Abstract
The brain transforms sensory input to motor coordinates to accommodate for changes of posture and gaze direction. Neurophysiological and neuropsychological evidence supports the existence of multiple representations of space. A debated issue regards whether objects that we see are encoded in egocentric frames only or also maintain an object-centered frame of reference. Previous clinical studies were unable to discriminate between these models as the stimuli used to determine object-based (allocentric) biases are contaminated by body-centered (egocentric) effects. To address this, we used stimuli where allocentric side was elicited by gestalt configuration rather than egocentric location. We then displayed these stimuli at different egocentric positions allowing us to independently measure the effects of allocentric position, egocentric position as well as their interaction. In a group of stroke patients with neglect we demonstrate that allocentric biases are modulated as a function of egocentric position. These findings help adjudicate between the different models of space representation, demonstrating that specific allocentric deficits not only exist but also often co-exist with egocentric biases.
Keywords: Spatial neglect, Reference frames, Attention, Allocentric, Egocentric, Space representation, Stroke, Human
Introduction
Accurate limb and eye movements require the brain to transform perceptual inputs to account for changes in eye, head, trunk and limb position. While initial visual information is encoded retinotopically, coherent responses to an object (e.g. catching a ball) need to take into account both the movements of the individual (changes in posture and gaze direction) as well as movement of the object (trajectory of the ball). There has been considerable interest regarding the frames of reference used by higher levels of the brain to encode perceptual information. Two very obvious frames use egocentric coordinates (coding a feature relative to the viewer's perspective) and allocentric coordinates (coding a feature on an object relative to the object itself). Individuals who suffer spatial neglect following brain injury provide a unique insight into this question. These individuals ignore information on their contralesional side (e.g., Karnath, 1994). Based on experiments with neglect patients allocentric coding has further been subdivided into reference frames that are (i) object-centred in the sense of operating relative to the principal axis of a shape and (ii) object-centred in the sense of operating relative to an intrinsic side of a stimulus. This second form of object-centered coding has been demonstrated with letters and words Patients have been described who neglected the contralesional side of a canonical representation of a word, irrespective of whether the word was presented in standard (horizontal) print, vertical print, mirror-reversed print, or even read out loud to the patient (Caramazza & Hillis, 1994). It has been interpreted as evidence for coding of information in a word-centered coordinate system, i.e. for a canonical, orientation-invariant representation of the word.
The present work focuses on the first aspect of allocentricity (see above; i.e. the coding of visual information relative to the principal axis of a shape). There have been numerous reports of spatial neglect in this allocentric frame of reference as well as in egocentric, body related frames of reference. Nevertheless, the relationship between these two forms of coding neglect behavior remained debated. Specifically, there is disagreement regarding whether egocentric neglect and allocentric neglect are dissociable or reflect a unitary deficit. The strongest evidence for the dissociation between allocentric and egocentric neglect comes from Hillis and co-workers. For example, in a study of 50 individuals with right hemisphere injury it was found that eleven exhibited only egocentric neglect, four suffered from allocentric neglect and only one had both deficits (Hillis et al., 2005). Moreover, results from a meta-analysis study indicated that, while egocentric symptoms were associated with damage within the perisylvian network and damage within sub-cortical structures, more posterior lesions were associated with allocentric symptoms (Chechlacz et al., 2012a,b). On the other hand, in a study of 110 acute right hemisphere patients (Yue et al., 2012) allocentric neglect was only observed in conjunction with egocentric neglect. Likewise, Rorden et al. (2012) examined 36 patients with continuous measures for these deficits and found a strong association between the severity of egocentric and allocentric neglect. One explanation for the discrepancy in the literature may be that the dissociations reported by Hillis et al. (2005) reflect stringent categorical thresholds applied to noisy measures that miss subtle forms of these deficits (Joula, 2000). On the other hand, the associations reported by Yue et al. (2012) and Rorden et al. (2012) may reflect the fact that the `allocentric' tasks used in all these studies are in fact contaminated by egocentric biases.
Pouget et al. (1999) noted that clinical measures of allocentric neglect could be parsimoniously explained by models that do not encode the objects' frame of reference: according to this “relative egocentric neglect” theory one sees poor allocentric performance simply because the objects' impaired side is egocentrically contralesional to its other side. According to this account, the evidence for associations found in clinical studies (Yue et al., 2012; Rorden et al., 2012) might merely reflect that the `allocentric' measure is contaminated by egocentric biases. Therefore, common tests cannot discriminate between this simple one-dimensional egocentric model from the two-dimensional models that attempt to encode both egocentric as well as allocentric information.
