be poorly tolerated. All patients received broad-spectrum antibiotics intravenously. First-line antibiotic treatment was usually Impinem 500 mg × 3 mg, which thereafter was changed depending on culture results, resistance patterns and need for fungus prophylaxis. Some patients were given enteral nutrition via a feeding jejunostomy. Otherwise, total parenteral nutrition was given until the leak was considered to be under control. Moreover, percutaneous thoracic drains were placed whenever a pleural effusion was documented, which also allowed for pleural lavage. All SEMS were inserted under general anesthesia and the positioning of the stent was controlled by fluoroscopy. Whenever needed, double stents were deployed for better coverage of the defect. Over the years different types of covered SEMS have been used. As soon as it was feasible from a practical and clinical perspective, swallow test with water-soluble contrast medium was performed to assess whether the defect had been sealed by the stent(s). If the leak was not entirely under control, this strategy harbored additional attempts either to reposition the SEMS or to insert another one. In order to minimize difficulties retrieving SEMS, extraction was generally performed within 4-6 wk[22,23]. If the leakage persisted, a new stent was applied.
Following these procedures, the patient’s general condition was assessed daily (in the ICU or in a high dependency unit), where the critical question was raised whether damage control had been obtained. However, as part of this stent based strategy, when it was considered indicated to drain contained mediastinitis and/or mediastinal abscesses, this was performed through a posterior mini-thoracotomy, alternatively via laparotomy and transhiatal drainage. More recently the debridement of the mediastinum and pleural spaces was accomplished by use of thoracoscopy.
Failure and outcome measures
The primary outcome studied was failure of the stent therapy. This was defined as a radical change of treatment strategy (see above) due to uncontrolled mediastinitis, which in this setting meant esophagectomy with end-esophagostomy or death as a consequence of the perforation following uncontrolled sepsis. Cases with persistent leakage after SEMS that could be managed successfully with only drainage procedures (see above) were not considered to be failures. The following parameters were analyzed as potential predicting factors of unsuccessful SEMS therapy; time between perforation and placement of SEMS; cause and location of perforation (traumatic vs spontaneous vs iatrogenic perforation and middle vs distal esophagus); age; gender; co-morbidities, smoking or alcohol abuse; body mass index (BMI); C-reactive protein (CRP), albumin and creatinine at admission and American Society of Anesthesiologists physical status classification (ASA grade) prior to the EPR. Descriptive data were secondary outcomes and included frequency of SEMS as first-line treatment, characteristics of the underlying background upper GI lesions, number and type of drainage procedures, number of endoscopic re-interventions, days of hospitalization, days in ICU, days with mechanical ventilator support, hemodialysis treatment and in-hospital mortality.
Statistical analysis
Numerical data is presented using median and interquartile range. Continuous variables were compared using the non-parametric Mann-Whitney U-test for ordinal data/categorical variables the Fisher’s exact test was used. Possible predicting factors for stent treatment failure were evaluated by uni-variate logistic regression analysis. Variables with P < 0.2 in the uni-variate logistic regression analysis, were further analyzed in a multi-variate analysis. P < 0.05 was considered statistically significant. SPSS software was used for the statistical calculations. This study was part of a quality control audit commissioned by the Chairman of the Department and therefore did not need a formal ethical approval according to the regulations of the hospital.
RESULTS
Between 1st of March 2003 and 1st of March 2013, 48 patients with EPR were enrolled. Seventeen of these patients (35%) were referred from other hospitals. Eight patients did not receive stent with an intention to heal the perforation as primary treatment (Table 1). One patient with severe cardiopulmonary co-morbidity had a spontaneous perforation measuring 5 cm, involving 75% of the esophageal circumference, which was treated with a stent. This patient differed from all others, since the stent was applied with a purely palliative intent and the patient died three days after stent insertion.
Table 1.
