Abstract
Purpose
Morphological assessment of human blastocysts has been effective for selecting embryos with high potential. However, they often show repeated shrinkage and expansion toward their hatching. Here we assessed whether capturing morphological changes over time of vitrified–warmed blastocysts could lead to a better selection of viable embryos from shrunken blastocysts.
Methods
The implantation rates of vitrified–warmed blastocysts that were shrunken or expanded (developing) at the time of loading for transfer were compared among 2,729 cycles that were subjected to single blastocyst transfer. Vitrified (107) and fresh blastocysts (17) were donated for the experimental study. To assess the relationship between morphology (expanded vs. shrunken) and the mitochondrial respiration of blastocysts, the oxygen consumption rate (OCR) was analyzed for 55 specimens using an uncoupler of oxidative phosphorylation. The remaining 69 blastocysts were used for recording morphological changes every 15 min for 48 h after warming.
Results
Because there were no surplus embryos, 7 % of the vitrified–warmed blastocysts were shrunken and transferred. The shrunken embryos had sufficient implantation ability (40 %). The OCR of the shrunken embryos was significantly lower than that of their expanded counterparts. Upon exposure to the uncoupler, the OCR of some shrunken embryos increased to levels similar to the expanded specimens. Time-lapse images revealed some shrunken embryos which formed blastocoel by 5 h following warming exhibited developmental competence to the hatched stage.
Conclusions
Data of the present study suggest a group of shrunken blastocysts contains many viable and clinically available embryos and time-lapse observation of vitrified–warmed blastocysts is a potential method to distinguish viable embryos from shrunken blastocysts.
Keywords: Vitrified–warmed blastocyst, Oxygen consumption, Time-lapse images, Embryo selection, Embryo transfer
Introduction
Assisted reproductive technology (ART) is associated with a risk of multiple pregnancies and births that are associated with adverse maternal and infant outcomes [1]. The principal factor contributing to this risk is the policy of transferring more than one embryo into the uterus. There is a growing trend toward transferring the minimum number of embryos possible in many countries, but the practice of single embryo transfer (SET) remains uncommon [2]. This policy should be balanced against the acceptance by patients and clinicians of the reduction in the pregnancy rate [3]. The contribution of supernumerary cryopreserved embryos to cumulative reproductive outcomes is essential for SET. Consequently, the selection criteria for cryopreserved embryos on the basis of its functional and morphological changes have increased importance since more embryos are available for cryopreservation. Therefore, the establishment of a new parameter is required for the selection of single embryos that have high developmental potential after cryopreservation, especially shrunken blastocysts.
The morphological assessment of human blastocysts has been effective in the selection of high potential embryos [4]. However, murine and human blastocysts often repeatedly alternate between shrinkage and expansion before hatching [5, 6]. Thus, it can be difficult to predict the subsequent development of embryos by only morphological observation. On the other hand, it is possible to select vitrified–warmed blastocysts with high developmental potential based on their respiratory activity [7]. From this point of view, the measurement of the oxygen consumption of shrunken blastocysts could provide information about their developmental competence.
Incorporation of time-lapse imaging of the development of human embryos provides useful information about their development [8–13]. However, no report about time-lapse imaging of the development of cryopreserved embryos after warming has been completed.
The present work describes the implantation potential of shrunken blastocysts after vitrification and warming. The oxygen consumption of the shrunken blastocysts after vitrification and warming was examined in the presence and absence of mitochondrial toxins. Moreover, time-lapse imaging was used to investigate the relationship between morphological properties and developmental competence of vitrified–warmed blastocysts.
Materials and methods
This study was approved by the ethics committee of the IVF Namba Clinic (approval number: 2013–3). Embryos donated by patients who had completed fertility treatment were used for the in vitro study. For the in vivo study, patients received a full explanation of the methodology and informed consent to their participation and all donors provided signed informed consent.
