Abstract
Recent work supports the idea that cisternae of the Golgi apparatus can be assigned to three classes, which correspond to discrete stages of cisternal maturation. Each stage has a unique pattern of membrane traffic. At the first stage, cisternae form in association with the ER at multifunctional membrane assembly stations. At the second stage, cisternae synthesize carbohydrates while exchanging material via COPI vesicles. At the third stage, cisternae of the trans-Golgi network segregate into domains and produce transport carriers with the aid of specific lipids and the actin cytoskeleton. These processes are coordinated by cascades of Rab and Arf/Arl GTPases.
Introduction
The Golgi apparatus is fascinating but enigmatic. A working assumption is that operational principles of the Golgi are conserved between species, and that model organisms can serve to illuminate these principles [1, 2]. From this perspective, the goal is to synthesize diverse lines of evidence into a “generic” picture of how the Golgi works.
Such a multifaceted approach has been applied to understanding the flow of membrane and cargo through the Golgi [3, 4]. Perhaps the most widely embraced model for Golgi traffic is cisternal maturation, which proposes that cis-Golgi cisternae arise de novo, then progressively mature into trans-Golgi cisternae while carrying secretory cargoes forward, then ultimately disintegrate into transport carriers at the trans-Golgi network (TGN) stage. As the cisternae mature, COPI vesicles are thought to recycle resident Golgi membrane proteins. Maturation of Golgi cisternae has been directly observed in Saccharomyces cerevisiae, which has non-stacked Golgi cisternae that can be optically resolved [5, 6]. In plant and animal cells, the ability of large cargoes to transit through the Golgi provides some of the clearest evidence that Golgi cisternae serve as anterograde carriers [7, 8]. However, experimental and theoretical arguments suggest that other data are inconsistent with a simple maturation mechanism [9 •, 10], and alternatives such as the cisternal progenitor model and the kiss-and-run model have been proposed [11, 12], so this issue remains unresolved.
A pair of recent studies tested the cisternal maturation model in mammalian cells [13 ••, 14 ••]. Both groups used transmembrane proteins fused to a multiple copies of a dimerizing mutant of FK506-binding protein (FKBP). In one case, an FKBP-tagged secretory cargo self-associated to form “staples” that linked the two faces of a cisterna together [13 ••]. Those staples did not progress through the Golgi, consistent with the idea that Golgi cisternae are long-lived compartments. In the other case, an FKBP-tagged resident Golgi protein aggregated in the plane of the membrane [14 ••]. Those aggregates moved forward through the Golgi, and when they were subsequently dissolved, the tagged Golgi proteins recycled to earlier cisternae, consistent with the idea that Golgi cisternae carry large cargoes forward while recycling resident proteins. The discrepancies between the two studies presumably center on technical details. Resolution of this debate will be an important step toward a unified picture of Golgi traffic.
Our review focuses on Golgi compartmentation and identity. These issues are closely linked to Golgi traffic, and the cisternal maturation model will serve as the basis for the discussion, but we will highlight open questions.
The Golgi as a tripartite organelle
In both stacked and nonstacked Golgi organelles, the cisternae vary in composition [15, 16]. Mammalian cells contain multiple Golgi cisternae plus the ER-Golgi intermediate compartment (ERGIC) [17]. These findings raise the question of how many fundamentally different types of compartments exist in the Golgi. There are compelling reasons to distinguish between the TGN and earlier cisternae [18, 19], and an early review suggested that the Golgi is actually a tandem pair of organelles [20], which would now be designated the Golgi stack and the TGN. However, differences can also be seen among cisternae that precede the TGN [21, 22], leading to the suggestion that the Golgi consists of four or more different kinds of compartments [15, 23]. This uncertainty highlights a major gap in our understanding.
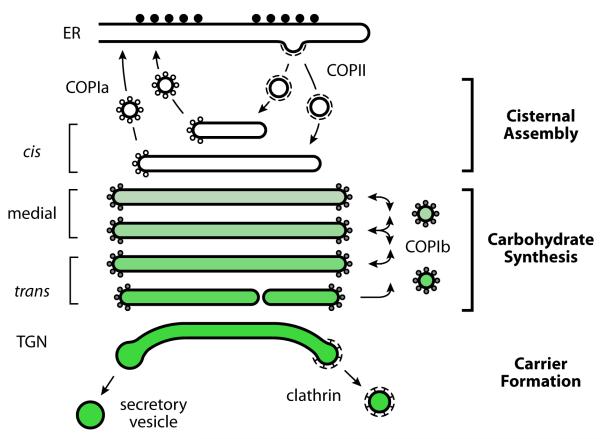
We recently updated an old concept [19] by postulating that Golgi cisternae can be divided into three classes, which represent discrete stages of maturation [24]. Each stage is defined by the membrane traffic pathways to and from the cisternae (Fig. 1). At the “cisternal assembly” stage, which includes the ERGIC and the cis-Golgi cisternae, ER-derived COPII vesicles deliver secretory proteins to the nascent cisterna, while vesicles of the COPIa type bud from the nascent cisterna to recycle trafficking components and resident ER proteins back to the ER. At the “carbohydrate synthesis” stage, which includes the medial and trans-cisternae, vesicles of the COPIb type transfer resident Golgi proteins between cisternae. This transfer is presumably nondirectional for each individual vesicle, but as young cisternae acquire the ability to receive COPIb vesicles while old cisternae lose this ability, COPIb vesicles mediate a net recycling of resident Golgi proteins from old to young cisternae. Finally, at the “carrier formation” stage, which corresponds to the TGN as traditionally defined, the cisternae produce clathrin-coated vesicles and secretory carriers while receiving incoming traffic from endosomes. Within a given stage, cisternae can evolve in terms of their resident protein composition, but they share a common machinery for membrane traffic. Current knowledge of the Golgi traffic machinery is consistent with the three-stage model [24].