Summary of patients who received other primary treatment than stenting with intention to heal
| Cause of perforation | Localization | Treatment | Reason other treatment |
| Iatrogenic | Cervical | Drainage and antibiotics | Stent not well tolerated in cervical part |
| Boerhaave | Distal | Drainage and antibiotics | Treated at another hospital |
| Foreign body | Cervical | Suture | Stent not well tolerated in cervical part |
| Boerhaave | Distal | Suture | Large, longstanding lesion |
| Swallowed knives | Cervical | Suture | Required surgical removal of knife |
| Unknown | Cervical | Suture | Stent not well tolerated in cervical part |
| Boerhaave | Distal | Stent | Palliation |
| Boerhaave | Distal | Esophagectomy | Large, circumferential lesion |
Clinicopathological findings of the patients treated with stent
In total, 40 out of the 48 patients received stent as the primary treatment, of whom 33 recovered and left the hospital with an intact esophagus. In 7 cases the stent treatment failed. There was no significant difference between the groups (failures vs success) regarding age, sex, diabetes, pulmonary disease, alcohol abuse, primary rupture into the pleural space, BMI, CRP, creatinine and albumin levels at admission (Table 2). Smoking and cardiovascular disease seemed to be more common in the group where stent treatment failed, who also had a higher ASA, but these differences did not reach statistical significance. There was no significant difference in alcohol abuse between the groups, although both groups displayed a relatively high level (around 1/3) of alcohol consumption. Spontaneous perforation (Boerhaave’s syndrome) was the most common underlying cause. Although we experienced difficulty in capturing uniform data on the size of the esophageal lesion, the size ranged from 0.5-5.5 cm in patients who received stent as primary treatment. The largest perforation that recovered with SEMS treatment measured 5.5 cm. Regardless of first treatment strategy, 8 out of 16 patients (50%) with an iatrogenic perforation and 9 out of 23 patients (39%) with Boerhaave’s syndrome had a history of previous esophageal disease, which included stricture, achalasia or subjective dysphagia.
Table 2.
Patient and lesion characteristics according to stent treatment outcome
| Stent and recovery | Stent and failure | P value | |
| n = 33 (83%) | n = 7 (17%) | ||
| Age, median (range) | 62 (10-82) | 68 (35-76) | 0.193 |
| Sex, M:F | 23:10 | 5:02 | 1.000 |
| ASA score1 | 2 (1-2) | 2 (2-3) | 0.338 |
| Smoker, n = 35 | 8 (29) | 4 (57) | 0.200 |
| Alcohol misuse, n = 31 | 8 (32) | 2 (33) | 1.000 |
| Cardiovascular disease | 12 (36) | 5 (71) | 0.113 |
| Diabetes | 3 (9) | 1 (14) | 0.552 |
| Pulmonary disease | 5 (15) | 1 (14) | 1.000 |
| Localization | 0.224 | ||
| Distal | 17 (57) | 6 (86) | |
| Cause of perforation | 0.570 | ||
| Trauma | 1 (3) | 0 | |
| Boerhaave | 20 (61) | 3 (43) | |
| Iatrogenic | 12 (36) | 4 (57) | |
| Foreign body | 0 | 0 | |
| Primary pleural rupture, n = 36 | 15 (50) | 3 (50) | 1.000 |
| Time to stent2 (d), n = 38 | 1 (1-1) | 3 (1-10) | 0.020 |
| CRP (mg/L) | 63 (21-251) | 122 (48-280) | 0.572 |
| Albumin (g/L), n = 29 | 25 (21-33) n = 22 | 28 (19-35) n = 7 | 0.878 |
| Creatinine (μmol/L), n = 38 | 80 (69-137) | 80 (46-103) | 0.522 |
| BMI (kg/m2), n = 32 | 24.8 (22.3-27.9) | 23.9 (21.7-26.3) | 0.698 |
Before the perforation;
Time between symptom onset or injury and placement of stent. Values are n (%) or median (IQR) unless otherwise indicated. CRP: C-reactive protein; ASA: American Society of Anesthesiologists; M: Male; F: Female.
Patient and lesion characteristics as related to stent treatment outcome are shown in Table 2. In our study, we found that the time elapsed between perforation and placement of stent seemed to be the only significant predicting factor for failed stent therapy. The time period was significantly shorter in patients who recovered with stent compared to patients who experienced stent treatment failure (P = 0.020). In 4 of the 7 cases with failed stent strategy, placement of stent was delayed by more than 1 d after the damage, whereas 22 out of the 30 patients who recovered had placement of the stent within 1 d from symptom onset. In the treatment failure group, all but one patient (6/7) had a perforation in the distal part of esophagus. The remaining patient where stenting failed had a large perforation in the middle esophagus, measuring 4 cm × 2 cm.