The blastocysts from full to hatching stages and their inner cell mass (ICM), which were categorized into tightly packed and many cells or loosely grouped and several cells before vitrification, were included in the analysis except for fresh samples. Morphologically-poor blasyocysts which were excluded from the option of transfer were used for fresh samples. The blastocysts were vitrified and warmed as previously described using the open vitrification device (Cryotop® Kitazato corporation, Tokyo, Japan), [14]. When the morphology of the vitrified–warmed blastocysts recovered to the pre-vitrification levels at the time of loading for transfer or 5–6 h after warming, the embryos were categorized as expanded. In the case of exhibiting shrinkage at the time of loading for transfer or 5–6 h after warming, the embryos were categorized as shrunken.
Retrospective analysis of clinical data
To assess the implantation potential after ET of shrunken blastocysts following vitrification and warming, 2,729 patients received single vitrified–warmed blastocyst transfers under hormone-replacement cycles from January 2006 to December 2012. During the period subject to this study, 2,822 blastocysts were warmed and 2,703 samples survived (the survival rate: 95.8 %). The proportion of shrunken embryos at the time of transfer was 7.5 % (202/2,703). An additional warming was performed in 46 cases because there were surplus cryopreserved blastocysts. All blastocysts survived in the case of additional warming. The proportion of transferred shrunken specimens was 6.7 % (182/2,729). Clinical pregnancy was confirmed by the presence of a gestation sac (GS) by ultrasound analysis at 3 weeks after ET. The endometrium was prepared as previously described [15].
Blastocyst scoring
To define a numerical blastocyst morphology grading system based on Gardner’s grading system [4], the blastocyst grade was converted to the multiplicative blastocyst quality score (BQS) [16]. BQS is a metric of blastocyst quality based on established morphological criteria; it is defined by the degree of expansion, hatching status, and ICM and TE grades, where grade A, B, and C are given values of 3, 2, and 1, respectively. For a 4AB blastocyst, the BQS is 4 × 3 × 2, giving a BQS score of 24.
Donated blastocysts
Seventeen fresh blastocysts and 38 vitrified samples were used for measuring oxygen consumption and 69 vitrified specimens were used for the time-lapse imaging analysis. The mean age of the patients was 34.4 years old when they produced these blastocysts, which were either on day 5 or day 6 after fertilization. After warming, they were cultured individually (Blastocyst Medium; COOK Medical, Queensland, Australia) at 37.0 °C under 5 % CO2, 5 % O2, and 90 % N2. Morphologically-poor blastocysts were not used clinically but their oxygen consumption was measured as a reference (fresh blastocysts).
Oxygen consumption rate (OCR)
To assess whether the viability of shrunken blastocyst could be predicted based on their OCRs, the OCR of expanded and shrunken blastocysts were measured using mitochondrial toxins. Stock solutions of 1 mM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; C2920, Sigma- Aldrich, St. Louis, MO, USA) and 1 M sodium cyanide (380970, Sigma-Aldrich) were prepared in ethanol and water, respectively. During the recording of oxygen consumption by embryos in the respiration medium, either FCCP or cyanide was added to give final concentrations of 1 μM and 1 mM, respectively. Cyanide at 1 mM completely inhibits mitochondrial cytochrome oxidase activity.
The OCR of vitrified–warmed blastocysts was measured using scanning electrochemical microscopy (SCEM: CRAS-1.0; Clino Ltd., Miyagi, Japan) at 37 °C as previously described [7]. In brief, each blastocyst was transferred into a well filled with human tubal fluid-buffered with 21 mM HEPES containing 2.7 mM glucose and 0.33 mM pyruvate (Modified HTF Medium; Irvine Scientific, CA, USA) containing 5 % Serum Substitute Supplement (SSS, IS Japan, Tokyo, Japan) (v/v), where the blastocyst sank to the bottom of the cone-shaped microwell and remained at the lowest point. A platinum microdisk electrode was loaded into 5 mL of HEPES-buffered medium, and the tip potential was maintained at −0.6 V versus Ag/AgCl with a potentiostat to monitor the local oxygen concentration. The microelectrode scanned along the z-axis from the edge of the sample, and the oxygen consumption rate was calculated with custom software based on the spherical diffusion theory [17]. The OCR was measured at three arbitrary points around the embryos.
