Abstract
The Hedgehog (Hh) pathway has become an important model to study diverse aspects of cell biology of the primary cilium, and reciprocally, the study of ciliary processes provides an opportunity to solve longstanding mysteries in the mechanism of vertebrate Hh signal transduction. The cilium is emerging as an unique compartment for G-protein—coupled receptor (GPCR) signaling in many systems. Two members of the GPCR family, Smoothened and Gpr161, play important roles in the Hh pathway. We review the current understanding of how these proteins may function to regulate Hh signaling and also highlight some of the critical unanswered questions being tackled by the field. Uncovering GPCR-regulated mechanisms important in Hh signaling may provide therapeutic strategies against the Hh pathway that plays important roles in development, regeneration and cancer.
1. Introduction
The unexpected discovery that vertebrate Hedgehog (Hh) signaling is dependent on primary cilia a decade ago has had a profound impact on our understanding of this key signaling pathway in development and disease [1]. Primary cilia function as compartments for Hh signaling, with transduction of the signal driven by a set of choreographed protein trafficking events. Indeed, nearly all events in Hh signaling prior to target gene transcription have been linked to ciliary mechanisms. In the absence of signaling, Patched 1 (Ptch1), a 12-pass transmembrane protein that receives Hh ligands along with co-receptors [2, 3], is concentrated in and around primary cilia [4]. In this OFF state, Protein Kinase A (PKA) and Suppressor of Fused (SuFu) restrain the activity of the Gli family of transcription factors and promote the formation of truncated Gli repressors (GliR) [5-9]. The orphan rhodopsin family G-protein-coupled receptor (GPCR) Gpr161 localizes to cilia and promotes the PKA-mediated generation of GliR [10]. Reception of Hh ligands, such as sonic hedgehog (Shh), causes the displacement of Ptch1 and Gpr161 away from the primary cilium [2, 8]. This allows accumulation of Smoothened (Smo), a member of the frizzled (class F) family of GPCRs, to high levels in the ciliary membrane [11]. Smo concentration at cilia ultimately leads to activation of the Gli family of transcription factors. Activated Smo has to overcome two negative regulators, SuFu and PKA. Smo signaling promotes the transport of Gli-SuFu complexes to the tips of cilia, allowing Glis to dissociate from SuFu and enter the nucleus to transcribe target genes [12-15]. Ciliary mechanisms are likely critical to understanding the following unsolved mysteries in vertebrate Hh signaling: (a) how is PKA activity regulated at cilia during Gli processing, (b) how is Smo regulated by Ptch1, and (c) how does Smo transmit the signals to the Gli family of transcription factors. A challenge going forward is to understand the biochemical mechanisms that regulate each of these signaling steps at cilia and to understand how these mechanisms are integrated with the dynamic trafficking changes that have been revealed by protein localization studies. We discuss the emerging view that the cilium serves as a unique platform for GPCR signaling with an emphasis on its roles in the Hh pathway. We focus on regulatory mechanisms both upstream and downstream of these two GPCRs, Smo and Gpr161, in the context of their localization and functioning in cilia. We also discuss mechanisms that link cellular GPCR-generated signaling to the transcriptional output of the Hh pathway in different tissues and in the pathophysiology of Hh-dependent cancers.
2. Mechanisms underlying Smoothened activity in primary cilia
2.1. Regulation of Smoothened by Patched 1
An enduring mystery in Hh signaling in all animals revolves around the mechanism by which Ptch1 regulates Smo. Notably, the Ptch1-Smo interaction is the most frequently damaged step in two Hh-driven cancers, medulloblastoma (MB) and basal cell carcinoma (BCC). Current models propose that Ptch1 regulates Smo activity by modulating the concentration or localization of a (yet to be discovered) small molecule ligand. This conclusion has been derived from the following observations: Ptch1 can inhibit Smo catalytically rather than stoichiometrically [16], Ptch1 and Smo do not physically interact in conventional biochemical assays, and Ptch1 demonstrates distant homology to the bacterial Resistance, Nodulation, Division (RND) family of small-molecule pumps [17].
The activity of Smo itself is subject to regulation by a bewildering diversity of synthetic and endogenous small molecules. The plant alkaloid cyclopamine was the first described direct antagonist of Smo and subsequently became the inspiration for a class of anti-Hh cancer drugs that are now in clinical use [18-21]. A number of small molecule screens have since uncovered both direct Smo agonists and antagonists [22-26]. While the endogenous Smo ligand is unknown, sterol-related molecules have been proposed to have a role in Smo activation. In vertebrates, pharmacological or genetic depletion of cholesterol from cells blocks both ligand-induced Hh signaling and constitutive Smo signaling in Ptch1−/− cells [19, 27, 28]. Oxysterols, a class of enigmatic oxidized cholesterol derivatives, were discovered to be potent activators of Hh signaling in multiple systems [29, 30]. While one initial study suggested that oxysterols could not be Smo agonists [30], subsequent mechanistic analysis convincingly demonstrated that oxysterols were direct ligands and allosteric modulators of Smo [31]. Surprisingly, detailed pharmacological analysis showed that oxysterols likely bound to a site that was physically distinct from the cyclopamine binding site that was the focus of research and therapeutic intervention for the prior decade [31]. Finally, Vitamin D3 and derivative analogs have been implicated as Smo antagonists [32]. Interestingly, glucocorticoids, which share a tetracyclic ring skeleton with sterols, have also been identified as synthetic Smo ligands [33].
How do these molecules influence Smo activity? Smo is composed of an N-terminal, extracellular Cysteine-Rich Domain (CRD), homologous to the CRD of the Frizzled (Fz) proteins that bind to Wnt ligands [34]. A linker connects the CRD to the membrane-spanning 7-helix bundle (7TM), which in turn is followed by a cytoplasmic tail. Structures of both the isolated CRD and the isolated 7-helix bundle have been solved and provided views of two distinct ligand binding sites on Smo [35-37]. Liganded structures show that the 7TM bundle, associated extracellular loops, and the CRD linker comprise the “cyclopamine-binding site,” which engages ligands that compete with cyclopamine for binding to Smo [35, 38]. Oxysterols, on the other hand, bind to the CRD in a hydrophobic groove that corresponds to the groove used by the Fz CRD to bind to the palmitoleyl moiety of Wnt ligands [36, 37, 39, 40]. While present on physically separable domains in Smo, pharmacological studies show that the oxysterol- and cyclopamine-binding sites are allosterically linked [31]. While a Smo structure containing both the CRD and 7TM segments is not yet available, one possibility is that the CRD can influence the 7TM site by interacting with the loops that form the extracellular end of the 7-helix bundle. A Smo molecule lacking the CRD or containing mutations in the 7TM site can still be inhibited by cholesterol depletion, suggesting that the effect of cholesterol may be mediated through a completely distinct mechanism or site on Smo [40]. In this regard, cholesterol-binding sites with regulatory potential have been identified within the transmembrane segments of GPCRs, channels, and transporters (reviewed in [41]).