Outcome of stent therapy
Treatment and outcomes for patients who received stent as primary treatment are presented in Table 3. The overall in-hospital-mortality was 10.4% (5/48) and 7.5% (3/40) for patients who received stent as primary treatment with an intention to heal the perforation. The median time of in-hospital stay as well as the median time from initial SEMS placement and eventual change of treatment strategy or death is presented in Table 3. In 6 out of 7 cases where stent treatment failed, esophagectomy with cervical esophagostomy was performed. One patient had a concomitant carcinoma localized at the base of the tongue and was therefore considered unfit for esophagectomy, and subsequently died in-hospital. Three of the patients who were treated with esophagectomy died in-hospital. The causes of death were respiratory insufficiency without sepsis (n = 1) and sepsis and multi-organ failure (n = 2). All these three patients had significant co-morbidity (including concomitant malignancy and cardio-respiratory incapacity).
Table 3.
Overall outcome and in-hospital treatment strategies
| Stent and recovery | Stent and failure | P value | |
| n = 33 (83%) | n = 7 (17%) | ||
| In hospital mortality | 0 | 3 (43) | 0.004 |
| Time to recovery | 29 (19-55) | - | |
| Time to new strategy | - | 12 (7-15) | |
| Days of hospitalization | 29 (15-48) | 54 (18-91) | 0.227 |
| Days in ICU | 1 (0-7) | 14 (11-38) | 0.001 |
| Dialysis | 0 | 2 (29) | 0.027 |
| Ventilator support | 9 (27) | 7 (100) | 0.001 |
| Sealed leakage after first stent | 0.026 | ||
| Yes n = 22 | 17 (71) | 1 (17) | |
| No n = 8 | 7 (29) | 5 (83) | |
| Endoscopic reintervention1 | 0.234 | ||
| 0 | 19 (57) | 2 (29) | |
| 1 | 8 (24) | 3 (43) | |
| 2 | 4 (12) | 2 (29) | |
| 3 | 2 (6) | 0 | |
| Number of drainage procedures2 | 3 (2-5) | 4 (2-8) | 0.552 |
Including change, adjustment or addition of stent;
Including percutaneous drainage, laparotomy, thoracoscopy and thoracotomy. Values are n (%) or median (IQR). ICU: Insensive Care Unit.
For the majority of all patients who were treated with stents, the leakage was sealed directly after deployment of the first stent (Table 3). However, 43% of the patients in the recovery group underwent up to three subsequent endoscopic re-interventions, which was even more frequent among those whose stent treatment failed (Table 3). As expected, the stent failure group had a longer median hospital stay (54 d vs 29 d) and all these patients required intensive care and ventilator support.
Complications of stent therapy
No stent-related complications occurred. One patient initially treated at our unit was scheduled for stent extraction at a county hospital. Due to difficulties with the procedure, the patient was referred back to us where the extraction was performed uneventfully.
Risk factors of stent treatment failure
Stent treatment failure correlated significantly with time to stent (OR = 1.368, 95%CI: 1.016-1.843) by uni-variate analysis (Table 4). Factors with relatively small P values (P < 0.2) in the uni-variate analysis were selected as co-variables in the multi-variate analysis, which identified time to stent (OR = 1.802, 95%CI: 1.802-3.264) as the most predominant risk factor of a higher rate of treatment failure with stent (Table 5).
Table 4.