After the morphological evaluation, the OCR of vitrified–warmed blastocysts was measured at 6 h after warming as described above. The OCR of the vitrified–warmed blastocysts recovered to their pre-vitrification levels approximately 6 h after warming [7]. After adding FCCP, the OCR was measured every 10 min for 40 min, and then for 30 min in the presence of 1 μM cyanide. The blastocysts were transferred to microwells filled with fresh medium to avoid CO2 accumulation around the embryo. The data were shown as relative values to the initial values obtained in the absence of respiratory toxins.
Time-lapse imaging
To investigate the relationship between morphological properties and developmental competence of vitrified–warmed blastocysts, time-lapse images were taken. Time-lapse images of 69 blastocysts were captured as previously described [10] using a commercially available incubator with a built-in microscope and camera connected to a computer (EmbryoScope: Unisense Fertilitech, Aarhus, Denmark and Primo Vision Time-Lapse Embryo Monitoring System: Vitrolife AB, Göteborg, Sweden). Vitrified–warmed blastocysts were cultured in 25-μL culture medium under 1.2-mL mineral oil (Kitazato BioPharma Co., Tokyo, Japan) at 37 °C under 5 % CO2, 5 % O2, and 90 % N2.
The capturing of the blastocyst images was started soon after their warming; these images were recorded every 15 min for 48 h. The embryos were divided into two groups according to their morphology (shrunken or expanding) at 5–6 h after the warming. When a shrunken blastocyst was found, we retrospectively checked the initial images to determine whether the blastocyst showed expanding morphology or not before 5 h.
Statistical analysis
The data were shown as mean ± SEM. The OCR, donor age, and BQS data were analyzed using student’s t test and the endometrial thickness was analyzed using Welch’s t test. The developmental rate to the hatched stage was compared using χ2-test. P < 0.05 was considered significant.
Results
Retrospective analysis of clinical data
Human blastocysts often show repeated shrinkage and expansion toward their hatching. The implantation potential of shrunken blastocysts after vitrification and warming was retrospectively assessed. About 7 % of vitrified–warmed blastocysts (202/2,868) were shrunken at the time of transfer. Approximately 6.7 % of vitrified–warmed blastocysts (182/2,729) had been transferred despite their shrunken morphology at SET when there were no surplus embryos. The implantation rate of the shrunken embryos at SET (40 %, n = 72) was significantly lower (P < 0.01) than that of their expanded counterparts (51 %, n = 1,289). The morphologically shrunken embryos showed a slight decrease in their implantation rate despite no difference in the age of female patients (shrunken: 35.2 ± 0.3 y.o. vs. expanded: 35.3 ± 0.1 y.o.), the endometrial thickness (shrunken: 11.3 ± 0.2 mm vs. expanded: 11.1 ± 0.1 mm), and the embryo score before vitrification (shrunken: 16.1 ± 0.5 vs. expanded: 16.7 ± 0.1) between the shrunken and expanded groups. These results indicate that there are many shrunken blastocysts with full implantation potential.