At first glance, this relative egocentric model of neglect appears to predict a fixed gradient of salience based on egocentric neglect (see Figure 1A of Driver & Pouget, 2000). This seems incompatible with the finding that when responding to multi-element arrays (such as copying drawings or defect detection tasks) many patients will neglect information when it occurs on the left side of an object yet respond to stimuli at the same horizontal coordinates if it is on the right side of an object. However, this is explained by consequences of the attention/fixation that occurs after an item is selected (see Figure 1C of Driver & Pouget, 2000): the ipsilesional side of an object has a competitive advantage for perceptual selection.
Figure 1.
Simple lesion overlaps of the 5 neglect patients and the 6 right brain damaged control subjects investigated.
Moreover, Pouget et al. (1999) suggested that the only way to distinguish between the egocentric and allocentric alternatives, or to convincingly establish object-centered neglect, is to rotate the object such that the left-right axis of the object is no longer lined up with the left-right axis of the subject. Ultimately, there was only the paradigm of Driver et al. (1994) that required models to include this specific allocentric encoding.
Here we leverage this paradigm to examine how allocentric neglect interacts with egocentric biases when both are present. In this task, the participant simply reports whether a central triangle has a gap or not. Flanking triangles are used to bias the perceived left-right axis of the triangle (and therefore whether the gap appears to be on the left or right side of the triangle). The interesting aspect of this paradigm is that the gap stays always at the same egocentric position relative to the subject's trunk. In this way, we can disentangle egocentric position from the object-centered axis, which means we are able to obtain specific allocentric values without contamination of egocentric information. Our aim was to use this paradigm to see if the severity of allocentric bias is modulated as a function of egocentric position.
Materials and Methods
Participants
We tested eleven consecutive acute right hemisphere stroke patients admitted to the Center of Neurology at Tübingen University. Five of them showed spatial neglect (NEG); six had no spatial neglect (right brain damage, RBD). Patients with a left-sided stroke, patients with diffuse or bilateral brain lesions, as well as patients who were unable to follow the instructions to finish the experiment were excluded. In addition, thirteen neurologically healthy subjects (NBD) were recruited. All 24 subjects gave their informed consent to participate in the study, which was performed in accordance with the ethical standard of the 1964 Declaration of Helsinki. Demographic and clinical data of all subjects are presented in Table 1. Simple lesion density overlap maps of all 11 stroke patients with right hemisphere lesions are presented in Figure 1.
Table 1.
Demographic and clinical data of all 24 participants
| Spatial Neglect | Controls |
||
|---|---|---|---|
| NEG | RBD | NBD | |
| Number | 5 | 6 | 13 |
| Sex (m/f) | 3/2 | 4/2 | 10/3 |
| Age (years) | 67.2 (7.8) | 65.8 (10.8) | 70.9 (5.9) |
| MMSE | 27.2 (1.6) | 28.7 (1.2) | 29.3 (0.8) |
| Etiology | 5 infarct | 6 infarct | |
| Time since lesion (days) | 5.2 (3.1) | 2.7 (1.0) | |
| Hemiparesis (% present) | 100% | 50% | |
| Visual field defects (% present) | 0 | 0 | |
| Spatial neglect scores | |||
|
| |||
| Letter cancellation (CoC) | 0.405 (0.264) | −0.004 (0.004) | |
| Bells test (CoC) | 0.388 (0.325) | 0.011 (0.029) | |
| Copying (% omitted) | 43.75 (31.46) | 0 | |
| Ota defect detection task-1 (A) | 0.023 (0.042) | 0.005 (0.012) | |
| Ota defect detection task-2 (A) | 0.096 (0.161) | 0 | |
Data are presented as mean (SD). CoC, Center of Cancellation (Rorden and Karnath, 2010); NEG, right brain damage with spatial neglect; RBD, right brain damage without spatial neglect; NBD, non-brain damage; m, male; f, female.
Clinical measures
All right brain damaged patients were assessed with the following clinical tests: Letter Cancellation Task (Weintraub and Mesulam, 1985), Bells Test (Gauthier et al, 1989), and Copying Task (Johannsen and Karnath. 2004). All three tests were presented on a horizontally oriented 21*29.7cm sheet of paper. For the Letter Cancellation Task and the Bells Test, we calculated the Center of Cancellation (CoC) using the procedure and software by Rorden and Karnath (2010). This measure is sensitive to both the number of omissions and the location of these omissions. CoC scores > 0.09 in the Letter Cancellation Task and the Bells Test were taken to indicate neglect behavior (cf. Rorden and Karnath, 2010). In the Copying Task, omission of at least one of the contralateral features of each figure was scored as 1, and omission of each whole figure was scored as 2. One additional point was given when contralesional figures were drawn on the ipsilesional side of the test sheet. The maximum score was 8. Any score higher than 1 (i.e., >12.5% omissions) indicated spatial neglect (Johannsen & Karnath, 2004). For a firm diagnosis of spatial neglect in the acute stage of the stroke, the patients had to fulfill the above criteria in at least two of the three tests. Table 1 demonstrates that the 5 neglect patients investigated in the present study all showed CoC value and omission scores that were considerably above these formal criteria.