Uni-variate logistic regression analysis
| Variables | OR |
95%CI |
P value | |
| Lower | Upper | |||
| Age | 1.035 | 0.972 | 1.102 | 0.279 |
| Sex | 1.087 | 0.180 | 6.576 | 0.928 |
| ASA score | 2.495 | 0.709 | 8.778 | 0.154 |
| Smoker, n = 35 | 3.333 | 0.605 | 18.371 | 0.167 |
| Alcohol misuse, n = 31 | 1.062 | 0.160 | 7.061 | 0.950 |
| Cardiovascular disease | 4.375 | 0.733 | 26.116 | 0.105 |
| Diabetes | 1.667 | 0.147 | 18.874 | 0.680 |
| Pulmonary disease | 0.933 | 0.092 | 9.507 | 0.954 |
| Localization | 4.421 | 0.477 | 40.980 | 0.191 |
| Cause of perforation | 2.368 | 0.479 | 11.707 | 0.290 |
| Primary contaminated pleura | 1.000 | 0.173 | 5.772 | 1.000 |
| Time to stent, days n = 38 | 1.368 | 1.016 | 1.843 | 0.039 |
| CRP, mg/L n = 38 | 1.000 | 0.995 | 1.006 | 0.902 |
| Albumin, g/L n = 29 | 1.015 | 0.902 | 1.142 | 0.805 |
| Creatinine, μmol/L n = 38 | 0.992 | 0.973 | 1.011 | 0.409 |
| BMI, kg/m2, n = 32 | 0.936 | 0.746 | 1.173 | 0.564 |
CRP: C-reactive protein; BMI: Body mass index.
Table 5.
Multi-variate analysis
| Variables | OR |
95%CI |
P value | |
| Lower | Upper | |||
| ASA score | 3.355 | 0.392 | 28.749 | 0.269 |
| Cardiovascular disease | 4.247 | 0.188 | 95.721 | 0.363 |
| Smoker | 0.672 | 0.672 | 9.515 | 0.768 |
| Localization | 350.103 | 0.192 | 637196.344 | 0.126 |
| Time to stent, d, n = 38 | 1.802 | 1.802 | 3.264 | 0.052 |
As seen in Figure 1, the predicted probability for stent treatment failure increases, rather rapidly, with time lapsed between perforation and insertion of stent. A delay in insertion of stent from day one to day three leads to a nearly two-folded increase in predicted probability for stent treatment failure.
Figure 1.
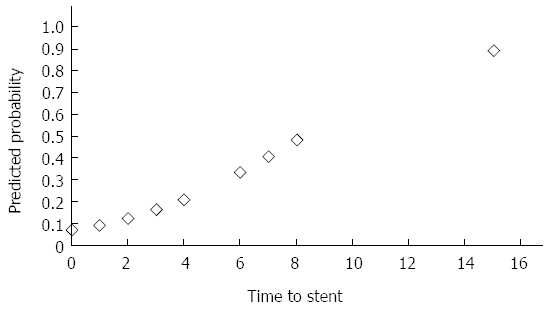
Predicted probability for stent treatment failure plotted against time to stent in days.
DISCUSSION
We have consistently applied an esophageal SEMS-based strategy as a part of a multi-modal first-line treatment of EPR, with minimal risk of introducing a selection bias of patients. Thereby we have tried to analyze the efficacy of stent treatment and predictors for failure of this therapeutic concept in benign esophageal transmural damage, including spontaneous rupture, iatrogenic and trauma. In the present study we defined treatment failure as a radical change of treatment strategy due to uncontrolled mediastinitis, which in this setting meant emergency esophagectomy with end-esophagostomy or death as a consequence of the perforation following uncontrolled sepsis. The major finding was that the time between EPR and the positioning of the stent was the most predominant risk factor for treatment failure. There were also three potentially important trends, i.e. that distal esophageal defects and the cardio-vascular comorbidity and ASA score of the patients exerted an unfavorable influence on the SEMS outcome.