Figure 1.
We propose that the Golgi can be divided into three stages of maturation. During the Cisternal Assembly stage, COPII vesicles bud from the ER and fuse with one another to generate a new Golgi cisterna, while COPIa vesicles bud from this nascent cisterna to recycle trafficking proteins and resident ER proteins to the ER. This stage includes cis-Golgi cisternae, as well as ERGIC membranes in animal cells. During the Carbohydrate Synthesis stage, most of the oligosaccharide remodeling reactions take place, and resident Golgi proteins are transferred between cisternae by COPIb vesicles. This stage includes cisternae that are often designated medial and trans. During the Carrier Formation stage, the cisterna disintegrates to produce cargo carriers that include clathrin-coated and secretory vesicles. This stage includes cisternae that are traditionally designated TGN. Adapted from [24].
It remains to be seen whether this view of the Golgi as a tripartite organelle will endure. The classification of Golgi cisternae has been an elusive quest, and indeed, the names of the three stages shown in Fig. 1 are imperfect. For instance, even though carbohydrate synthesis occurs mainly at the second stage, Golgi glycosylation in yeast is initiated in the earliest Golgi compartments [23], and terminal glycosylation in mammalian cells can occur in the TGN [25]. Yet diverse eukaryotes may all have three types of Golgi cisternae as defined by their membrane traffic machinery. According to this hypothesis, an understanding of Golgi compartmentation and identity will rely on deciphering the mechanisms that generate the three stages.
To the Golgi: the cisternal assembly stage
Golgi cisternae are thought to assemble by the homotypic fusion of COPII vesicles, which are produced at long-lived domains known as transitional ER (tER) sites or ER exit sites [26]. Intermediates in this cisternal assembly pathway were visualized by electron tomography of plant and algal Golgi stacks [27 ••]. An assembling cisterna produces COPIa vesicles that recycle trafficking components to the ER [26, 28]. These retrograde carriers may be targeted to tER sites [29 •, 30]. Regardless of whether tER sites are unidirectional or bidirectional portals, they are clearly associated with early Golgi or ERGIC membranes in diverse eukaryotes [17, 31, 32].
A long-standing mystery is how COPII vesicles become clustered at tER sites. Normal tER organization requires Sec16, a peripherally membrane-associated COPII binding protein [33-35]. Sec16 has been proposed to act as a scaffold that clusters COPII components at tER sites [34-37]. However, recent data challenge this notion, suggesting instead that Sec16 is recruited by individual COPII vesicles as a regulator of COPII coat dynamics [37, 38 •, 39, 40]. When Sec16 was inactivated in the yeast Pichia pastoris, tER sites became smaller and more dynamic, but they persisted [38 •]. The implication is that a Sec16-independent mechanism establishes tER sites. This mechanism might involve the tethering of nascent and budded COPII vesicles to adjacent early Golgi or ERGIC membranes [41] (Fig. 2). If multiple COPII vesicles are all tethered to the same membrane structure, these vesicles will naturally cluster. Some of the tethering events may be controlled by cycles of phosphorylation and dephosphorylation of COPII coat subunits [42 •]. Studies of Golgi stack formation suggest that tethering proteins help to nucleate cisternal assembly [43, 44]. The tethering model postulates that tER sites form in conjunction with early Golgi or ERGIC membranes by an integrated self-organization pathway [41].
Figure 2.
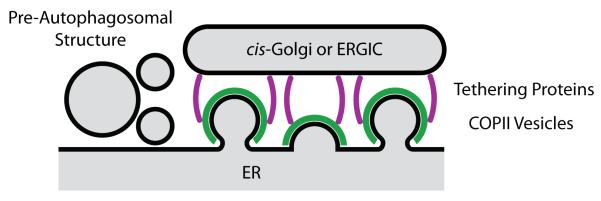
The ER contains multifunctional membrane assembly stations. COPII vesicles bud from tER sites, which may be generated by the tethering of nascent COPII vesicles to adjacent cis-Golgi or ERGIC membranes. A subset of the tER sites are associated with preautophagosomal structures.
The tER plus the early Golgi/ERGIC seems to be part of a larger structure that includes nascent autophagosomes (Fig. 2). Starved yeast cells contain a compartment for unconventional protein secretion (CUPS) that associates with tER sites and contains autophagy-related proteins [45], and pre-autophagosomal structures are functionally and physically linked to tER sites in yeast and mammalian cells [46 ••, 47 •, 48 ••]. COPII vesicles evidently contribute to forming these structures [49]. Moreover, the yeast Ypt1 GTPase, which is implicated in ER-to-Golgi transport, has an additional autophagy-related function at the ER [50 •, 51 ••, 52 •]. Thus, Golgi cisternae may form together with other compartments at a multifunctional membrane assembly station (Fig. 2).
Through the Golgi: the carbohydrate synthesis stage
After cisternal assembly is complete, the main task is to synthesize and remodel carbohydrates. Conversion of a Golgi cisterna to the carbohydrate synthesis stage involves multiple changes: a loss of the ability to receive COPII vesicles, a transition from producing COPIa to COPIb vesicles, and a gain of the ability to receive COPIb vesicles (Fig. 1). At the carbohydrate synthesis stage, COPIb vesicles are thought to exchange resident Golgi glycosylation enzymes between cisternae, thereby generating gradients of enzyme composition across Golgi stacks [53]. However, fundamental questions remain about intra-Golgi traffic at this stage.