To clinically measure allocentric biases we also applied the Defect Detection Tasks (Ota et al, 2001) and computed an allocentric score (A) based on the formula of Rorden et al. (2012). In the Defect Detection Task-1, 20 complete circles, 20 incomplete circles with a left-side gap and 20 incomplete circles with a right-side gap were arranged evenly on either side of the vertical midline of the test sheet. The patients were asked to find all the complete circles in a first copy of the test sheet and all the incomplete circles in a second copy of the test sheet. The Defect Detection Task-2 is similar to the Defect Detection Task-1; instead of circles, there were 20 equilateral triangles, 20 pseudo-triangles with a missing portion on the left-side and 20 pseudo-triangles with a missing portion on the right-side. Patients were asked to find all the equilateral triangles in a first copy of the test sheet and all the pseudo-triangles in a second copy of the test sheet.
Apparatus and stimuli
For all subjects stimuli were generated by software E-Prime 2.0 and displayed on a ThinkPad laptop (type 8932) with a screen size of 1280*800 pixel. Two button-boxes were connected to the laptop. There were two response-buttons (one yellow, one green) representing `with gap' and `no gap' as well as a red resting-point in between the two response-buttons. Subjects had to place the index finger on this resting-point before and after each response to stimuli.
The stimuli applied were identical to those introduced by Driver et al. (1994). They consisted of 1 target-triangle that was embedded in the middle of a diagonal line of 6 outline context-triangles. There are two possible configurations for the diagonal arrays (illustrated in the bottom of Figure 2). One ran from south-west to north-east (SW-NE); the other configuration ran from south-east to north-west (SE-NW). On the basis of previous research with normal observers (Palmer et al., 1980, 1981, 1985), the triangles should be seen as pointing towards the top-left of the screen with the SW-NE configuration and towards the top-right with the SE-NW configuration. As a result of this perceived pointing, gaps in the target-triangle should fall on the allocentric right of the object-centered axis along which the triangle points with the SW-NE configuration, whereas gaps should fall on the allocentric left of the object-centered axis with the SE-NW configuration. The interesting aspect of this paradigm is that the gap stays always at the same egocentric position relative to the subject's trunk.
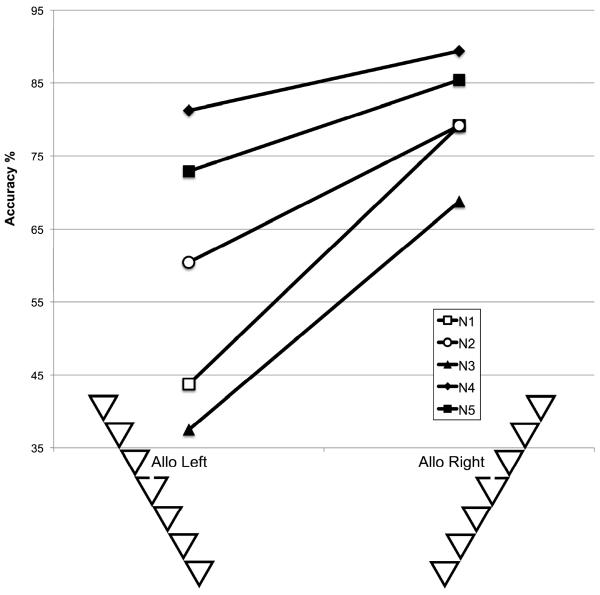
Figure 2.
Accuracy (vertical axis) for each neglect patient (lines) to detect a gap (target) that appeared on the allocentric left (left column) or right (right column) of an object when targets presented directly in front of the participant (egocentric center). Each patient exhibits pronounced allocentric neglect, missing gaps more frequently when they appear on the left side of the object's axis relative to gaps on the right side of the object's axis. Note that the egocentric location of the gap is identical for the two configurations, with left or right allocentric positoins defined by the configural axis. Despite over 100 citations, this is the first replication of the findings of Driver et al. (1994).