The overall survival rate of our EPR patients was high (89.6%) and even higher (92.5%) among those treated with SEMS as the initial therapy. It is uncontentious that the sooner therapy is applied in EPR the better are the chances for survival. This was shown in a systematic review and meta-analysis of 75 studies, where treatment started within 24 h after the perforation resulted in a pooled mortality rate of 7.4% compared with 20.3% in patients who did not receive treatment within 24 h[3]. These observations were basically based on analyses of case series where surgical treatment was the gold standard. In this context it shall be emphasized that even with the traditional surgical approach there are large variations depending on the local tradition, location of the lesion, severity of the contamination and the length of history[3,4,7,24]. Subsequently, it can be rather difficult to separate the different confounding risk factors from each other. Therefore, when a predefined prospective strategy is applied, we can establish the pivotal importance of the length of the EPR history to critically determine the outcome when a SEMS based therapy is practiced. This information is of vital clinical importance since SEMS placement can be completed in hospitals where endoscopic service is available and stents are regarded to be affordable. Through prompt sealing of the perforation and drainage of the pleural cavity (if concomitant pleural effusion), the time lag can be effectively influenced. We also witnessed a relatively high proportion of patients referred from other hospitals, and we firmly believe that these referrals represent another important step in a successful SEMS based strategy. After sealing of the lesion at the local hospital, the patient can be safely referred to a specialized center with esophageal surgical expertise, provided that this is possible from a logistic point of view. Alternatively, daily telephone consultations can be conducted, if the patient’s general condition allows so.
Several important trends that affected outcome were observed. However, these did not reach a statistical significance, which could be due to low power of the study. One of these trends was that fewer patients with distal EPR healed on SEMS as compared to those located in the middle section of the esophagus. There seems to be a rational behind this trend, since damage to the distal esophagus often engages the gastroesophageal junction as well. As such, it entails a situation where it is difficult to cover the damaged area with stent, resulting in a persistent leak of gastric content outside the stent reaching up to the perforation. This problem was also recognized in a recent study on stent-based therapy, including anastomotic leakages[25]. The development of stent technology with more specialized, custom-made devices could be a possible solution to this problem. The current stent treatment in the middle esophagus only failed in a single case with a very large perforation, out of a total of twelve, which shows the potential of the therapy. However, a liberal attitude towards early conventional surgical intervention, in a situation where the lesion straddles the GE junction, could be motivated.
The high proportion of patients presenting with an EPR that received stent as first-line treatment, is one of the strengths of this study. It allowed us to perform an analysis regarding predictors for failure of the stent-based therapy. The definition of treatment failure for this strategy can, of course, be questioned, but we believe it is clinically valid. The stent-based therapy was abandoned only as a last resort, often followed by esophagectomy and cervical esophagostomy. The implementation of a successful SEMS based strategy entails a huge clinical commitment, as illustrated by the frequent repositioning and adjustment of the SEMS to accomplish damage control. This reiterates the notion that patients with EPR shall, whenever possible, be referred to centers with extensive experience of managing these complex cases.
The indication for stent therapy should be individualized and treatment tailored for each patient. There are no strict criteria for when, for instance, only drainage is sufficient and thus there may have been some “over-treatment” with stent in a few cases. Due to the unpredictable clinical course, the potential severity of this condition and the absence of stent-related complications according to our experience, we believe that the risk of redundant placements of stents is not a major issue.
In conclusion, this study shows that a stent treatment, when possible, is a successful strategy for treatment of EPR. Delayed treatment increases the risk that the injury will not heal. It is possible that the location of the lesion in the distal esophagus and cardiovascular co-morbidity may be additional risk factors for failure. Nevertheless, the management of EPR with stents requires an individualized treatment, pleural-mediastinal minimal invasive drainage procedures and preparedness for endoscopic re-intervention in the event that the leakage persists. In patients with delayed diagnosis of EPR and possible risk factors for stent treatment failure, who do not improve after stenting, emergency esophagectomy should be considered at an early stage.
COMMENTS
Background
Esophageal perforation/rupture (EPR) is a rare condition associated with high morbidity and mortality. The disruption of the esophageal wall and the subsequent contamination by gastric and oral secretions can rapidly progress to systemic sepsis and death. Despite advancements in diagnostic technology, surgical techniques, endoscopy and critical care, both diagnosis and treatment of EPR still present challenges.
Research frontiers
Application of a covered self-expanding metal stent over the EPR has been suggested, by several authors, to yield promising results. However, most of the literature concerning stent treatment consists of small, selected and heterogeneous case series and there is a need for more studies that also identify risk factors associated with poor outcome of treatment with stents.