One question concerns the molecular distinction between COPIa and COPIb vesicles. This terminology comes from morphological studies of the plant secretory pathway [22], but studies of mammalian cells also provide evidence for two classes of COPI vesicles [54-57]. In mammalian cells, COPI subunit isoforms apparently interact with specific partners during different COPI-dependent pathways [58 •]. More generally, the properties of a COPI vesicle may be dictated by the composition of the parental compartment, with the same basic machinery generating either COPIa or COPIb vesicles at different stages of maturation.
A second question concerns the directionality of COPIb vesicles. If these vesicles move one step at a time from older to younger cisternae, the observed gradients of resident Golgi proteins across the stack can be conveniently explained [53]. However, no mechanism for such orderly movement has been described. A plausible alternative is that COPIb vesicles “percolate” stochastically between cisternae at the carbohydrate synthesis stage [59]. To explain how stochastic vesicle movement generates gradients of protein composition across the stack, one can postulate that time-dependent changes in cisternal pH or lipid composition kinetically trap resident Golgi proteins in cisternae of a particular age [24, 60].
Finally, a third question is whether COPI vesicles contain secretory cargoes. If so, vesicle-mediated transport could provide an anterograde intra-Golgi transport pathway that is complementary to cisternal maturation [61]. The literature on this topic has been contradictory [4, 62], but a study of transfer between Golgi stacks in fused cells suggested that COPI vesicles could carry both resident Golgi enzymes and small secretory cargoes [63 •]. This finding supports the idea that unlike COPII vesicles, COPI vesicles can act as long-range carriers.
Some abundant secretory proteins such as albumin and insulin are not glycosylated, so cells could conserve resources by enabling these proteins to move quickly through the carbohydrate synthesis stage. The Golgi stacks of actively secreting mammalian cells contain narrow tubular connections between heterologous cisternae [64-66], and such tubules could permit the rapid anterograde transport of small soluble cargoes [67, 68]. Intriguingly, the COPI machinery can cooperate with lipid modifying enzymes to generate either vesicles or tubules [69]. Heterologous tubular connections between cisternae have been convincingly demonstrated only in mammalian cells, so this mechanism might be a specialization rather than a conserved property of the Golgi.
In a Golgi stack, the number of cisternae at the carbohydrate synthesis stage varies from one or two to a dozen or more, depending on the cell type [27 ••]. The mechanisms that regulate the number of cisternae per stack are obscure. However, a study of S. cerevisiae revealed that altering the kinetics of cisternal maturation influenced the copy number of Golgi cisternae, suggesting that an analogous process operating in a stacked Golgi could modulate the number of cisternae per stack [70 •].
What holds the cisternae together in a stacked Golgi? The most prominent candidates have been peripheral membrane proteins of the GRASP family [71]. GRASP proteins promote homotypic interactions between apposing membranes [72-74]. However, plant cells have Golgi stacks but lack GRASP proteins, and deletion of the P. pastoris GRASP homolog Grh1 did not perturb Golgi stacking [31]. The results with mammalian cells have been conflicting, with some researchers finding that GRASP inactivation disrupted Golgi stacking while others saw effects only on the lateral linking of Golgi stacks [71, 75]. It seems likely that cisternal stacking depends on weak adhesive interactions that involve a variety of proteins in different cell types. For example, in mammalian cells, GRASPs seem to cooperate with coiled-coil “golgin” proteins to mediate cisternal stacking [76]. Golgi stacking has been repeatedly lost during eukaryotic evolution [77], implying that this structural feature is dispensable for the basic operation of the Golgi.
From the Golgi: the carrier formation stage
At the final stage, Golgi cisternae produce transport carriers that deliver secretory cargo proteins to plasma membrane domains, regulated secretory granules, and endosomes or lysosomes/vacuoles. These sorting events occur in cisternae that are traditionally designated the TGN [19, 78]. The definition of the TGN is sometimes expanded to include earlier cisternae [79], but we will treat the TGN as being synonymous with the carrier formation stage. TGN cisternae differ from younger cisternae in that they produce clathrin-coated vesicles but do not produce or receive COPI vesicles [18, 24]. Moreover, only TGN cisternae receive membrane traffic from endosomes [80]. In mammalian cells and P. pastoris, TGN cisternae peel off from the Golgi stack, and in plant cells, the TGN is often fully dissociated from the Golgi stack [22, 81-83], implying that stacking interactions are reduced or eliminated during the carrier formation stage.
The transition from the carbohydrate synthesis stage to the carrier formation stage seems to be abrupt. Electron tomography of the mammalian Golgi revealed a sharp morphological divide in which the trans-most cisterna produced only clathrin-coated vesicles while earlier cisternae produced only COPI vesicles [18]. Moreover, video microscopy of the S. cerevisiae Golgi showed a rapid conversion in which cisternae lost Vrg4, which participates in carbohydrate synthesis, while acquiring Sec7, which participates in clathrin-coated vesicle formation [5]. These results support the idea that Golgi maturation involves “quantized” transitions between stages.
TGN cisternae segregate both resident proteins and secretory cargoes into membrane domains, which can generate multiple types of transport carriers [78, 84]. These processes rely on the localized metabolism of specific lipids [85-87], and on the regulated flipping of phospholipids between the two leaflets of the membrane [88]. A lipid crucial for the carrier formation stage is phosphatidylinositol-4 phosphate (PI4P), which binds multiple effectors and helps to coordinate exocytic vesicle formation [85, 86, 89, 90]. TGN domain formation also involves luminal Ca2+ gradients generated by interactions of calcium pumps with the actin cytoskeleton [91 •, 92 ••]. Thus, a variety of processes contribute to the functional segregation of the TGN.