We used a viewing distance of approximately 60 cm, i.e. the sides of each triangle were 1.9°, while the entire diagonal array of triangles was 13° long by 6.5° wide. Additionally, a solid fixation point (0.3° in diameter) was presented at the center of the target-triangle and the center of the screen. The fixation point disappeared when stimuli (triangles) were displayed. The triangles were black, the fixation point was white, with the background in grey color.
Procedure
During a training phase a diagram of the two possible stimulus configurations (illustrated in the bottom of Figure 2) was shown. Subjects were asked: `In which direction do all of the triangles point together?' The answers from all subjects in the present study were consistent with previous research (Palmer et al., 1980, 1981, 1985), i.e. the triangles with the SE-NW configuration were seen as pointing towards the top-right and the triangles with the SW-NE configuration as pointing towards the top-left of the screen. The diagram was then used to explain that the gap in the target-triangle was sometimes absent and the task was simply to decide whether the gap was there or not. It was emphasized that gaps occurred only on one of the edges of the target-triangle. When specifying where the gap could appear, no directional terms such as `top' or `horizontal' were used. Instead, the experimenter pointed to the upper horizontal edge of the target-triangle and emphasized that `the gap will only appear on this edge'.
There were 13 blocks of stimulus presentation in the whole experiment with 36 trials presented in each block. The experimenter sat face-to-face of the subjects to control their fixation and attention by observation. Following the experimenter's button press, the fixation point was presented for 1000 msec. On its offset, the diagonal array of triangles was presented for 175 msec. Subsequently the target-triangle disappeared while the context-triangles continued to be displayed for a full 2000 msec. Then the screen was cleared until the experimenter confirmed that the patient was ready for the next trial (eyes on the fixation point and attention not distracted) by pressing the control-button-box to initiate the next trial. The subjects were free to rest between blocks whenever they wished. In each block, the 36 trials displayed the two configurations equiprobably and intermingled randomly. On one third of all trials, there was no gap in the central target-triangle. The subjects were not informed about the probabilities for the presence or absence of gaps.
The first block served as titration block and was not considered for later data analysis. The purpose of this block was to avoid ceiling and floor effects, making the task difficulty comparable between individuals. During this block the target gap size was dynamically adjusted after each trial, regardless of condition. If the participant made an incorrect answer the gap in the following trial would be bigger (easier to detect), whereas if the participant made the correct answer on two successive trials the subsequent trial would use a smaller gap size (harder to detect). This algorithm is designed to settle on a criterion that yields a 71% accuracy rate (Levitt, 1971). The starting size of gap was 0.68°, the adjusting size was 0.028°, the maximum size of gap after total adjustment was 1.46° (easiest) and the minimum size of gap after total adjustment was 0.028° (hardest). The final gap size used in this titration block was applied to the following 12 experimental blocks.
The egocentric position of the display was individually adjusted between blocks. In the first titration block, the horizontal position of the fixation point was aligned with the subject's trunk midline. In the following 12 experimental blocks, the horizontal position of the display was moved in pseudo-random order (counter balanced between participants) to one of three locations with respect to the subject's trunk midline, namely −40° left, +40° right, or at 0°, i.e. in line with the subject's trunk midline. In total, 4 blocks were performed at each egocentric location (excluding the titration block). The subjects were requested to orient their heads and gaze toward the display at the three different egocentric positions while keeping trunk position fixed (thus this manipulation shifted the position relative to the trunk, but not relative to the head or retina).
Overall, there was a total of 432 experimental trials in 12 blocks for each individual: 216 displayed the SW-NE configuration, 216 the SE-NW configuration, and 72 trials a configuration without a gap. At each of the three egocentric positions, 144 trials were performed.
Results
With the display located at 0°, i.e. with the fixation point in line with the subject's trunk midline, we were able to replicate the observations by Driver et al. (1994). Figure 2 illustrates the accuracy of each patient with spatial neglect separately for the two stimulus configurations. As in the study by Driver et al. (1994) the patients failed to detect the presence of the gap more often with the SE-NW (`allo'centric left) configuration than with the SW-NE (`allo'centric right) configuration. In other words, they missed more gaps to the left of the axis (as defined by the configural grouping), providing a clear demonstration of object-based neglect.