Despite this progress, the binding site on Smo regulated by Ptch1 remains to be identified. Mutations in the 7TM segment that abrogate the binding of several 7TM ligands have little influence on the ability of Smo to be regulated by Shh, and thus by Ptch1 [40, 42]. While some point mutations in the CRD can significantly reduce Shh responsiveness, other mutations that completely abrogate oxysterol binding have no effect [36, 39]. Moreover, a truncated Smo molecule lacking the CRD can still be repressed by over-produced Ptch1 and remains weakly responsive to Shh [39, 40]. Taken together, these data show that neither of these two sites can be entirely responsible for mediating the inhibitory effect of Ptch1 on Smo.
In addition to ligand-mediated regulation, changes in the levels of Ptch1 and Smo in the ciliary membrane seem to be critical for Smo activation. Ptch1 and Smo undergo a characteristic reciprocal change in localization at the ciliary membrane when signaling is initiated [4, 11]. Without Hh ligands, Ptch1 is localized in punctate structures along the length of the ciliary membrane and is found in vesicles around the ciliary base, while Smo is present at low levels. When cells are exposed to Hh, Ptch1 is cleared from cilia and instead Smo accumulates to high levels in the ciliary membrane [4].
There is consensus that the accumulation of Smo in the ciliary membrane is required for downstream signaling. For instance, Drosophila Smo, which is normally inactive and not localized in cilia in vertebrate cells, can activate Hh signaling when recruited to cilia by replacing its C-terminal tail with that of vertebrate Smo [39]. While cilia localization may be required for Hh signaling, it is not sufficient. First, Smo appears to be cycling through the cilium even in the absence of Hh ligands. Genetic [43, 44] or pharmacological blockade [45] of the retrograde intraflagellar transport (IFT) motor dynein 2, which mediates transport of cargoes from the tip to the base of cilia, leads to Smo accumulation in the ciliary membrane without triggering signaling. Also, certain Smo antagonists, such as cyclopamine, can themselves induce Smo accumulation in cilia without triggering downstream signaling [46]. Finally, the loss of IFT25, an intraflagellar transport protein (a subunit of the IFT complex B, see section 3.1) that does not seem to be required for ciliogenesis or for ciliary integrity, leads to the accumulation of both Smo and Ptch1 in cilia and a loss of signaling efficacy [47]. The IFT25/27 subcomplex is emerging as an accessory module of the IFT machinery that regulates the trafficking of Hh pathway proteins through cilia, perhaps by primarily regulating ciliary egress.
Is there a way in which these two views of Smo activation—regulation by small molecules and regulation by ciliary localization—can be integrated? One possibility is that Ptch1 gates Smo entry into the cilium, perhaps by controlling a preciliary trafficking step. This model is unlikely due to the above-mentioned observation that Smo seems to show trafficking through cilia even in the absence of Hh ligands. In addition, Smo over-expression leads to the constitutive accumulation of Smo in cilia, but only to a very modest level of target gene induction [46]. In other words, Ptch1 is able to exert an inhibitory influence on Smo even when its levels in the cilia membrane are artificially increased, making a purely gating function unlikely. Another possibility is that Ptch1 regulates the concentration or availability of a Smo ligand in the ciliary membrane, which could then control whether the Smo that is also cycling through this membrane adopts an active conformation and interacts with the downstream signaling machinery. When Hh ligands inactivate Ptch1 and/or trigger its removal from the cilia membrane, Smo would become active and accumulate in cilia. The concept that the ciliary membrane may serve as a unique, privileged lipidic environment could have regulatory implications for the activity of other GPCRs and signaling receptors that are localized in cilia.
The mechanisms by which Ptch1 and Smo are trafficked to cilia are not well understood. A major challenge is that defects in the structure or function of cilia, which can be caused by mutations in a myriad of cilia genes, can often lead to indirect effects on the accumulation of proteins within cilia. Other than the work on IFT25/27 noted above [47], which implicated this complex in the exit of Ptch1 from cilia, very little is known about the machinery that regulates Ptch1 trafficking. In line with its genealogy as a GPCR, Smo accumulation in cilia has been linked to a G-protein coupled receptor kinase 2 (Grk2)-β-arrestin mechanism [48, 49]. In this model, phosphorylation of the C-terminal tail of Smo by Grk2 leads to recruitment of β-arrestin and the consequent association with the anterograde IFT motor Kif3a. Phosphorylation of the Smo tail by both Grk2 and CK1α has also been linked to ciliary Smo accumulation and Smo activation through a conformational change involving its C-terminal tail [48, 50, 51]. Integrin-linked Kinase (ILK) [52] and the Bardet-Biedl Syndrome (Bbsome [53]) protein complex [54, 55], which may link the core IFT machinery to ciliary cargoes, have also been implicated in Smo ciliary trafficking. The diversity of factors implicated in Smo ciliary trafficking remains to be reconciled into a biochemically coherent mechanism.
2.2. Activation of downstream signaling by ciliary Smo
A variety of experiments have shown that Smo is capable of coupling to the Gαi family of heterotrimeric G-proteins [56, 57]. Given that Protein Kinase A (PKA) is a rate-limiting negative regulator of the Gli proteins, Smo-Gαi coupling (predicted to lead to a drop in cAMP levels and consequently PKA activity) would provide a very straightforward mechanism by which Hh signaling could trigger Gli protein activation. These changes in PKA activity could be locally confined to the ciliary compartment, insulating Hh responses from the other signaling pathways that are regulated by the cAMP-PKA system. In Drosophila, the single Gαi has been implicated directly downstream of Smo [58]. However, the requirement for Gαi in vertebrates Hh signaling is uncertain. Multiple Gαi paralogs in vertebrates makes loss of function approaches challenging. Pertussis toxin (PTX), which inactivates all Gαi paralogs except Gz, has an incomplete inhibitory effect on Hh signaling in some cultured cells [56, 59]. Expression of PTX in the limb mesenchyme progenitor cells with a Prx1 promoter, in combination with the knock out of Gz, has no effect on Hh-dependent limb patterning [60]. An important consideration is that Hh signaling is exquisitely sensitive to the basal level of PKA activity in the cell. This basal level of activity is set by the balance of inputs into Gαi and Gαs from the panel of GPCRs and their states of activity present in a specific cell type. Thus, Hh responsiveness can show significant changes when the Gαi-Gαs balance is perturbed, without this being a reflection of direct Smo-Gαi coupling. Taken together, the balance of data suggests that Smo-Gαi coupling is likely not universally required for canonical Hh signaling in vertebrates, though it may play a tissue-specific or context-specific role.
In Drosophila, Smo has been shown to assemble a signaling complex containing the kinesin-like protein Cos2 at its C-terminal, cytoplasmic tail [61, 62]. Though the C-terminal tails of Drosophila and human Smo have diverged significantly, Kif7 has emerged as the vertebrate homolog of Drosophila Cos2 [63-66]. Kif7, which forms a complex with Gli2 and Gli3, is important for the formation of both repressor and activator forms of the Gli proteins, at least in specific tissues. Interestingly, Kif7 shows dynamic trafficking changes at cilia in response to Hh signaling and may scaffold a signaling complex at cilia, in a manner similar to Cos2 in flies. However, the biochemical mechanism by which Kif7 mediates the communication between Smo and Gli proteins remains to be described.