A puzzle concerns how proteins are localized to the TGN. As a cisterna matures from the carbohydrate synthesis stage to the carrier formation stage, it receives peripheral and transmembrane TGN proteins from maturing TGN cisternae [5, 93 ••]. Moreover, mutant forms of some plasma membrane proteins accumulate in the TGN [94, 95 •], consistent with the existence of active recycling from older to younger TGN cisternae. Such a recycling pathway could explain why secretory proteins exit the Golgi with exponential rather than linear kinetics [10]. Components that might play a role in recycling from older to younger TGN cisternae include clathrin-coated vesicles containing the AP-1 adapter [96, 97] and a subcomplex of the COG tether [98]. Much remains to be learned about membrane dynamics at the carrier formation stage.
Role of GTPases in Golgi compartmentation and identity
Golgi-associated GTPases of the Rab and Arf/Arl families are likely to coordinate multiple events: the recruitment of resident Golgi proteins at each stage of maturation, the transitions between stages, and the partitioning of Golgi cisternae into membrane domains. This topic was recently reviewed in depth [99], so we will highlight only a few key points.
Rab GTPases, many of which have the prefix “Ypt” in yeast, act throughout the secretory pathway. They regulate the tethering of vesicles to their target compartments and the recruitment of motor proteins to secretory vesicles. As a consequence of these and other activities, Rab GTPases help to define the identities of Golgi cisternae [100-102]. It has been proposed that Rab proteins also define membrane domains within individual cisternae [12]. An appealing hypothesis is that Rab proteins operate in cascades, with a given Rab recruiting the guanine nucleotide exchange factor (GEF) for the next Rab, which in turn recruits a GTPase activating protein (GAP) that inactivates the first Rab [99, 101, 102]. Such a Rab cascade could drive the conversion from one Golgi stage to the next. Evidence was presented for a cascade at a late stage of the yeast Golgi, with the late-acting Ypt32 recruiting a GAP for the early-acting Ypt1 [103]. Ypt32 also recruits a GAP for Ypt6, which acts at intermediate and late stages of the yeast Golgi [104 ••]. In mammalian cells, the Rab33B GTPase in the medial Golgi recruits a GEF for the trans-Golgi/TGN protein Rab6, which is the homolog of yeast Ypt6 [105 •]. Thus, Rab proteins seem to be central players in choreographing Golgi membrane dynamics.
The Arf/Arl family of GTPases is equally important for Golgi function. Class I and II Arf proteins recruit the COPI coat at ERGIC and Golgi compartments, with the aid of the GEF Gea1/2 in yeast or GBF1 in mammalian cells [106]. Arf proteins also recruit clathrin adaptors at the TGN with the aid of the GEF Sec7 in yeast or BIG1/2 in mammalian cells [93 ••, 106]. In addition, Arf-like Arl GTPases cooperate with Rab GTPases to recruit vesicle tethers at the TGN [107-109]. The Arf GEFs show interesting behavior in two respects. First, Gea2 and GBF1 are apparently present on all Golgi compartments, including the TGN [110 •, 111 •], presumably because they remain associated with cisternae throughout the maturation process. Second, Arf and Arl help to recruit the TGN-localized Arf GEFs [112 ••, 113 ••]. The implication is that like Rab GTPases, Arf and Arl GTPases operate in cascades during Golgi maturation [114].
Conclusions
A three-stage model for Golgi maturation and function is useful for guiding ongoing research. These stages are defined by distinct membrane trafficking pathways. At each stage, a set of GTPases recruits specific components while regulating the transition to the next stage. Challenges for the future include identifying the components that establish each stage, and determining how membrane and proteins are recycled within and between the three stages.
Acknowledgements
Thanks to the Glick lab for helpful feedback. This work was supported by grant R01 GM104010 from the U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Glick BS, Nakano A. Membrane traffic within the Golgi stack. Annu. Rev. Cell Dev. Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Klute MJ, Melançon P, Dacks JB. Evolution and diversity of the Golgi. Cold Spring Harb. Perspect. Biol. 2011;3:a007849. doi: 10.1101/cshperspect.a007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb. Perspect. Biol. 2011;3:a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat. Rev. Mol. Cell Biol. 2005;6:812–817. doi: 10.1038/nrm1735. [DOI] [PubMed] [Google Scholar]
- [5].Losev E, Reinke CA, Jellen J, et al. Golgi maturation visualized in living yeast. Nature. 2006;22:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- [6].Matsuura-Tokita K, Takeuchi M, Ichihara A, et al. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;22:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- [7].Becker B, Bölinger B, Melkonian M. Anterograde transport of algal scales through the Golgi complex is not mediated by vesicles. Trends Cell Biol. 1995;5:305–307. doi: 10.1016/s0962-8924(00)89047-9. [DOI] [PubMed] [Google Scholar]
- [8].Bonfanti L, Mironov AA, Jr., Martínez-Menárguez J, et al. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- [9 •].Dmitrieff S, Rao M, Sens P. Quantitative analysis of intra-Golgi transport shows intercisternal exchange for all cargo. Proc. Natl. Acad. Sci. USA. 2013;110:15692–15697. doi: 10.1073/pnas.1303358110. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors formulated a mathematical model for intra-Golgi transport, and used this framework to evaluate published experimental data. They concluded that cisternal progression is insufficient and that secretory cargoes must exchange between cisternae