The primary statistical analyses used the accuracy of responses as the dependent variable. We performed a 3 (egocentric positions: −40°, 0°, +40°) × 2 (allocentric positions: gap on left, gap on right) repeated measures ANOVA with group (NEG, RBD, NBD) as the between-subject factor. The interaction between these three factors was significant (F(4,42)=4.45, P=0.004). We then performed two separate two-way repeated measures ANOVAs for the two stimulus configurations respectively, using the 3 egocentric positions as within-subject variable (−40°, 0°, +40°) and group (NEG, RBD, NBD) as the between-subject factor. For object right configuration (SW-NE) neither the interaction (F(4,42)=2.30, P=0.075) nor the two main effects (egocentric position: F(2,42)=2.70, P=0.078 / group: F(2,21)=0.81, P=0.457) were statistically significant. For allocentric left configuration (SE-NW) the analysis revealed a significant interaction (F(4,42)=10.39, P<0.0001). We thus performed three separate paired t-tests for each of the three subject groups for this stimulus configuration. Figure 3 shows the accuracies of responses as a function of object orientation and egocentric positions (−40°, 0°, +40°). The differences of accuracies among egocentric positions in groups RBD and NBD were not significant (all P>0.05). In group NEG, the differences between egocentric positions 0° and +40° (t(4) = −2.88, P=0.045) as well as between −40° and +40° (t(4)= −5.96, P=0.004) both were significant. The third comparison between egocentric position −40° and 0° showed no statistically significant result but a numerical tendency in the expected direction (t(4)= −2.45, P=0.071).
Figure 3.
The top panel shows accuracy (vertical axis) for detecting gaps that appeared on the allocentric left (black lines) or right (gray lines) of an object when targets presented with different egocentric positions (horizontal axis, with targets located either 40° left, center, or 40° right of trunk midline). The three panels show the performance of the three groups: neglect patients, right brain injured patients without neglect and healthy controls who did not have brain injury. Note that neglect patients show profoundly poorer performance (reduced accuracy) for the allocentric left side of objects, as well as for objects presented on the egocentric left side of the body. The lower panel shows response times (vertical axis) with the same layout as the top panel. Note similar pattern with poorer performance (slower responses) for the gaps on the left side of the objects' axis relative to the right side of the axis, as well as poorer performance for stimuli presented on the left side of the body relative to the right side of the body.
To tease apart the effects of allocentric and egocentric biases we conducted a 3 (egocentric positions: −40°, 0°, +40°) × 2 (allocentric positions: gap on left, gap on right) repeated measures ANOVA of the error rate for the patients who exhibited neglect. We found a significant interaction (F(2,8)=7.88 p=0.013) as well as main effects of egocentric position (F(2,8)=9.9 p=0.007) and of the object-axes side (F(1,4)=69.63 p<0.001). In order to understand the main effects in the presence of what appears to be an ordinal interaction, we conducted t-tests to look at the simple main effects of allocentric configuration. These reveal that responses to allocentric left items were less accurate than for allocentric right items at the egocentric left (t(4)=8.8, p=0.0009), egocentric center (t(4)=4.83 p=0.0085) and egocentric right positions (t(4)=4.88 p=0.0081). To better understand the main effect of egocentric position in the presence of an interaction we conducted t-tests on data where we collapsed across allocentric configuration. The resulting t-test were not significant for left versus center (t(4)=2.37 p=0.077) and center versus right (t(4)=2.04 p=0.1113) positions but was significant between the left and right (t(4)=4.46 p=0.0111) egocentric positions. Descriptively, the interaction reflects an increasingly severe allocentric bias for more leftward egocentric positions. On the other hand, there was a reliable effect for more errors for gaps on the left side of the triangle's axis and equivocal trends for better performance on the right versus left side of the body. To rule out speed/error tradeoffs we conducted an analogous ANOVA on the reaction times and found the same pattern of results (Figure 3): a main effect of egocentric position (F(2,8)=7.93 p = 0.013) and a trend in the predicted direction of object-axes side (F(1,4)=5.05 p = 0.087 [two-tailed]) but no firm evidence for an interaction (F(2,8)=2.08 p = 0.19). Therefore, the analysis of response times is consistent with the analysis of error rates and provides reliable evidence for a main effect of egocentric position.
In order to document that the statistically proven effects based on the group average results (illustrated in Fig. 3) are valid observations also on the individual level, Figure 4 illustrates the performance for each patient with spatial neglect (N1-N5) for gaps appearing on the allocentric left or right, for the three egocentric display positions. Indeed, all individuals were numerically better at detecting gaps on the left side of objects when the display was on the egocentric right than on the egocentric left side of their trunk.
Figure 4.
Performance for each individual with neglect (N1–N5) for gaps appearing on the allocentric left (_L) or right (_R), for three egocentric display positions: Ego Left (stimuli 40° to the left of the tunk midline), Ego Center (stimuli straight ahead with respect to trunk) and Ego Right (stimuli 40° to right of trunk midline). To help visualize speed-accuracy tradeoffs this figure shows the inverse efficiency score (response time/accuracy fraction [Townsend and Ashby, 1978]) such that higher values indicate poorer performance, with values being normalized so that each individual has a mean efficiency score of one. Note that numerically all individuals were better at detecting gaps on the allocentric left side of objects when the display was on the egocentric right than on the egocentric left side of their trunk.