Innovations and breakthroughs
Traditionally, the standard treatment option for EPR has been surgical repair, including resection of the esophagus, or conservative management with cessation of oral intake, antibiotic therapy, various drainage procedures and intensive care for multiple organ failure. During the last decade, endoscopic stent placement has increasingly become the treatment-of-choice at many centers, offering an alternative approach to aggressive operative intervention or the conservative, non-operative management that often entails a prolonged hospital stay. Previously, stent therapy in the esophagus has most commonly been used for palliation in patients with malignancies that lead to obstruction and problems with eating. The developments of stent materials, which now consist of specialized metal alloy compounds and durable polymers, have improved these results, whilst also having expanded the potential indications to include benign esophageal diseases such as EPR. In case the EPR does not heal with the stent, the patient usually undergoes a long period of repeated attempts to adjust or replace the stent. The failure of the stent therapy might eventually result in a need to take out the esophagus. A more prompt operation could be considered of benefit for such a patient, whilst also consuming less intensive care resources. Thus, the problem is to identify subjects at high risk of failure after stent-based therapy. An important question is whether there are any predictors for failure of stent-based treatment, implying that the EPR will not heal with a stent. Presently, there is a lack of studies with analyses on stent treatment failure.
Applications
The study suggests that application of a self-expandable metal stent is a safe and effective method, with a relatively low mortality, that can be applied to the majority of unselected patients presenting with EPR.
Terminology
Esophageal perforation/rupture (EPR): a hole or tear in the esophagus that is caused by injury or vomiting, with or without an underlying pathological process. Stent: a device that is inserted into a tubular structure, such as the esophagus, to provide support and open the tubular structure or, as in this case, to seal a hole or rupture in the esophageal wall.
Peer review
This is an interesting manuscript because a large referral unit has been able to manage complex patients with esophageal perforations with a single protocol for 10 years.
Footnotes
P- Reviewer: Hsiao KCW, Meshikhes AWNN, Ross CS, Tam PKH S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Søreide JA, Konradsson A, Sandvik OM, Øvrebø K, Viste A. Esophageal perforation: clinical patterns and outcomes from a patient cohort of Western Norway. Dig Surg. 2012;29:494–502. doi: 10.1159/000346479. [DOI] [PubMed] [Google Scholar]
- 2.Vidarsdottir H, Blondal S, Alfredsson H, Geirsson A, Gudbjartsson T. Oesophageal perforations in Iceland: a whole population study on incidence, aetiology and surgical outcome. Thorac Cardiovasc Surg. 2010;58:476–480. doi: 10.1055/s-0030-1250347. [DOI] [PubMed] [Google Scholar]
- 3.Biancari F, D’Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051–1059. doi: 10.1007/s00268-013-1951-7. [DOI] [PubMed] [Google Scholar]
- 4.Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77:1475–1483. doi: 10.1016/j.athoracsur.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med. 2011;19:66. doi: 10.1186/1757-7241-19-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sepesi B, Raymond DP, Peters JH. Esophageal perforation: surgical, endoscopic and medical management strategies. Curr Opin Gastroenterol. 2010;26:379–383. doi: 10.1097/MOG.0b013e32833ae2d7. [DOI] [PubMed] [Google Scholar]