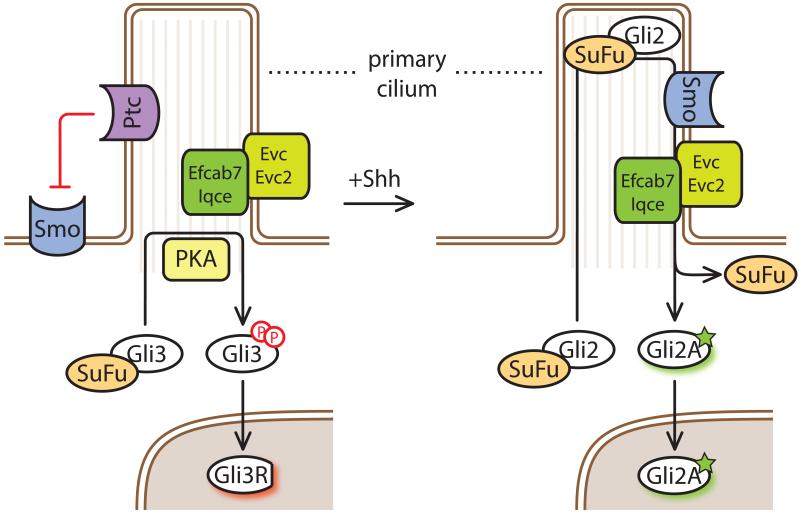
Another Smo-binding complex with a possible scaffolding function at cilia is the Evc-Evc2 complex, which plays a tissue-specific role in Hh signaling [67-69]. Evc and Evc2 are type I transmembrane proteins with extensively coiled-coil cytoplasmic domains. The EVC and EVC2 genes are mutated in two ciliopathies, Ellis van Creveld syndrome and Weyers Acrodental Dysostosis, characterized by impaired Hh signaling in skeletal, cardiac and orofacial tissues (reviewed in [70]). Smo binds to the Evc-Evc2 complex in response to Hh signal activation in a compartment near the base of cilium termed the “EvC Zone.” This region may represent a microdomain where Hh signaling complexes are assembled at primary cilia (Figure 1). A disordered segment located at the C-terminus of Evc2 tethers Evc-Evc2 in the EvC Zone through a complex composed of the proteins Iqce and Efcab7 [71]. Remarkably, patients with Weyer’s syndrome carry a deletion of this localization sequence in one allele of Evc2, leading to a dominant block in Hh signaling and dispersal of the Evc-Evc2 complex throughout the ciliary membrane [72]. This human disease allele highlights the importance of precise protein localization in Hh signaling at the primary cilia. As with Kif7, the biochemical mechanism by which the Smo-Evc2 interaction regulates downstream components such as PKA, SuFu and the Glis remains to be elucidated.
Figure 1. Hh signaling at primary cilia.
A model for Hh signaling in the absence (left) or presence (right) of Shh. In the absence of Hh ligands, PKA phosphorylates the Gli proteins (red circles) and initiates their processing to repressor forms (GliR) in a cilia-dependent manner. Gli3R suppresses target genes upon nuclear translocation. Upon binding of Shh with Ptch1, Ptch1 is removed from cilia, and Smo activation results in its accumulation in cilia and the consequent decrease in PKA activity towards the Gli proteins. The recently described Efcab7-Iqce module anchors the Evc-Evc2 complex in a signaling microdomain at the base of cilia, transducing downstream signals for Smo-dependent Gli2 activation [71, 72]. Recent results with Efcab1/Iqce knockouts not inhibiting Gli3 processing but preventing Shh pathway activation [72], and the Shh-dependent removal of Gpr161 from cilia (Figure 2) [10] strongly suggest that the inhibition of Gli3 processing is uncoupled from Gli2 activation downstream of Smo. Gli proteins traffic through the cilia in a Gli-Sufu complex, and activation by Shh results in dissociation of Gli from SuFu, allowing them to enter the nucleus and activated target genes.
3. Role of Gpr161 as a negative regulator of Shh signaling
3.1. Ventral neural tube patterning by Shh and the role of cilia
Shh morphogen signals from notochord and floor plate provide spatiotemporal information organizing gene expression and cellular differentiation in the ventral region of the vertebrate neural tube [73, 74]. The graded Shh signal patterns the ventral neuroepithelium into five progenitor domains generating distinct neuronal subtypes [75]. The key factors that determine the final output of Shh signaling are concentration and duration of the morphogen activity. Progressively higher concentrations of Shh induce genes encoding the transcription factors NK6 homeobox 1 (Nkx6.1), oligodendrocyte transcription factor 2 (Olig2) and NK2 homeobox 2 (Nkx2.2), whereas increasing duration of high Shh levels results in increased Nkx2.2 expression over Olig2 [76, 77]. In the absence of Shh signaling, ventral neural cell fates are lost [74]. This concentration- and time-dependent response is fine-tuned by feedback expression of the negative regulator Ptch1 [76], which is a direct transcriptional output of Shh signaling, and by the ratio of the repressor to activator forms of the Gli proteins [78]. The transcriptional factor Gli1, considered a pure activator, is also a direct target of Shh signaling [79]. Of the other Gli transcriptional factors, Gli2 functions mostly as an activator, while Gli3 acts predominantly as a repressor in the neural tube. The effect of Gli2 in ventral patterning is apparent in Gli2 knock out embryos, where the most ventral floor plate cells that express Shh and FoxA2 are lost [80]. Expression of one copy of Gli1 can rescue the Gli2 mutant floor plate defects, suggesting that the primary effect of Gli2 in neural tube patterning is mediated by its function as an activator [80]. The primary effect of Gli3 as a repressor is apparent in mouse embryos lacking Shh, where the removal of Gli3 recovers the expression of some target genes [78]. Finally, recent studies show that the final interpretation of the Shh morphogen in the neural tube is an emergent property of a downstream transcriptional circuit, such that the expression domains are maintained due to hysteresis of this regulatory circuit [81]. This mechanism coupled with subsequent positional sorting of cells [82] maintains the characteristic precision of the Shh-mediated pattern in the presence of the inherent noise of developmental signaling.