Discussion
The present study examined whether or not allocentric neglect varies with egocentric position. Unlike previous studies we used a paradigm to determine allocentric neglect that cannot be explained as merely a relative egocentric effect. In a group of neglect patients, we observed that allocentric neglect indeed varied with egocentric position. The poor accuracy for detecting features on the left side of the object's axis was most severe for objects presented on the contralesional side of the body. These findings clearly illustrates that not only were allocentric and egocentric biases present simultaneously, but that egocentric information can influence the severity of allocentric neglect.
Three previous studies have suggested that egocentric and allocentric neglect interact, suggesting that these deficits may rely on the same substrates. Two have demonstrated that task instructions can modulate whether a patient exhibits egocentric or allocentric neglect. Karnath and Niemeier (2002) examined exploratory eye movements when patients were directed via the experimenter's instructions to either attend to the whole space or only a restricted part of it. They observed that the same physical stimulus was attended to or, in another situation, neglected, just depending on the individuals' goal. Likewise Baylis et al. (2004) asked patients with spatial neglect to either search for a letter across the entire expanse of a computer screen, or within a particular object presented on that display. Patients exhibited egocentric deficits when searching globally yet allocentric deficits for physically identical stimuli when searching within an object. In a third experiment, neglect patients' eye and head movements were recorded while they explored objects at five egocentric positions along the horizontal dimension of space (Karnath et al., 2011). They found that allocentric neglect varied with egocentric position. Although the neglect of the objects' left side was severe at contralesional egocentric positions, it ameliorated at more ipsilesional egocentric positions of the objects. However, the results from these three studies do not provide unambiguous support. As with other clinical measures of allocentric neglect the measures of allocentric neglect in these three studies were contaminated – according to “relative egocentric neglect” theory – by egocentric biases. Poor allocentric performance could simply due to the fact that the objects' impaired side is egocentrically contralesional to its other side (Pouget et al., 1999).
In order to avoid the contamination of egocentric biases and to acquire the specific allocentric effect, the present study applied the stimuli of Driver et al. (1994) which has been suggested as the only paradigm to distinguish between egocentric and allocentric alternatives. Though Driver et al.'s original work documented that all their left-neglect patients showed positive results in their experiment, they did not contrast this behavior with control groups to test whether or not it is specific for neglect subjects. Therefore, in the present study, we included two control groups (brain damaged patients without neglect and healthy subjects) to provide a powerful proof that positive results in the paradigm of Driver et al. were not due to other post-stroke deficits or some general cognitive effects. In fact, we replicated Driver et al.'s result in each acute neglect patient but not in any subject from our control groups.
Our present findings based on 5 neglect patients consecutively admitted to our Center of Neurology are incompatible with the one-dimensional model of Pouget et al. (1999). On the other hand, it accords with the two-dimensional models of Niemeier and Karnath (2002a,b) and of Deneve and Pouget (1998, 2003). Niemeier and Karnath (2002a) proposed a representation concept coding visual input in two kinds of coordinates simultaneously, with no need for further transformation. This “integrated space-object map” (ISO-map) codes the position of an object in egocentric space along a head- and/or trunk-centered dimension and the object's within-object coordinates along an object-based dimension. In neglect patients, the salience functions were assumed to be altered in a particular way, namely, the bell-shaped pattern along the horizontal egocentric axis with the peak shifted to the ipsilesional right side and the ramp-shaped pattern along the horizontal object-based axis increasing monotonically from left to right. A central prediction of the model was, thus, a direct interaction between allocentric and egocentric coordinates, namely, that the left-right asymmetry in neglect should improve with more ipsilesional positions of the objects.
In contrast, the model by Deneve and Pouget (1998, 2003) did not assume an explicit object-centered representation but rather implemented a neural network in which the input contains a V1-like of the image of the object, a set of cells tuned to the orientation of the object, and a set of cells encoding the current command. It further assumed a layer that contains gain-modulated cells similar to those observed in physiological recording. Therefore, in their model object orientation modulates the activity of cells that have different (e.g. retinotopic) receptive fields, without any cells showing an explicit object-based receptive field.
While different in their designs these two models coincide in that both claim that allocentric information should be influenced by egocentric manipulations. The present observations provide evidence for this claim, showing for the first time that specific allocentric deficits not only exist but also can actually co-exist an egocentric bias. In fact, the severity of allocentric bias was modulated as a function of egocentric position.