- [10].Patterson GH, Hirschberg K, Polishchuk RS, et al. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mironov AA, Sesorova IV, Beznoussenko GV. Golgi’s way: a long path toward the new paradigm of the intra-Golgi transport. Histochem. Cell Biol. 2013;140:383–393. doi: 10.1007/s00418-013-1141-6. [DOI] [PubMed] [Google Scholar]
- [12].Pfeffer SR. How the Golgi works: a cisternal progenitor model. Proc. Natl. Acad. Sci. USA. 2010;107:19614–19618. doi: 10.1073/pnas.1011016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13 ••].Lavieu G, Zheng H, Rothman JE. Stapled Golgi cisternae remain in place as cargo passes through the stack. eLife. 2013;2:e00558. doi: 10.7554/eLife.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14 ••].Rizzo R, Parashuraman S, Mirabelli P, et al. The dynamics of engineered resident proteins in the mammalian Golgi complex relies on cisternal maturation. J. Cell Biol. 2013;201:1027–1036. doi: 10.1083/jcb.201211147. [DOI] [PMC free article] [PubMed] [Google Scholar]; Both of these studies took advantage of dimerizing FKBP to aggregate membrane proteins within mammalian Golgi cisternae. The cisternal maturation model predicts that such aggregates should move readily through the stack. Lavieu et al. found that the aggregates were immobile, whereas Rizzo et al. found that the aggregates moved forward
- [15].Farquhar MG. Progress in unraveling pathways of Golgi traffic. Annu. Rev. Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- [16].Papanikou E, Glick BS. The yeast Golgi apparatus: insights and mysteries. FEBS Lett. 2009;583:3746–3751. doi: 10.1016/j.febslet.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- [18].Ladinsky MS, Mastronarde DN, McIntosh JR, et al. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mellman I, Simons K. The Golgi complex: in vitro veritas? Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rothman JE. The Golgi apparatus: two organelles in tandem. Science. 1981;213:1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- [21].Dunphy WG, Rothman JE. Compartmental organization of the Golgi stack. Cell. 1985;42:13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- [22].Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brigance WT, Barlowe C, Graham TR. Organization of the yeast Golgi complex into at least four functionally distinct compartments. Mol. Biol. Cell. 2000;11:171–182. doi: 10.1091/mbc.11.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Day KJ, Staehelin LA, Glick BS. A three-stage model of Golgi structure and function. Histochem. Cell Biol. 2013;140:239–249. doi: 10.1007/s00418-013-1128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rabouille C, Hui N, Hunte F, et al. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- [26].Barlowe CK, Miller EA. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics. 2013;193:383–410. doi: 10.1534/genetics.112.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27 ••].Donohoe BS, Kang BH, Gerl MJ, et al. cis-Golgi cisternal assembly and biosynthetic activation occur sequentially in plants and algae. Traffic. 2013;14:551–567. doi: 10.1111/tra.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]; Electron tomography of algal and plant Golgi stacks revealed likely intermediates in cisternal formation. Evidence was presented that carbohydrate processing is initated in medial rather than cis cisternae
- [28].Spang A. Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013;5:a013391. doi: 10.1101/cshperspect.a013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29 •].Lerich A, Hillmer S, Langhans M, et al. ER import sites and their relationship to ER exit sites: a new model for bidirectional ER-Golgi transport in higher plants. Front. Plant Sci. 2012;3:143. doi: 10.3389/fpls.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]; To identify ER import sites, the authors tagged ER-localized SNAREs and tethers. The resulting localization data suggested that recycling COPI vesicles are targeted to the ERGolgi interface
- [30].Mardones GA, Snyder CM, Howell KE. cis-Golgi matrix proteins move directly to endoplasmic reticulum exit sites by association with tubules. Mol. Biol. Cell. 2006;17:525–538. doi: 10.1091/mbc.E05-05-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Levi SK, Bhattacharyya D, Strack RL, et al. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 2010;11:1168–1179. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Okamoto M, Kurokawa K, Matsuura-Tokita K, et al. High-curvature domains of the ER are important for the organization of ER exit sites in Saccharomyces cerevisiae. J. Cell Sci. 2012;125:3412–3420. doi: 10.1242/jcs.100065. [DOI] [PubMed] [Google Scholar]
- [33].Connerly PL, Esaki M, Montegna EA, et al. Sec16 is a determinant of transitional ER organization. Curr. Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- [34].Hughes H, Budnik A, Schmidt K, et al. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J. Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ivan V, de Voer G, Xanthakis D, et al. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol. Biol. Cell. 2008;19:4352–4365. doi: 10.1091/mbc.E08-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Whittle JRR, Schwartz TU. Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat. J. Cell Biol. 2010;190:347–361. doi: 10.1083/jcb.201003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yorimitsu T, Sato K. Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites. Mol. Biol. Cell. 2012:2930–2942. doi: 10.1091/mbc.E12-05-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38 •].Bharucha N, Liu Y, Papanikou E, et al. Sec16 influences transitional ER sites by regulating rather than organizing COPII. Mol. Biol. Cell. 2013;24:3406–3419. doi: 10.1091/mbc.E13-04-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sec16 has been proposed to act as a scaffold that recruits COPII to tER sites. A detailed analysis of P. pastoris Sec16 indicated that in fact, COPII recruits Sec16 to tER sites as a regulator of COPII turnover