The primary cilium is fundamentally important in mediating the transduction of Hh signals in the ventral neuroepithelium. Primary cilia are assembled by an active process called intraflagellar transport (IFT), consisting of trains of multipolypeptide particles that move continuously along axonemal microtubules. Anterograde transport of these particles is mediated by kinesin-II, whereas retrograde transport is powered by the dynein 2 motor (for review, see [83, 84]). IFT particles are organized into two complexes, called complex A (IFT-A) and complex B (IFT-B). The IFT-B complex is implicated in anterograde IFT [83]. In a forward genetic screen for mouse embryogenesis defects, Kathryn Anderson’s group first discovered that mutations in ciliary assembly affect ventral neural tube patterning [1]. Mouse mutants that affect the intraflagellar transport (IFT) machinery, including the IFT-B complex and IFT motors, exhibit loss of the ventral cell types specified by high levels of Shh [1, 85]. However, in contrast to most other cilia mutants, mutations in the IFT-A complex paradoxically result in increased basal Shh signaling [44, 86-88]. The IFT-A complex is organized as a core complex (Ift144, Ift140, and Ift22) with additional peripheral subunits (Ift139, Ift121, and Ift43) [89, 90] (Figure 2). The IFT-A complex is implicated in retrograde IFT, and its disruption causes axonemal bulges, similar to the retrograde IFT motor dynein 2 mutants. However, in contrast to the dynein 2 mutants, null mutations in two IFT-A genes (Thm1/Ift139alien and Ift122sopb), and a hypomorphic allele of the IFT-A subunit Ift144 (Ift144twt), exhibit dorsal expansion of the floor plate, V3 progenitor, and motor neuron domains in the caudal neural tube [44, 86-88]. Even in IFT-A alleles with severe disruption of the complex (e.g. a null allele of Ift144, Ift144dmhd, or a combined disruption of two IFT-A subunits), which result in stunted ciliary morphology, the motor neurons that require intermediate levels of Shh signaling are expanded in the caudal neural tube [87]. The opposing neural tube phenotypes of IFT-A mutants from the other mutants of the IFT machinery suggest that the IFT-A complex may have additional “preciliary” functions.
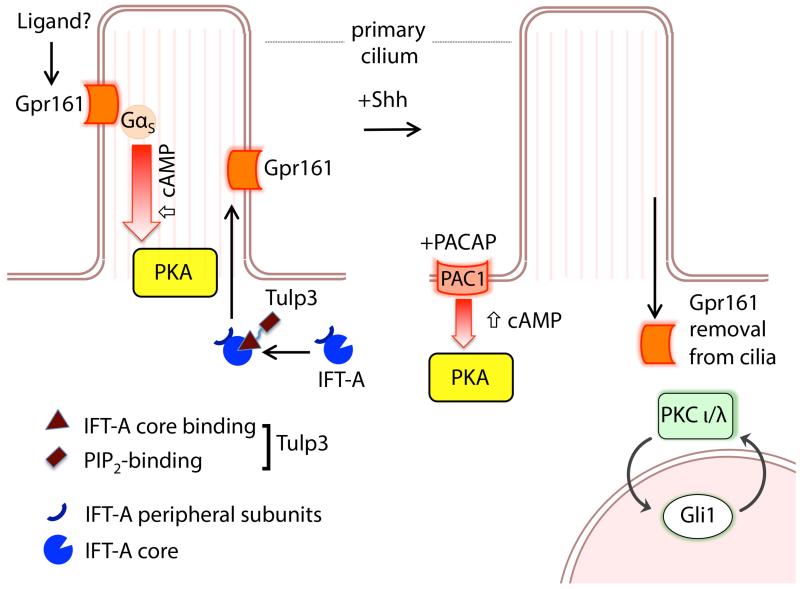
Figure 2. Gpr161 functions as a negative regulator of the Hh pathway in the neural tube.
Left, Gpr161 localizes to primary cilia in a Tulp3/IFT-A-dependent manner [10]. Constitutive activity of Gpr161 leads to increase in cAMP levels in a Gαs-coupled manner. Gαs also functions as a negative regulator of Hh signaling in the neural tube [60]. Currently, ligands that could modulate Gpr161 activity are unknown. Gpr161 activity in cilia could increase ciliary cAMP pools, resulting in PKA activity in close proximity in the basal body [120], promoting Gli3 processing. Right, Shh activity removes Gpr161 from cilia [10], possibly preventing ciliary activation of PKA. Other factors that modulate Shh-mediated activity include activity of PACAP receptor, PAC1 that can affect global PKA activity in the cerebellar granular progenitor neurons [135], and atypical protein kinase C τ/λ (aPKC-τ/λ) that functions in a positive feedback circuit to phosphorylate and activate Gli1 in the context of basal cell carcinoma [139].
3.2. Discovery of Gpr161
Mutations in the tubby family protein Tulp3 cause increased Shh signaling in the caudal neural tube phenocopying IFT-A mutants [91, 92]. Using a tandem affinity purification and mass-spectrometry-based approach, Tulp3 was found to bind to the IFT-A subcomplex [89]. In addition to its known role in retrograde transport, the core IFT-A subcomplex recruits Tulp3 to the cilia through this direct interaction [88, 89]. Furthermore, Tulp3 promotes trafficking of specific rhodopsin family (class A) GPCRs to cilia, and this process requires both the IFT-A- and phosphoinositide-binding domains of Tulp3 [89] (Figure 2). These results combined with the fact that trafficking of Smo (class F GPCR) is not affected by Tulp3/IFT-A [88, 89] suggested the role of a novel GPCR in the coordinated function of IFT-A/Tulp3 as negative regulators of Shh signaling [89]. Screening for GPCRs expressed early during development resulted in the discovery of Gpr161 as this key regulator [10]. Gpr161 is broadly expressed early during neural tube development and is localized predominantly in the nervous system after mid-gestation. Gpr161 is localized to cilia broadly in a wide variety of cultured cells, and the ciliary localization is reduced upon Tulp3 knockdown and in core IFT-A mutant (Ift122sopb) fibroblasts. Interestingly, a null mouse knock out of the receptor causes embryonic lethality by E10.5, and results in increased Shh signaling throughout the rostro-caudal extent of the neural tube. Double mutant analyses indicate that the increased Shh signaling phenotype in Gpr161 mutants is dependent on the cilia, and is independent of Smo. As Smo plays a critical role in the activation of Shh signaling (Figure 1), and the Gpr161 mutant phenotype is mediated independent of Smo activation, Gpr161 primarily functions in the basal repression mechanism in the Shh pathway (i.e. Gli3 processing into Gli3R). In addition, Shh signaling results in removal of Gpr161 from the cilia, similar to the other negative regulator of Shh signaling, Ptch1 [4]. Thus, Gpr161 functions as a negative regulator of Shh signaling in the neural tube, while itself being regulated by Shh signaling in a positive feedback circuit (Figure 2).
3.3. Role of Gpr161 in PKA-mediated Gli3 processing
How does Gpr161 act as a negative regulator of Shh signaling in the neural tube? Gpr161 knockout prevents Gli3 processing, and Gli2/3 full-length stability. Overall, these effects look similar, but less severe than PKA [93, 94] and Sufu mutants [9, 95], suggesting that Gpr161 could be regulating Gli3 processing and/or stability by either PKA or Sufu. Primary cilia are not required for Sufu to inhibit Gli activity [96, 97], whereas the neural tube phenotype in the Gpr161 mutant is cilia-dependent, suggesting that the effects of Gpr161 are not mediated primarily through the action of Sufu. Gli3 processing is also regulated by the cAMP-activated kinase PKA [6]. In the absence of a known ligand for Gpr161, assays testing for constitutive activity of the receptor using inducible clonal cell lines detected significant increase in cAMP levels in induced cells, and a concomitant reduction in the basal cAMP activity by knocking down Gαs, suggesting that Gpr161 is primarily Gαs-coupled [10]. These experiments suggest that Gpr161 is probably regulating the Shh pathway in the neural tube by activation of PKA (Figure 2).