OCR
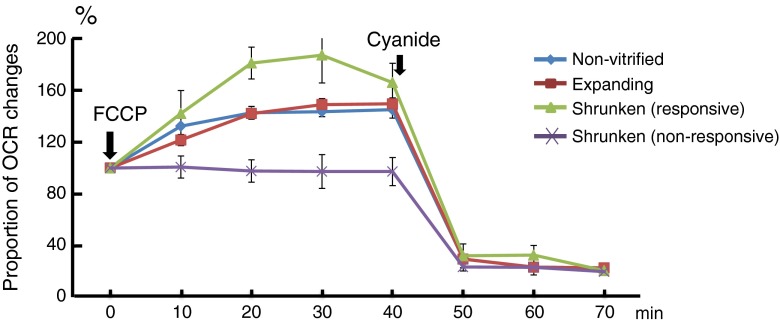
The OCR of the shrunken and expanded blastocysts was measured at 6 h after warming because the vitrified-warmed blastocysts recovered their OCR to their pre-vitrification levels about 6 h after warming [7]. The OCR of the shrunken embryos (n = 10, 4.61 ± 0.48 fmol/s) was significantly lower than that of the expanded specimens (n = 28, 6.30 ± 0.38 fmol/s) despite no difference in BQS between shrunken (12.4 ± 1.4) and expanded (12.1 ± 1.0). In the presence of FCCP, the shrunken embryos were classified depending on their oxygen consumption potential. As shown in Fig. 1, the OCR of responsive shrunken-embryos increased to 187 ± 21 % (n = 5) 30 min after adding FCCP. The extent of this increase is similar to those of expanded blastocysts (149 ± 5 %) and non-vitrified blastocysts which were excluded from the option of transfer because of poor morphology (144 ± 4 %) although the BQS of non-vitrified specimens (7.1 ± 0.9) was lower (P < 0.01) than those of expanded (12.1 ± 1.0) and responsive shrunken samples (n = 5, 13.6 ± 1.6). On the other hand, the OCR of unresponsive shrunken-embryos (97 ± 13 %; n = 5) was significantly lower (P < 0.05) than those of their responsive counterparts and expanded blastocysts and the BQS of unresponsive shrunken-embryos (11.2 ± 2.4) was not different compared with others. In the presence of sodium cyanide, the mitochondrial respiration of both the expanded and responsive shrunken-embryos was inhibited. The OCR of the responsive shrunken-embryos decreased to 20 ± 2 %; this decrease was similar to that of the expanded embryos (23 ± 1 %). The OCR of the responsive shrunken-embryos before FCCP treatment (4.85 ± 0.63 fmol/s) was similar to that of the unresponsive shrunken-embryos (4.36 ± 0.78 fmol/s).
Fig. 1.
The proportion of the oxygen consumption rate (OCR) changes based on the OCR at 5–6 h after warming. The OCRs of shrunken blastocysts (n = 10) were measured after an addition of carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP). The uncoupler was treated at 5–6 h after warming. The OCR of several shrunken blastocysts (responsive, n = 5) increased to more than 180 % and was similar to that of the expanded and non-vitrified blastocysts. On the other hand, most shrunken blastocysts did not show any changes in their OCR (P < 0.01 during FCCP treatment, n = 5). Cyanide was added at 40 min after FCCP treatment. The OCRs of all embryos decreased to the lowest level after cyanide treatment
Time-lapse imaging
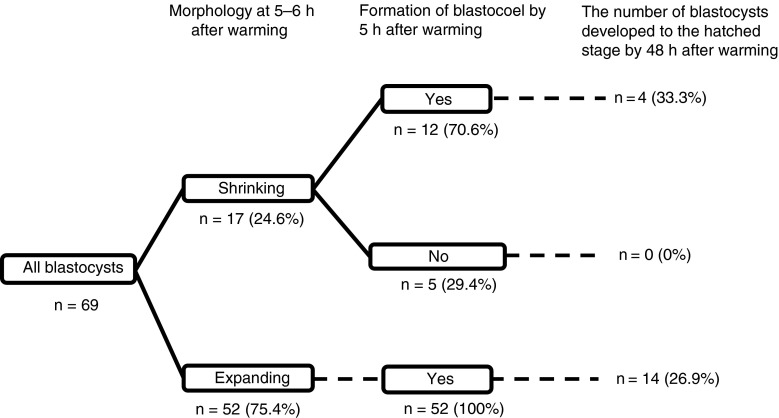
Time-Lapse Imaging revealed that 17 (25 %; Fig. 2) of the vitrified–warmed blastocysts remained shrunken at 5–6 h of warming despite no significant difference in grading between the shrunken (13.4 ± 1.4) and expanded (n = 52, 17.0 ± 1.0) blastocysts (Fig. 3). Twelve (71 %) of the shrunken blastocysts formed their blastocoels within 5 h after warming. As shown in Fig. 3, four blastocoel-collapsed embryos (33 %) developed to their hatched stage within 24 h. This value was similar to that of the expanded blastocysts between 5 and 6 h after warming (27 %). The BQS of the shrunken blastocysts which formed their blastocoels within 5 h after warming (12.4 ± 1.1) was significantly lower (P < 0.01) than that of expanded counterparts at 5–6 h (17.0 ± 1.0). However, the BQS of shrunken blastocysts which did not form their blastocoels within 5 h after warming (15.6 ± 4.1) was no statistical difference compared with other groups.