- 7.Chirica M, Champault A, Dray X, Sulpice L, Munoz-Bongrand N, Sarfati E, Cattan P. Esophageal perforations. J Visc Surg. 2010;147:e117–e128. doi: 10.1016/j.jviscsurg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Abbas G, Schuchert MJ, Pettiford BL, Pennathur A, Landreneau J, Landreneau J, Luketich JD, Landreneau RJ. Contemporaneous management of esophageal perforation. Surgery. 2009;146:749–755; discussion 755-756. doi: 10.1016/j.surg.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 9.D'Cunha J, Rueth NM, Groth SS, Maddaus MA, Andrade RS. Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg. 2011;142:39–46.e1. doi: 10.1016/j.jtcvs.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Leers JM, Vivaldi C, Schäfer H, Bludau M, Brabender J, Lurje G, Herbold T, Hölscher AH, Metzger R. Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc. 2009;23:2258–2262. doi: 10.1007/s00464-008-0302-5. [DOI] [PubMed] [Google Scholar]
- 11.van Heel NC, Haringsma J, Spaander MC, Bruno MJ, Kuipers EJ. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol. 2010;105:1515–1520. doi: 10.1038/ajg.2010.104. [DOI] [PubMed] [Google Scholar]
- 12.Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc. 2009;23:1526–1530. doi: 10.1007/s00464-009-0432-4. [DOI] [PubMed] [Google Scholar]
- 13.Fischer A, Thomusch O, Benz S, von Dobschuetz E, Baier P, Hopt UT. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg. 2006;81:467–472. doi: 10.1016/j.athoracsur.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Freeman RK, Ascioti AJ. Esophageal stent placement for the treatment of perforation, fistula, or anastomotic leak. Semin Thorac Cardiovasc Surg. 2011;23:154–158. doi: 10.1053/j.semtcvs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Johnsson E, Lundell L, Liedman B. Sealing of esophageal perforation or ruptures with expandable metallic stents: a prospective controlled study on treatment efficacy and limitations. Dis Esophagus. 2005;18:262–266. doi: 10.1111/j.1442-2050.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuppusamy MK, Hubka M, Felisky CD, Carrott P, Kline EM, Koehler RP, Low DE. Evolving management strategies in esophageal perforation: surgeons using nonoperative techniques to improve outcomes. J Am Coll Surg. 2011;213:164–171; discussion 171-172. doi: 10.1016/j.jamcollsurg.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 17.Koivukangas V, Biancari F, Meriläinen S, Ala-Kokko T, Saarnio J. Esophageal stenting for spontaneous esophageal perforation. J Trauma Acute Care Surg. 2012;73:1011–1013. doi: 10.1097/TA.0b013e318265d176. [DOI] [PubMed] [Google Scholar]
- 18.Schweigert M, Solymosi N, Dubecz A, Stadlhuber RJ, Muschweck H, Ofner D, Stein HJ. Endoscopic stent insertion for anastomotic leakage following oesophagectomy. Ann R Coll Surg Engl. 2013;95:43–47. doi: 10.1308/003588413X13511609956255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg. 2008;12:1168–1176. doi: 10.1007/s11605-008-0500-4. [DOI] [PubMed] [Google Scholar]
- 20.Siersema PD, Homs MY, Haringsma J, Tilanus HW, Kuipers EJ. Use of large-diameter metallic stents to seal traumatic nonmalignant perforations of the esophagus. Gastrointest Endosc. 2003;58:356–361. doi: 10.1067/s0016-5107(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 21.Ben-David K, Lopes J, Hochwald S, Draganov P, Forsmark C, Collins D, Chauhan S, Wagh MS, Carreras J, Vogel S, et al. Minimally invasive treatment of esophageal perforation using a multidisciplinary treatment algorithm: a case series. Endoscopy. 2011;43:160–162. doi: 10.1055/s-0030-1256094. [DOI] [PubMed] [Google Scholar]
- 22.Yoon CJ, Shin JH, Song HY, Lim JO, Yoon HK, Sung KB. Removal of retrievable esophageal and gastrointestinal stents: experience in 113 patients. AJR Am J Roentgenol. 2004;183:1437–1444. doi: 10.2214/ajr.183.5.1831437. [DOI] [PubMed] [Google Scholar]
- 23.Song HY, Park SI, Do YS, Yoon HK, Sung KB, Sohn KH, Min YI. Expandable metallic stent placement in patients with benign esophageal strictures: results of long-term follow-up. Radiology. 1997;203:131–136. doi: 10.1148/radiology.203.1.9122381. [DOI] [PubMed] [Google Scholar]
- 24.Sung SW, Park JJ, Kim YT, Kim JH. Surgery in thoracic esophageal perforation: primary repair is feasible. Dis Esophagus. 2002;15:204–209. doi: 10.1046/j.1442-2050.2002.00251.x. [DOI] [PubMed] [Google Scholar]
- 25.Freeman RK, Ascioti AJ, Giannini T, Mahidhara RJ. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg. 2012;94:959–964; discussion 964-965. doi: 10.1016/j.athoracsur.2012.05.047. [DOI] [PubMed] [Google Scholar]