- [39].Kung LF, Pagant S, Futai E, et al. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 2011;31:1014–1027. doi: 10.1038/emboj.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Takagi J, Renna L, Takahashi H, et al. MAIGO5 functions in protein export from Golgi-associated endoplasmic reticulum exit sites in Arabidopsis. Plant Cell. 2013;25:4658–4675. doi: 10.1105/tpc.113.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Glick BS. Integrated self-organization of transitional ER and early Golgi compartments. BioEssays. 2014;36:129–133. doi: 10.1002/bies.201300131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42 •].Bhandari D, Zhang J, Menon S, et al. Sit4p/PP6 regulates ER-to-Golgi traffic by controlling the dephosphorylation of COPII coat subunits. Mol. Biol. Cell. 2013;24:2727–2738. doi: 10.1091/mbc.E13-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Phosphorylation of the COPII coat subunit Sec23 releases COPII into the cytosol, and dephosphorylation is needed for another round of COPII assembly. This study identified yeast Sit4p and its mammalian orthologue PP6 as the relevant phosphatase
- [43].Ho HH, He CY, de Graffenried CL, et al. Ordered assembly of the duplicating Golgi in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA. 2006;103:7676–7681. doi: 10.1073/pnas.0602595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tängemo C, Ronchi P, Colombelli J, et al. A novel laser nanosurgery approach supports de novo Golgi biogenesis in mammalian cells. J. Cell Sci. 2011;124:978–987. doi: 10.1242/jcs.079640. [DOI] [PubMed] [Google Scholar]
- [45].Bruns C, McCaffery JM, Curwin AJ, et al. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 2011;195:979–992. doi: 10.1083/jcb.201106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46 ••].Graef M, Friedman JR, Graham C, et al. ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell. 2013;24:2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47 •].Suzuki K, Akioka M, Kondo-Kakuta C, et al. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J. Cell Sci. 2013;126:2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- [48 ••].Tan D, Cai Y, Wang J, et al. The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc. Natl. Acad. Sci. USA. 2013;110:19432–19437. doi: 10.1073/pnas.1316356110. [DOI] [PMC free article] [PubMed] [Google Scholar]; A series of papers demonstrated that the pre-autophagosomal structure (phagophore) assembles in proximity to tER sites, and that COPII vesicles provide membrane for this structure
- [49].Wang J, Tan D, Cai Y, et al. A requirement for ER-derived COPII vesicles in phagophore initiation. Autophagy. 2014;10 doi: 10.4161/auto.28103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50 •].Lipatova Z, Belogortseva N, Zhang XQ, et al. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc. Natl. Acad. Sci. USA. 2012;2012:6981–6986. doi: 10.1073/pnas.1121299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51 ••].Lipatova Z, Shah AH, Kim JJ, et al. Regulation of ER-phagy by a Ypt/Rab GTPase module. Mol. Biol. Cell. 2013;24:3133–3144. doi: 10.1091/mbc.E13-05-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52 •].Wang J, Menon S, Yamasaki A, et al. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc. Natl. Acad. Sci. USA. 2013;110:9800–9805. doi: 10.1073/pnas.1302337110. [DOI] [PMC free article] [PubMed] [Google Scholar]; The yeast Rab GTPase Ypt1 is known to function in ER-to-Golgi transport, and these studies also implicate Ypt1 in autophagy-related processes, some of which occur at the ER. Thus, a Rab protein can regulate multiple pathways originating at the same compartment
- [53].Glick BS, Elston T, Oster G. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 1997;414:177–181. doi: 10.1016/s0014-5793(97)00984-8. [DOI] [PubMed] [Google Scholar]
- [54].Lanoix J, Ouwendijk J, Stark A, et al. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol. 2001;155:1199–1212. doi: 10.1083/jcb.200108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- [56].Moelleken J, Malsam J, Betts MJ, et al. Differential localization of coatomer complex isoforms within the Golgi apparatus. Proc. Natl. Acad. Sci. USA. 2007;104:4425–4430. doi: 10.1073/pnas.0611360104. [DOI] [PubMed] [Google Scholar]
- [57].Orci L, Stamnes M, Ravazzola M, et al. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- [58 •].Hamlin JN, Schroeder LK, Fotouhi M, et al. Scyl1 scaffolds class II Arfs to selective subcomplexes of coatomer via the γ-COP appendage domain. J. Cell Sci. 2014 doi: 10.1242/jcs.136481. in press. [DOI] [PubMed] [Google Scholar]; Scy1-like 1 (Scyl1), a catalytically inactive protein kinase, was shown to link an Arf GTPase to the γ2 COPI isoform, thereby promoting the specific assembly of retrograde COPI vesicles at the ERGIC and cis-Golgi
- [59].Orci L, Ravazzola M, Volchuk A, et al. Anterograde flow of cargo across the Golgi stack potentially mediated via bidirectional “percolating” COPI vesicles. Proc. Natl. Acad. Sci. USA. 2000;97:10400–10405. doi: 10.1073/pnas.190292497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sharpe HJ, Stevens TJ, Munro S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell. 2010;142:158–169. doi: 10.1016/j.cell.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pelham HR, Rothman JE. The debate about transport in the Golgi--two sides of the same coin? Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- [62].Popoff V, Adolf F, Brügger B, Wieland F. COPI budding within the Golgi stack. Cold Spring Harb. Perspect. Biol. 2011;3:a005231. doi: 10.1101/cshperspect.a005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63 •].Pellett PA, Dietrich F, Bewersdorf J, et al. Inter-Golgi transport mediated by COPI vesicles carrying small cargoes. eLife. 2013;2:e01296. doi: 10.7554/eLife.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analysis of fused mammalian cells indicated that small secretory cargoes and resident Golgi enzymes can transfer between Golgi stacks, probably in COPI vesicles, but that large secretory cargoes stay within a given Golgi stack