Fig. 2.
Time-Lapse imaging of 69 vitrified–warmed blastocysts. They were classified by whether they had shrunk or not at 5–6 h after warming. Moreover, shrunken blastocysts were classified according to whether they recovered or not by 5–6 h after warming. There was no significant change in the proportion of blastocysts that developed to the hatched stage between the expanded (26.9 %) and the shrinking blastocysts (33.3 %) at 5–6 h but there was a difference in the recovery rate before 5 h. On the other hand, the number of blastocysts that had not recovered by 5–6 h after warming and that developed to the hatched stage was zero
Fig. 3.
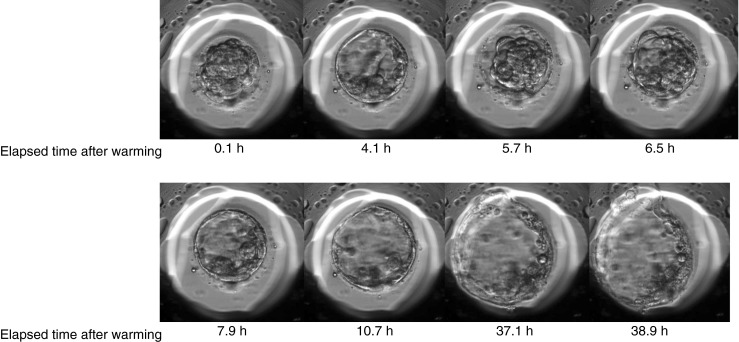
Temporal changes of a vitrified–warmed blastocyst. Pictures show representative images of a blastocyst, which showed temporal expansion at 4.1 h after warming and shrinkage at 5–6 h and re-expansion toward hatched stage at 6.5 h
Discussion
Produced blastocysts from mice and human often show repeated shrinkage and expansion toward hatching [5, 6]. Thus, the prediction of the subsequent development of embryos using simple morphological observation can be difficult. In clinical practice, physicians often have no choice except for transferring a shrunken embryo. Therefore, to assess whether shrunken blastocysts at the time of transfer have the developmental competence, we retrospectively examined the implantation potential of shrunken blastocysts. To our surprise, the implantation rate of the shrunken embryos was fairly high (40 %). This observation suggested that a group of shrunken embryos may contain a number of viable and clinically available embryos.
In the first place, we assessed whether the viable shrunken embryos could be distinguishable based on its OCR or not. Unfortunately, there were no shrunken embryos exhibiting remarkably high OCR. However, FCCP markedly increased the OCR of some shrunken embryos. Moreover, inhibition of mitochondrial cytochrome oxidase by cyanide strongly decreased their OCR, suggesting that some shrunken embryos retained mitochondrial activity similar to that of expanded blastocysts at the time of transfer. Judging from high implantation potential of shrunken blastocysts, those retaining high respiration potential could have clinical competence for implantation.