It is currently unknown if Gpr161 activity in the neural tube is constitutive or is further modulated by ligands. In cultured cells, overexpression of Gpr161 results in constitutive basal activity of the receptor resulting in high cAMP levels of upto ~70% of maximal forskolin-induced response [10]. Agonist-independent constitutive signaling has been observed for a wide variety of GPCRs [98, 99]. A recent study analyzed constitutive GPCR activity for its role in olfactory GPCR-driven axonal projection of olfactory neurons [100]. Transgenic mice expressing mutants of the β2-adrenergic receptor (β2AR) that affect constitutive activity but not agonist-dependent activity changed the transcription levels of axon-targeting genes, causing shifts in glomerular locations along the anterior-posterior (A-P) axis, but not affecting glomerular segregation. A parallel study detected strong β2AR immunoreactivity in dendritic cilia of β2AR expressing olfactory neurons, suggesting that this effect could be mediated through constitutive activity of β2AR in olfactory cilia [101]. Similar to olfactory GPCRs, constitutive activity of Gpr161 could underlie its effect on cAMP response and PKA activation. However, ligand-dependent activation of Gpr161 could also regulate its function in the neural tube. The “DRY” motif flanking the third transmembrane helix in most rhodopsin family GPCRs, and the adjacent conserved valine in the second intracellular loop in the α1b adrenergic receptor, which is closely related to Gpr161, is critical in mediating activated conformations [102, 103]. The DRY motif is present in Gpr161 and mutation of the conserved valine (V158E) prevents its constitutive activity [10]. Physiological ligands that could increase (agonist) or decrease (inverse agonist) constitutive activity [104] of this receptor are currently unknown. However, the agonist would not need to be restricted in its localization in the dorso-ventral axis in activating Gpr161. Removal of ciliary Gpr161 by Shh would ensure that the receptor would be absent from cilia in the ventral regions, and result in activation in dorsal neural tube regions corresponding to high Gli3R activity.
3.4. Models for Gpr161 function in cilia in regulation of the Shh pathway
Although Gpr161 activity could be regulating PKA activation in the neural tube, and PKA-mediated Gli3 processing is cilia-dependent [85, 105], it is presently unclear if Gpr161 function is required in the cilia. Gpr161 is also localized to vesicles close to the ciliary base [10], and could potentially function from this location. cAMP has been predicted to be freely diffusible between ciliary and extra-ciliary compartments, using a FRET-based membrane-anchored biosensor capable of resolving intra- and extra- ciliary cAMP pools [106]. However, downstream targets of cAMP are better predictors of subcellular cAMP response. The activation of PKA by cAMP can be spatiotemporally regulated by A-kinase-anchoring protein (AKAP) anchored PKA regulatory subunits [107, 108], reducing the functional activity of cAMP to microdomains as apparent in case of PKA activation in T tubules of cardiac myocytes [109]. Recent work suggests that the cilium acts as a signaling compartment by local increases in second messengers in the cilia. Pkd2l1 functions as a calcium channel in cilia and generates a local increase in calcium in this compartment with respect to the cytoplasm [110, 111]. In the case of the Shh pathway, Gli3 processing into Gli3R involves a series of coordinated steps in which the Gli3-Sufu complex shuttles through the cilia [13, 15], is phosphorylated by PKA [6, 112, 113], and subsequently by Gsk3β and CKI [112], is recognized by the β-TrCP/cul1 E3 ubiquitin ligase [112, 114], and finally the ubiquitinated protein is partially proteolysed by the proteasome till it reaches a restriction domain in Gli3 [115, 116]. The precise location of PKA-mediated processing of Gli3 is unknown, but the proteosomal machinery has been shown to dynamically associate with the centrosome [117, 118], and the Drosophila homolog of β-TrCP, SCFSlimb localizes to centrioles [119]. There could be two ways by which cilia may be contributing to Gli3 processing. First, localization of Gpr161 in cilia may create a ciliary cAMP gradient that could result in activation of PKA in close proximity to the cilia, such as the basal body [94, 120]. If ciliary Gpr161 drives PKA-mediated Gli3 processing, the regulated removal of Gpr161 from the cilia upon Shh signaling would provide a logical explanation for preventing Gli3 processing upon Shh activity (Figure 2). Further studies using precise mutants of Gpr161 that prevents its ciliary localization or removal from cilia would be needed to resolve these issues. Second, Sufu and Gli2/3 shuttle in and out of the cilia by the IFT machinery. This becomes apparent upon cytoplasmic dynein 2 knockdown, where both Sufu, Gli2 and Gli3 start accumulating in the tips of cilia, even without Shh pathway activation [13, 121, 122]. The role of cilia in Gli3 processing could be involving these shuttling steps as well.
4. GPCR-mediated PKA activity in other tissues during Shh signaling
If GPCR-mediated PKA activity is a robust mechanism for regulating the Shh pathway, a pressing issue is the identity of GPCRs regulating PKA activity in tissues other than the neural tube. In the developing cerebellum, Shh produced by the Purkinje neurons is required for proliferation of granule precursors in the external granule layer [123-125]. Lack of cilia results in severe cerebellar hypoplasia, primarily due to a failure of expansion of the granule cell progenitor populations [126]. Aberrant activation of Shh signaling in cerebellar granule cell progenitors causes medulloblastoma (MB) [127], and is cilia-dependent [128]. In tumor models of MB driven by constitutively active Smo, genetic ablation of primary cilia suppresses tumor development. In contrast, removal of cilia is required for MB growth by constitutively active Gli2. Thus, primary cilia can either prevent or enhance MB formation, depending on the initiating oncogenic event [128]. Similar results were also noted in tumor models of basal cell carcinoma (BCC) in the skin [129], emphasizing the role of cilia in Shh-dependent tumors.