Beside conceptual models, our present conclusions are also intuitively supported by functional brain activity research. In a recent fMRI study (Chen et al., 2012), BOLD responses were measured while healthy subjects performed egocentric or allocentric visuospatial judgments on a three-dimensional object (a fork on a plate). The authors observed that egocentric judgments and allocentric judgments conjointly activated both the ventral and the dorsal streams. By comparing with the allocentric judgments, they also found that egocentric judgments more strongly activated certain areas, while no significant activation was found in the reverse contrast. This indicated that obviously no isolated networks exists for allocentric judgments.
We suggest that apparent egocentric and allocentric behavior is emergent from our attentional selection. Consider how your visual search is different when you are looking for a note somewhere in your office (diffuse) versus when you were told the note is located on your computer screen (focused). Subsequent perceptual competition is biased within this selection (Driver and Pouget, 2000). Therefore, a single patient can appear to have either an allocentric or egocentric deficit depending on the selection strategy (Karnath and Niemeier, 2002; Niemeier and Karnath, 2002a; Baylis et al., 2004). According to this view, when faced with tasks that require both diffuse as well as focused selection (such as copying tasks or defect detection tasks) the performance of a neglect patient will reflect the strategic bias: does the patient attend to the `forest' (diffuse attention, appears more egocentric) versus the `trees' (focused attention, appears more allocentric).
Our behavioral data can not resolve the debate regarding whether allocentric and egocentric biases reflect deficits relying on disturbance of a unitary or of isolated neural networks. Certainly our findings are consistent with recent studies investigating large subject groups (Yue et al., 2012; Rorden et al., 2012) suggesting that at the acute stage allocentric biases are seen concomitant with egocentric biases. However, observing a neuropsychological association does not necessarily imply a unitary disorder. It is logically possible that these two dimensions are coded by separate modules, but that due to their anatomical location damage to one module typically also impacts the other module. Alternatively, these modules could be tightly coupled such that dysfunction to one module typically leads to knock-on effects in the other module. It is also possible that allocentric and egocentric biases reflect a single underlying disorder resulting from damage of a unitary module. Therefore, the strong correlations observed between allocentric and egocentric measures both in the present work as well as larger clinical studies (Yue et al., 2012; Rorden et al., 2012) could be explained by either a single unified module or two neighboring modules that are typically injured together. Indeed, this explanation would accord with anatomical studies (for review of the relevant literature, see Karnath and Rorden, 2012). Perhaps future brain stimulation studies can adapt our paradigm to directly test this question – for example demonstrating that focal stimulation transiently modulates only one of these frames of reference.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (KA 1258/10-1), and the National Institutes of Health (NS054266; DC017863).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baylis GC, Baylis LL, Gore CL. Visual neglect can be object based or scene-based depending on task representation. Cortex. 2004;40:237–246. doi: 10.1016/s0010-9452(08)70119-9. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. Spatial representation of words in the brain implied by studies of a unilateral neglect patient. Nature. 1990;346:267–269. doi: 10.1038/346267a0. [DOI] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Humphreys GW. Neuroanatomical dissections of unilateral visual neglect symptoms: ALE meta-analysis of lesion-symptom mapping. Front Hum Neurosci. 2012a;6:230. doi: 10.3389/fnhum.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Roberts KL, Bickerton W-L, Lau JKL, Humphreys GW. The prognosis of allocentric and egocentric neglect: evidence from clinical scans. PloS ONE. 2012b;7:e47821. doi: 10.1371/journal.pone.0047821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneve S, Pouget A. Neural Basis of Object-Centered Representations. In: Jordan MI, Kearns MJ, Solla S, editors. Advances in Neural Information Processing Systems. 10. MIT Press; Cambridge MA: 1998. pp. 24–30. [Google Scholar]
- Deneve S, Pouget A. Basis functions for object-centered representations. Neuron. 2003;37:347–359. doi: 10.1016/s0896-6273(02)01184-4. [DOI] [PubMed] [Google Scholar]