- [64].Marsh BJ, Volkmann N, McIntosh JR, Howell KE. Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet beta cells. Proc. Natl. Acad. Sci. USA. 2004;101:5565–5570. doi: 10.1073/pnas.0401242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].San Pietro E, Capestrano M, Polishchuk EV, et al. Group IV phospholipase A2α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol. 2009;7:e1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Trucco A, Polishchuk RS, Martella O, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat. Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- [67].Bechler ME, de Figueiredo P, Brown WJ. A PLA1-2 punch regulates the Golgi complex. Trends Cell Biol. 2012;22:116–124. doi: 10.1016/j.tcb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Martínez-Alonso E, Tomás M, Martínez-Menárguez JA. Golgi tubules: their structure, formation and role in intra-Golgi transport. Histochem. Cell Biol. 2013;140:327–339. doi: 10.1007/s00418-013-1114-9. [DOI] [PubMed] [Google Scholar]
- [69].Yang JS, Valente C, Polishchuk RS, et al. COPI acts in both vesicular and tubular transport. Nat. Cell Biol. 2011;13:996–1003. doi: 10.1038/ncb2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70 •].Bhave M, Papanikou E, Iyer P, et al. Golgi enlargement in Arf-depleted yeast cells is due to altered dynamics of cisternal maturation. J. Cell Sci. 2014;127:250–257. doi: 10.1242/jcs.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yeast cells deleted for the ARF1 gene have reduced levels of Golgi-associated Arf, and exhibit a pronounced enlargment of late Golgi cisternae. This effect was shown to result from a selective slowing of cisternal maturation for early cisternae
- [71].Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J. Cell Biol. 2010;188:237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Feng Y, Yu W, Li X, et al. Structural insight into Golgi membrane stacking by GRASP65 and GRASP55 proteins. J. Biol. Chem. 2013;288:28418–28427. doi: 10.1074/jbc.M113.478024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Heinrich F, Nanda H, Zan GH, et al. Myristoylation restricts orientation of the GRASP domain on membranes and promotes membrane tethering. J. Biol. Chem. 2014 doi: 10.1074/jbc.M113.543561. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang Y, Satoh A, Warren G. Mapping the functional domains of the Golgi stacking factor GRASP65. J. Biol. Chem. 2005;280:4921–4928. doi: 10.1074/jbc.M412407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jarvela T, Linstedt AD. Isoform-specific tethering links the Golgi ribbon to maintain compartmentalization. Mol. Biol. Cell. 2014;25:133–144. doi: 10.1091/mbc.E13-07-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lee I, Tiwari N, Dunlop MH, et al. Membrane adhesion dictates Golgi stacking and cisternal morphology. Proc. Natl. Acad. Sci. USA. 2014;111:1849–1854. doi: 10.1073/pnas.1323895111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mowbrey K, Dacks JB. Evolution and diversity of the Golgi body. FEBS Lett. 2009;583:3738–3745. doi: 10.1016/j.febslet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- [78].De Matteis MA, Luini A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- [79].Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE. Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic. 2004;5:338–345. doi: 10.1111/j.1398-9219.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- [80].Pfeffer SR. Entry at the trans-face of the Golgi. Cold Spring Harb. Perspect. Biol. 2011;3:a005272. doi: 10.1101/cshperspect.a005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mogelsvang S, Gomez-Ospina N, Soderholm J, et al. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol. Biol. Cell. 2003;14:2277–2291. doi: 10.1091/mbc.E02-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rambourg A, Clermont Y. Three-dimensional structure of the Golgi apparatus in mammalian cells. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser Verlag; Basel: 1997. pp. 37–61. [Google Scholar]
- [83].Uemura T, Suda Y, Ueda T, Nakano A. Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol. 2014 doi: 10.1093/pcp/pcu010. in press. [DOI] [PubMed] [Google Scholar]
- [84].Wakana Y, van Galen J, Meissner F, et al. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. EMBO J. 2012;31:3976–3990. doi: 10.1038/emboj.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 2011;21:113–121. doi: 10.1016/j.tcb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Johansen J, Ramanathan V, Beh CT. Vesicle trafficking from a lipid perspective: lipid regulation of exocytosis in Saccharomyces cerevisiae. Cell Logist. 2012;2:151–160. doi: 10.4161/cl.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Malhotra V, Campelo F. PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb. Perspect. Biol. 2011;3:a005280. doi: 10.1101/cshperspect.a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim. Biophys. Acta. 2012;1821:1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].De Matteis MA, Wilson C, D’Angelo G. Phosphatidylinositol-4-phosphate: the Golgi and beyond. BioEssays. 2013;35:612–622. doi: 10.1002/bies.201200180. [DOI] [PubMed] [Google Scholar]
- [90].Mesmin B, Bigay J, Moser von Filseck J, et al. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- [91 •].Curwin AJ, von Blume J, Malhotra V. Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast. Mol. Biol. Cell. 2012;23:2327–2338. doi: 10.1091/mbc.E11-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent work has implicated the actin cytoskeleton and luminal Ca2+ in cargo sorting at the TGN. This study showed that in yeast, the actin-severing protein cofilin and the Ca2+ pump Pmr1 are needed for normal TGN function