If ciliary regulation of the Shh pathway is a unifying feature during cerebellar development and in the pathogenesis of Shh-dependent tumors, which GPCRs could be involved in regulating PKA in these contexts? In this regard, the role of the pituitary adenylate cyclase activating polypeptide (PACAP) receptor 1 (PAC1) in cerebellar granular progenitor neurons is quite interesting. PACAP belongs to a peptide family that includes vasoactive intestinal peptide (VIP), and interacts via three G-protein-coupled receptors VPAC1, VPAC2, and PAC1 (reviewed in [130]). While, VPAC1 and VPAC2 have high affinity for both VIP and PACAP, PAC1 binds PACAP only with high affinity. PAC1 is expressed in granular progenitor neurons; however, its subcellular localization especially in the context of cilia is not known. VPAC2 is localized to neuronal cilia in various brain regions, including the suprachiasmatic nuclei and the thalamus, but not in the cerebellum [131]. Activation of PAC1 typically leads to robust Gαs-mediated cAMP elevation, but PAC1 can also link to other transduction pathways, such as Gαq-mediated phospholipase C (PLC) and calcium mobilization [132]. Differential pathway activation has been linked to different PAC1 splice isoforms [133, 134]. PACAP inhibits Shh-driven proliferation of granule cell progenitors [135], and acts as a tumor suppressor in murine MB [136]. In cultured cerebellar granular progenitors, PACAP blocks Shh signaling by affecting global PKA activity levels by PAC1 activation [135, 137] (Figure 2). Another chemokine SDF-1α (CXC12), and its receptor CXCR4, increase proliferation and migration of granule precursors by Gαi-mediated decrease in cAMP levels [138]. PAC1 activity through Gαq may also moderately stimulate mitogenesis via the phospholipase C/PKC pathway [135]. Thus, modulation of activity of PKA by GPCRs fine-tunes the Shh pathway during cerebellar proliferation.
GPCR-regulated pathways directly affecting the transcriptional amplification of Gli1 could also be impacting on Hh signaling. Growth of BCCs requires high levels of Hh signaling through Gli1. The atypical protein kinase C τ/λ (aPKC-τ/λ), itself a Hh pathway target, functions in a positive feedback circuit downstream of Smo to phosphorylate and activate Gli1 [139] (Figure 2). aPKC-τ/λ forms a complex and colocalizes at the basal body with missing-in metastasis (MIM), an actin regulatory protein that positively regulates Hh signaling and cilia maintenance [140]. Inhibition of aPKC-τ/λ function blocks Hh signaling and proliferation of BCC cells, and provides a novel therapeutic target for treatment of Smo-inhibitor-resistant tumors. GPCRs regulating PKC in Shh signaling are currently unknown, but chemical inhibitors targeting Smo-inhibitor resistant tumors could provide insights into these mechanisms.
5. Gαs-mediated regulation of Hh signaling and alternative pathways for cAMP regulation in the Hh pathway
Increase in cAMP levels that are mediated by GPCRs is dependent on coupling to Gαs. Interestingly, mouse Gnas (Gαs) knockout embryos are embryonic lethal by E9.5 with open neural tube and cardiac defects [60]. The E9.5 knockout embryos also show up regulation of Hh targets, and reduction of Wnt signaling targets. The neural tube in these embryos shows increased Hh signaling, as apparent from dorsal expansion of the ventral domains marked by Nkx2.2 and Shh expression, and loss of the dorsal Pax6 domain. Thus, Gαs functions as a negative regulator of Hh signaling in the neural tube (Figure 2). The neural tube phenotype of Gαs mutants is relatively more severe than that of the Gpr161 knockout, as Gpr161 presumably functions upstream of Gαs, and as Gαs is expected to have additional cellular functions. Currently, the subcellular location of Gαs during Hh signaling in the neural tube is unknown; however, Gαs has been recently described to be present in mammalian ciliary proteome [141]. Apart from its role in neural tube patterning, Gαs also functions in restricting bone formation to the skeleton by inhibiting Hh signaling in mesenchymal progenitors [60]. Progressive osseous heteroplasia, a human disease that results in extraskeletal ossification is caused by null mutations in GNAS, and the altered balance between Hh and Wnt signaling could result in these pathologies.
Apart from GPCRs that can regulate cAMP levels in the Hh pathway, cAMP phosphodiesterases (PDE) may also play a role in this process. The plausible role of the cAMP phosphodiesterase Pde4c in regulating ciliary cAMP levels is most clearly demonstrated in renal epithelial cells, where it localizes to primary cilia, and is transcriptionally regulated by the hepatocyte nuclear factor-1β (HNF-1β) [142]. Mutations in HNF-1β have been associated with kidney cysts. Pde4c is down regulated and cAMP levels are increased in HNF-1β mutant kidneys. While the contribution of Pde4c, and more so of its lack of ciliary localization in regulating the total cellular cAMP levels in the context of HNF-1β mutants are not clear, Pde4c also interacts with AKAP150, an AKAP that also localizes to cilia. Whether the regulation by PDEs and AKAPs is a common theme in cAMP regulation in the Hh pathway is currently unknown; however, the pool of PKA localized to the ciliary base of cerebellar granule progenitors and the disruption of AKAP anchoring to the PKA regulatory subunits plays an essential role in the integration of Hh signaling in these neurons [120]. More research on these interesting molecules promises to provide important insights into their regulation of PKA activity in the Hh pathway.
6. Conclusions and outstanding questions
With the discovery of the fundamental role of primary cilia and the key molecules that traffic through this compartment during Hh signaling, the major thrust in the near future is for elucidating the functional and biochemical roles of these signaling molecules in cilia. Studies of Hh signaling at cilia have provided a compelling illustration of how these ancient organelles have been co-opted by a more recently emerging signaling system to compartmentalize signaling events in a subcellular domain that maintains communication with the external environment but may be insulated from the cytoplasm. Moving forward, the development of additional tools that can precisely monitor and perturb signaling reactions at cilia will be important. Efforts to understand GPCR signaling at the ciliary membrane, and how it differs from plasma membrane signaling, will likely provide significant insights into both the biology and mechanism of ciliary signaling. Uncovering novel GPCR-regulated mechanisms important in Hh signaling in different tissue contexts and Hh-dependent tumors will provide novel druggable targets for treatment of Smo-inhibitor resistant cancers.
Box 1. Outstanding questions in the role of primary cilia in the vertebrate Hh pathway.
How does ciliary localization affect the biochemical activities and signaling functions of Ptch1, Smo, and Gpr161?
How does the unique lipidic composition of the ciliary membrane influence the activites of signaling proteins localized in cilia?
How is PKA activity regulated by the cilium during Gli processing?
Where does Gli processing occur in the Shh pathway?
How is Smo regulated by Ptch1?
How does Smo transmit the signals to the Gli family of transcription factors?
Which GPCRs regulate Shh signaling outside of the neural tube?
Highlights.
Hedgehog signaling is orchestrated at primary cilia in vertebrates.
Several G-protein coupled receptors (GPCRs) are found concentrated in the ciliary membrane.
The Hedgehog pathway transducer Smoothened, a member of the GPCR superfamily, accumulates in cilia upon ligand reception.
An orphan GPCR, Gpr161, leaves cilia in response to ligand reception.
Both ciliary and non-ciliary GPCRs likely regulate Hedgehog signaling by converging on Protein Kinase A.
Protein Kinase A activity regulates the Gli family of Hedgehog transcription factors.