- Driver J, Baylis GC, Goodrich SJ, Rafal RD. Axis-based neglect of visual shapes. Neuropsychologia. 1994;32:1353–1365. doi: 10.1016/0028-3932(94)00068-9. [DOI] [PubMed] [Google Scholar]
- Driver J, Pouget A. Object-centered visual neglect, or relative egocentric neglect? J Cogn Neurosci. 2000;12:542–545. doi: 10.1162/089892900562192. [DOI] [PubMed] [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11:49–54. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: Insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L, Karnath H-O. How efficient is a simple copying task to diagnose spatial neglect in its chronic phase? J Clin Exp Neuropsychol. 2004;26:251–6. doi: 10.1076/jcen.26.2.251.28085. [DOI] [PubMed] [Google Scholar]
- Juola P. Double dissociations and neurophysiological expectations. Brain Cogn. 2000;43:257–62. [PubMed] [Google Scholar]
- Karnath H-O. Spatial limitation of eye movements during ocular exploration of simple line drawings in neglect syndrome. Cortex. 1994;30:319–330. doi: 10.1016/s0010-9452(13)80202-x. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Niemeier M. Task-dependent differences in the exploratory behaviour of patients with spatial neglect. Neuropsychologia. 2002;40:1577–1585. doi: 10.1016/s0028-3932(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Mandler A, Clavagnier S. Object-based neglect varies with egocentric position. Journal of Cognitive Neuroscience. 2011;23:2983–2993. doi: 10.1162/jocn_a_00005. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467–477. [PubMed] [Google Scholar]
- Niemeier M, Karnath H-O. The exploration of space and objects in neglect. In: Karnath H-O, Milner AD, Vallar G, editors. The Cognitive and Neural Bases of Spatial Neglect. Oxford University Press; Oxford: 2002a. pp. 101–118. [Google Scholar]
- Niemeier M, Karnath H-O. Simulating and testing visual exploration in spatial neglect based on a new model for cortical coordinate transformation. Experimental Brain Research. 2002b;145:512–519. doi: 10.1007/s00221-002-1132-7. [DOI] [PubMed] [Google Scholar]
- Ota H, Fujii T, Suzuki K, Fukatsu R, Yamadori A. Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology. 2001;57:2064–2069. doi: 10.1212/wnl.57.11.2064. [DOI] [PubMed] [Google Scholar]
- Palmer SE. What makes triangles point: Local and global effects in configurations of ambiguous triangles. Cognit. Psychol. 1980;12:285–305. [Google Scholar]
- Palmer SE. The role of symmetry in shape perception. Acta Psychol. 1985;59:67–90. doi: 10.1016/0001-6918(85)90042-3. [DOI] [PubMed] [Google Scholar]
- Palmer SE, Bucher NM. Configural effects in perceived pointing of ambiguous triangles. J. exp. Psychol.: Hum. Percept. Perform. 1981;7:898–114. doi: 10.1037//0096-1523.7.1.88. [DOI] [PubMed] [Google Scholar]
- Pouget A, Driver J. Relating unilateral neglect to the neural coding of space. Current Opinion in Neurobiology. 2000;10:242–249. doi: 10.1016/s0959-4388(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Pouget A, Deneve S, Sejnowski TJ. Frames of reference in hemineglect: a computational approach. Progress in Brain Research. 1999;121:81–97. doi: 10.1016/s0079-6123(08)63069-1. [DOI] [PubMed] [Google Scholar]; Jim Reggia, Eytan Ruppin, Dennis Glanzman., editors. Neural modeling of brain disorders. Elsevier; [Google Scholar]
- Chen Q, Weidner R, Weiss PH, Marshall JC, Fink GR. Neural interaction between spatial domain and spatial reference frame in parietal-occipital junction. J Cogn Neurosci. 2012;24:2223–2236. doi: 10.1162/jocn_a_00260. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. A simple measure of neglect severity. Neuropsychologia. 2010;48:2758–63. doi: 10.1016/j.neuropsychologia.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Hjaltason H, Fillmore P, Fridriksson J, Kjartansson O, Magnusdottir S, Karnath H-O. Allocentric neglect strongly associated with egocentric neglect. Neuropsychologia. 2012;50:1151–7. doi: 10.1016/j.neuropsychologia.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG. Methods of modeling capacity in simple processing systems. In: Castellan J, Restle F, editors. Cognitive theory. Vol. 3. Hillsdale, N.J.; Erlbaum: 1978. pp. 200–239. [Google Scholar]
- Weintraub S, Mesulam M-M. Mental state assessment of young and elderly adults in behavioral neurology. In: Mesulam M-M, editor. Principles of behav- ioral neurology. F.,A., Davis; Philadelphia, PA: 1985. pp. 71–123. [Google Scholar]
- Yue Y, Song W, Huo S, Wang M. Study on the occurrence and neural bases of hemispatial neglect with different reference frames. Archives of Physical Medicine and Rehabilitation. 2012;93:156–162. doi: 10.1016/j.apmr.2011.07.192. [DOI] [PubMed] [Google Scholar]