- [92 ••].von Blume J, Alleaume AM, Kienzle C, et al. Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 2012;199:1057–1066. doi: 10.1083/jcb.201207180. [DOI] [PMC free article] [PubMed] [Google Scholar]; In mammalian cells, Cab45 is a soluble Ca2+-binding protein in the lumen of the TGN. The authors demonstrated that Cab45 binds and sorts secretory cargo proteins in a Ca2+- dependent manner
- [93 ••].Daboussi L, Costaguta G, Payne GS. Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat. Cell Biol. 2012;14:239–248. doi: 10.1038/ncb2427. [DOI] [PMC free article] [PubMed] [Google Scholar]; Live-cell imaging revealed that the composition of the yeast TGN evolves over time, with sequential recruitment of the GGA and AP-1 clathrin adaptors. Phosphatidylinositol 4-phosphate was implicated in this transition
- [94].Gut A, Kappeler F, Hyka N, et al. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95 •].Parmar HB, Barry C, Kai F, Duncan R. Golgi complex-plasma membrane trafficking directed by an autonomous, tri-basic Golgi export signal. Mol. Biol. Cell. 2014 doi: 10.1091/mbc.E13-07-0364. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analysis of the reovirus transmembrane protein p14 indicated that efficient export from the TGN requires a polybasic motif in the cytosolic tail. This result supports the existence of signal-mediated transport from the TGN to the plasma membrane
- [96].Myers MD, Payne GS. Clathrin, adaptors and disease: insights from the yeast Saccharomyces cerevisiae. Front. Biosci. 2013;18:862–891. doi: 10.2741/4149. [DOI] [PubMed] [Google Scholar]
- [97].Valdivia RH, Baggott D, Chuang JS, Schekman R. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- [98].Willett R, Kudlyk T, Pokrovskaya I, et al. COG complexes form spatial landmarks for distinct SNARE complexes. Nat. Commun. 2013;4:1553. doi: 10.1038/ncomms2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mizuno-Yamasaki E, Rivera-Molina F. P. N. GTPase networks in membrane traffic. Annu. Rev. Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Munro S. The Golgi apparatus: defining the identity of Golgi membranes. Curr. Opin. Cell Biol. 2005;17:395–401. doi: 10.1016/j.ceb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- [101].Pfeffer S. Rab GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Segev N. Coordination of intracellular transport steps by GTPases. Semin. Cell Dev. Biol. 2011;22:33–38. doi: 10.1016/j.semcdb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc. Natl. Acad. Sci. USA. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104 ••].Suda Y, Kurokawa K, Hirata R, Nakano A. Rab GAP cascade regulates dynamics of Ypt6 in the Golgi traffic. Proc. Natl. Acad. Sci. USA. 2013;110:18976–18981. doi: 10.1073/pnas.1308627110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ypt6 apparently acts upstream of Ypt32 in a Rab cascade at the yeast Golgi. The authors showed that a Ypt32 effector is Gyp6, which is a GAP for Ypt6, and that Gyp6 is necessary for displacing Ypt6 when Ypt32 is present
- [105 •].Pusapati GV, Luchetti G, Pfeffer SR. Ric1-Rgp1 complex is a guanine nucleotide exchange factor for the late Golgi Rab6A GTPase and an effector of the medial Golgi Rab33B GTPase. J. Biol. Chem. 2012;287:42129–42137. doi: 10.1074/jbc.M112.414565. [DOI] [PMC free article] [PubMed] [Google Scholar]; In mammalian cells, the Rab33B GTPase localizes to the medial Golgi. This paper showed that Rab33B recruits the GEF for Rab6A, which acts at the trans-Golgi. The results are consistent with a medial-to-trans Rab cascade
- [106].Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8:1476–1485. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- [107].Burd CG, Strochlic TI, Gangi Setty SR. Arf-like GTPases: not so Arf-like after all. Trends Cell Biol. 2004;14:687–694. doi: 10.1016/j.tcb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- [108].Burguete AS, Fenn TD, Brunger AT, Pfeffer SR. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell. 2008;132:286–298. doi: 10.1016/j.cell.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Houghton FJ, Chew PL, Lodeho S, et al. The localization of the Golgin GCC185 is independent of Rab6A/A’ and Arl1. Cell. 2009;138:787–794. doi: 10.1016/j.cell.2009.05.048. [DOI] [PubMed] [Google Scholar]
- [110 •].Lowery J, Szul T, Styers M, et al. The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN) J. Biol. Chem. 2013;288:11532–11545. doi: 10.1074/jbc.M112.438481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111 •].Tsai PC, Hsu JW, Liu YW, et al. Arl1p regulates spatial membrane organization at the trans-Golgi network through interaction with Arf-GEF Gea2p and flippase Drs2p. Proc. Natl. Acad. Sci. USA. 2013;110:E668–677. doi: 10.1073/pnas.1221484110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mammalian GBF1 and yeast Gea2 are Arf GEFs that act early in the Golgi to recruit COPI. Evidence was presented that GBF1 and Gea2 are also present at the TGN, suggesting that they persist on Golgi cisternae throughout the maturation process
- [112 ••].Christis C, Munro S. The small G protein Arl1 directs the trans-Golgi-specific targeting of the Arf1 exchange factors BIG1 and BIG2. J. Cell Biol. 2012;196:327–335. doi: 10.1083/jcb.201107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113 ••].Richardson BC, McDonold CM, Fromme JC. The Sec7 Arf-GEF is recruited to the trans-Golgi network by positive feedback. Dev. Cell. 2012;22:799–810. doi: 10.1016/j.devcel.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yeast Sec7 is the GEF for Arf at the TGN, and the animal homologs are BIG1 and BIG2. Recruitment of BIG1 and BIG2 was shown to depend on Arl. Recruitment of Sec7 was shown to depend on Arf, pointing to a positive feedback loop for Arf activation
- [114].Stalder D, Antonny B. Arf GTPase regulation through cascade mechanisms and positive feedback loops. FEBS Lett. 2013;587:2028–2035. doi: 10.1016/j.febslet.2013.05.015. [DOI] [PubMed] [Google Scholar]