Acknowledgements
Research in our laboratories are funded by endowed scholarship and first-time tenure track faculty recruitment funds (SM), and the NIH Common Fund, the Pew Foundation, Sontag Foundation and March of Dimes Foundation (RR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 2.Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Developmental cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Developmental cell. 2011;20:775–787. doi: 10.1016/j.devcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 5.Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 7.Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ, et al. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112(Pt 23):4437–4448. doi: 10.1242/jcs.112.23.4437. [DOI] [PubMed] [Google Scholar]
- 8.Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 9.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Developmental cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 12.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 17.Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- 18.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 19.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 20.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 21.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes & development. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Arvanites AC, Davidow L, Blanchard J, Lam K, Yoo JW, Coy S, Rubin LL, McMahon AP. Selective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocation. ACS chemical biology. 2012;7:1040–1048. doi: 10.1021/cb300028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu VM, Chen SC, Arkin MR, Reiter JF. Small molecule inhibitors of Smoothened ciliary localization and ciliogenesis. Proc Natl Acad Sci U S A. 2012;109:13644–13649. doi: 10.1073/pnas.1207170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Incardona JP, Gaffield W, Lange Y, Cooney A, Pentchev PG, Liu S, Watson JA, Kapur RP, Roelink H. Cyclopamine inhibition of Sonic hedgehog signal transduction is not mediated through effects on cholesterol transport. Dev Biol. 2000;224:440–452. doi: 10.1006/dbio.2000.9775. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 29.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 31.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of Smoothened by Patched-Dependent (Pro-)Vitamin D3 Secretion. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Davidow L, Arvanites AC, Blanchard J, Lam K, Xu K, Oza V, Yoo JW, Ng JM, Curran T, et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chemistry & biology. 2012;19:972–982. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, Siu FY, Roth BL, Cherezov V, Stevens RC. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachtergaele S, Whalen DM, Mydock LK, Zhao Z, Malinauskas T, Krishnan K, Ingham PW, Covey DF, Siebold C, Rohatgi R. Structure and function of the Smoothened extracellular domain in vertebrate Hedgehog signaling. eLife. 2013;2:e01340. doi: 10.7554/eLife.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana R, Carroll CE, Lee HJ, Bao J, Marada S, Grace CR, Guibao CD, Ogden SK, Zheng JJ. Structural insights into the role of the Smoothened cysteine-rich domain in Hedgehog signalling. Nature communications. 2013;4:2965. doi: 10.1038/ncomms3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weierstall U, James D, Wang C, White TA, Wang D, Liu W, Spence JC, Bruce Doak R, Nelson G, Fromme P, et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nature communications. 2014;5:3309. doi: 10.1038/ncomms4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol. 2013;9:557–564. doi: 10.1038/nchembio.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, Bazan JF, Beachy PA. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Developmental cell. 2013;26:346–357. doi: 10.1016/j.devcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Frontiers in physiology. 2013;4:31. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Developmental dynamics: an official publication of the American Association of Anatomists. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ocbina PJ, Eggenschwiler JT, Moskowitz I, Anderson KV. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43:547–553. doi: 10.1038/ng.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O’Donnell M, Gelfand VI, Kapoor TM, Chen JK. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell. 2012;22:940–951. doi: 10.1016/j.devcel.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler-Reya RJ, Lefkowitz RJ, Caron MG. Smoothened Signal Transduction is Promoted by G-Protein Coupled Receptor Kinase 2. Mol Cell Biol. 2006 doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252–258. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, Jiang J. Sonic Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS biology. 2011;9:e1001083. doi: 10.1371/journal.pbio.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barakat B, Yu L, Lo C, Vu D, De Luca E, Cain JE, Martelotto LG, Dedhar S, Sadler AJ, Wang D, et al. Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling. EMBO reports. 2013;14:837–844. doi: 10.1038/embor.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 54.Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum Mol Genet. 2012;21:1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen F, Cheng L, Douglas AE, Riobo NA, Manning DR. Smoothened is a fully competent activator of the heterotrimeric G protein G(i) Molecular pharmacology. 2013;83:691–697. doi: 10.1124/mol.112.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galpha(i) functions immediately downstream of Smoothened in Hedgehog signalling. Nature. 2008;456:967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Low WC, Wang C, Pan Y, Huang XY, Chen JK, Wang B. The decoupling of Smoothened from Galphai proteins has little effect on Gli3 protein processing and Hedgehog-regulated chick neural tube patterning. Dev Biol. 2008;321:188–196. doi: 10.1016/j.ydbio.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nature medicine. 2013;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogden SK, Ascano M, Jr., Stegman MA, Suber LM, Hooper JE, Robbins DJ. Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr Biol. 2003;13:1998–2003. doi: 10.1016/j.cub.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Molecular cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 63.Tay SY, Ingham PW, Roy S. A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development. 2005;132:625–634. doi: 10.1242/dev.01606. [DOI] [PubMed] [Google Scholar]
- 64.Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Law KK, Briscoe J, Hui CC. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 65.Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, Davis M, Scales SJ, Solloway MJ, de Sauvage FJ, et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19:1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 66.Liem KF, Jr., He M, Ocbina PJ, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13377–13382. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Developmental cell. 2012;23:823–835. doi: 10.1016/j.devcel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang C, Chen W, Chen Y, Jiang J. Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2. Cell research. 2012;22:1593–1604. doi: 10.1038/cr.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caparros-Martin JA, Valencia M, Reytor E, Pacheco M, Fernandez M, Perez-Aytes A, Gean E, Lapunzina P, Peters H, Goodship JA, et al. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum Mol Genet. 2013;22:124–139. doi: 10.1093/hmg/dds409. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet. 2009;151C:341–351. doi: 10.1002/ajmg.c.30226. [DOI] [PubMed] [Google Scholar]
- 71.Pusapati GV, Hughes CE, Dorn KV, Zhang D, Sugianto P, Aravind L, Rohatgi R. EFCAB7 and IQCE Regulate Hedgehog Signaling by Tethering the EVC-EVC2 Complex to the Base of Primary Cilia. Developmental cell. 2014 doi: 10.1016/j.devcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valencia M, Lapunzina P, Lim D, Zannolli R, Bartholdi D, Wollnik B, Al-Ajlouni O, Eid SS, Cox H, Buoni S, et al. Widening the mutation spectrum of EVC and EVC2: ectopic expression of Weyer variants in NIH 3T3 fibroblasts disrupts Hedgehog signaling. Hum Mutat. 2009;30:1667–1675. doi: 10.1002/humu.21117. [DOI] [PubMed] [Google Scholar]
- 73.Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- 74.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 75.Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb Symp Quant Biol. 1997;62:451–466. [PubMed] [Google Scholar]
- 76.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 77.Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 78.Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nature neuroscience. 2000;3:979–985. doi: 10.1038/79916. [DOI] [PubMed] [Google Scholar]
- 79.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 80.Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- 81.Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, Briscoe J, Ribes V. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell. 2012;148:273–284. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong F, Tentner AR, Huang P, Gelas A, Mosaliganti KR, Souhait L, Rannou N, Swinburne IA, Obholzer ND, Cowgill PD, et al. Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell. 2013;153:550–561. doi: 10.1016/j.cell.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenbaum JL, Witman GB. Intraflagellar transport. Nature reviews. Molecular cell biology. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 84.Bhogaraju S, Engel BD, Lorentzen E. Intraflagellar transport complex structure and cargo interactions. Cilia. 2013;2:10. doi: 10.1186/2046-2530-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nature genetics. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liem KF, Jr., Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol. 2012;197:789–800. doi: 10.1083/jcb.201110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1456–1461. doi: 10.1073/pnas.1011410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24:2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Behal RH, Miller MS, Qin H, Lucker BF, Jones A, Cole DG. Subunit interactions and organization of the Chlamydomonas reinhardtii intraflagellar transport complex A proteins. The Journal of biological chemistry. 2012;287:11689–11703. doi: 10.1074/jbc.M111.287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Norman RX, Ko HW, Huang V, Eun CM, Abler LL, Zhang Z, Sun X, Eggenschwiler JT. Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling. Human molecular genetics. 2009;18:1740–1754. doi: 10.1093/hmg/ddp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patterson VL, Damrau C, Paudyal A, Reeve B, Grimes DT, Stewart ME, Williams DJ, Siggers P, Greenfield A, Murdoch JN. Mouse hitchhiker mutants have spina bifida, dorso-ventral patterning defects and polydactyly: identification of Tulp3 as a novel negative regulator of the Sonic hedgehog pathway. Human molecular genetics. 2009;18:1719–1739. doi: 10.1093/hmg/ddp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Y, Roelink H, McKnight GS. Protein kinase A deficiency causes axially localized neural tube defects in mice. J Biol Chem. 2002;277:19889–19896. doi: 10.1074/jbc.M111412200. [DOI] [PubMed] [Google Scholar]
- 94.Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development. 2011;138:4921–4930. doi: 10.1242/dev.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang C, Pan Y, Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137:2001–2009. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen MH, Gao N, Kawakami T, Chuang PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25:7042–7053. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgard R, Liu A. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Developmental biology. 2009;330:452–460. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 99.Leurs R, Smit MJ, Alewijnse AE, Timmerman H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem Sci. 1998;23:418–422. doi: 10.1016/s0968-0004(98)01287-0. [DOI] [PubMed] [Google Scholar]
- 100.Nygaard R, Zou Y, Dror RO, Mildorf TJ, Arlow DH, Manglik A, Pan AC, Liu CW, Fung JJ, Bokoch MP, et al. The dynamic process of beta(2)-adrenergic receptor activation. Cell. 2013;152:532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Omura M, Grosmaitre X, Ma M, Mombaerts P. The beta2-adrenergic receptor as a surrogate odorant receptor in mouse olfactory sensory neurons. Molecular and cellular neurosciences. 2014;58:1–10. doi: 10.1016/j.mcn.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greasley PJ, Fanelli F, Scheer A, Abuin L, Nenniger-Tosato M, DeBenedetti PG, Cotecchia S. Mutational and computational analysis of the alpha(1b)-adrenergic receptor. Involvement of basic and hydrophobic residues in receptor activation and G protein coupling. The Journal of biological chemistry. 2001;276:46485–46494. doi: 10.1074/jbc.M105791200. [DOI] [PubMed] [Google Scholar]
- 103.Scheer A, Fanelli F, Costa T, De Benedetti PG, Cotecchia S. Constitutively active mutants of the alpha 1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J. 1996;15:3566–3578. [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 106.Marley A, Choy RW, von Zastrow M. GPR88 reveals a discrete function of primary cilia as selective insulators of GPCR cross-talk. PloS one. 2013;8:e70857. doi: 10.1371/journal.pone.0070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nature reviews. Molecular cell biology. 2012;13:646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nature reviews. Molecular cell biology. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 109.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 110.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tempe D, Casas M, Karaz S, Blanchet-Tournier MF, Concordet JP. Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP. Molecular and cellular biology. 2006;26:4316–4326. doi: 10.1128/MCB.02183-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R. Gli protein activity is controlled by multisite phosphorylation in vertebrate hedgehog signaling. Cell reports. 2014;6:168–181. doi: 10.1016/j.celrep.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 115.Pan Y, Wang B. A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. The Journal of biological chemistry. 2007;282:10846–10852. doi: 10.1074/jbc.M608599200. [DOI] [PubMed] [Google Scholar]
- 116.Schrader EK, Harstad KG, Holmgren RA, Matouschek A. A three-part signal governs differential processing of Gli1 and Gli3 proteins by the proteasome. J Biol Chem. 2011;286:39051–39058. doi: 10.1074/jbc.M111.274993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN. Activity and regulation of the centrosome-associated proteasome. The Journal of biological chemistry. 2000;275:409–413. doi: 10.1074/jbc.275.1.409. [DOI] [PubMed] [Google Scholar]
- 118.Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. The Journal of cell biology. 1999;145:481–490. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL. The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. The Journal of cell biology. 2009;184:225–239. doi: 10.1083/jcb.200808049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S. Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. Journal of cell science. 2010;123:62–69. doi: 10.1242/jcs.060020. [DOI] [PubMed] [Google Scholar]
- 121.Wen X, Lai CK, Evangelista M, Hongo JA, de Sauvage FJ, Scales SJ. Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol. 2010;30:1910–1922. doi: 10.1128/MCB.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Y, Yue S, Xie L, Pu XH, Jin T, Cheng SY. Dual Phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium. The Journal of biological chemistry. 2011;286:13502–13511. doi: 10.1074/jbc.M110.217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz i Altaba A. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 124.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 125.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Current biology: CB. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 126.Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 128.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nature medicine. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr., Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nature medicine. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. British journal of pharmacology. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soetedjo L, Glover DA, Jin H. Targeting of vasoactive intestinal peptide receptor 2, VPAC2, a secretin family G-protein coupled receptor, to primary cilia. Biology open. 2013;2:686–694. doi: 10.1242/bio.20134747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. The Japanese journal of physiology. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 133.Nicot A, DiCicco-Bloom E. Regulation of neuroblast mitosis is determined by PACAP receptor isoform expression. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4758–4763. doi: 10.1073/pnas.071465398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 135.Nicot A, Lelievre V, Tam J, Waschek JA, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9244–9254. doi: 10.1523/JNEUROSCI.22-21-09244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lelievre V, Seksenyan A, Nobuta H, Yong WH, Chhith S, Niewiadomski P, Cohen JR, Dong H, Flores A, Liau LM, et al. Disruption of the PACAP gene promotes medulloblastoma in ptc1 mutant mice. Developmental biology. 2008;313:359–370. doi: 10.1016/j.ydbio.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cellular signalling. 2013;25:2222–2230. doi: 10.1016/j.cellsig.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 139.Atwood SX, Li M, Lee A, Tang JY, Oro AE. GLI activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature. 2013;494:484–488. doi: 10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Developmental cell. 2010;19:270–283. doi: 10.1016/j.devcel.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ishikawa H, Thompson J, Yates JR, 3rd, Marshall WF. Proteomic analysis of mammalian primary cilia. Curr Biol. 2012;22:414–419. doi: 10.1016/j.cub.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi YH, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, Pontoglio M, Somlo S, Igarashi P. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10679–10684. doi: 10.1073/pnas.1016214108. [DOI] [PMC free article] [PubMed] [Google Scholar]