Because the embryos exposed to FCCP and/or cyanide for the analysis of their OCR cannot be used clinically, we observed the morphological changes of the cultured embryos that had been shrunken at the time of ET. Analysis using time-lapse imaging revealed that some shrunken embryos at 5–6 h after warming did develop to the hatched stage; these embryos were found to exhibit the expanded morphology at least once during 5 h after warming. On the other hand, embryos that did not expand by 5 h failed to develop to the hatched stage. These results suggest that the morphological change of vitrified–warmed blastocysts is a potential indicator for their subsequent development. However, in vivo developed blastocysts scarcely show contractions during the expanded and hatching period [18, 19]. Thus, the repeated contraction of embryos may be caused by suboptimal culture conditions. The incidence of such contractions could be decreased by reviewing the procedures of in vitro culture and vitrification. In time-lapse imaging, the BQS of the shrunken blastocysts which formed their blastocoels was low compared with expanded specimens. On the other hand, the BQS of FCCP-responsive embryos was similar to expanded counterparts in the measurement of the OCR experiment. Further investigation should be required whether the development of vitrified blastocysts following warming could be predicted based on their BQS before vitrification.
The present work suggested that monitoring morphological changes of vitrified blastocysts after warming is a potential method to distinguish viable embryos from shrunken blastocysts, which could be used clinically. Time-lapse observation requires at least 5 h. If the embryo did not show the blastocoel formation, an additional warming or a cancellation of embryo transfer would be necessitated. When an additional warming is performed, there is no time to observe morphological changes. Thus, another criterion to make a quick assessment of embryo viability following vitrification would be required. Further investigation is warranted to elucidate the fate of shrunken embryos and the subsequent development of the blastocysts. Short term observation by time-lapse imaging may permit better selection of shrunken blastocysts.
Acknowledgment
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
Part of this work was supported by a grant from the Japan Society for the Promotion of Science (JPS-RFTF 23580397 to S.H.).
Conflict of interest
None declared.
Footnotes
Capsule
Time-lapse imaging paves the way for possible selection of viable blastocysts from shrunken embryos after vitrification.
References
- 1.Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance–United States, 2005. MMWR Surveill Summ. 2008;57:1–23. [PubMed] [Google Scholar]
- 2.Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod. 2008;23:756–771. doi: 10.1093/humrep/den014. [DOI] [PubMed] [Google Scholar]
- 3.Pandian Z, Templeton A, Serour G, Bhattacharya S. Number of embryos for transfer after IVF and ICSI: a Cochrane review. Hum Reprod. 2005;20:2681–2687. doi: 10.1093/humrep/dei153. [DOI] [PubMed] [Google Scholar]
- 4.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 5.Mio Y, Iwata K, Yumoto K, Iba Y. Several issues highlighted by time-lapse cinematography of human blastocyst during extended in vitro culture. In Japanese. J Mamm Ova Res. 2011;28:152–158. doi: 10.1274/jmor.28.152. [DOI] [Google Scholar]
- 6.Niimura S. Time-lapse videomicrographic analyses of contractions in mouse blastocysts. J Reprod Dev. 2003;49:413–423. doi: 10.1262/jrd.49.413. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka M, Hashimoto S, Amo A, Ito-Sasaki T, Abe H, Morimoto Y. Developmental assessment of human vitrified-warmed blastocysts based on oxygen consumption. Hum Reprod. 2011;26:3366–3371. doi: 10.1093/humrep/der324. [DOI] [PubMed] [Google Scholar]
- 8.Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99:1035–1043. doi: 10.1016/j.fertnstert.2013.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz M, Garrido N, Herrero J, Perez-Cano I, Munoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25:371–381. doi: 10.1016/j.rbmo.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly (dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–337. doi: 10.1016/j.fertnstert.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99:1030–1034. doi: 10.1016/j.fertnstert.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 12.Hlinka D, Kalatova B, Uhrinova I, Dolinska S, Rutarova J, Rezacova J, et al. Time-lapse cleavage rating predicts human embryo viability. Physiol Res. 2012;61:513–525. doi: 10.33549/physiolres.932287. [DOI] [PubMed] [Google Scholar]
- 13.Pribenszky C, Matyas S, Kovacs P, Losonczi E, Zadori J, Vajta G. Pregnancy achieved by transfer of a single blastocyst selected by time-lapse monitoring. Reprod BioMed Online. 2010;21:533–536. doi: 10.1016/j.rbmo.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Kuwayama M, Vajta G, Leda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–614. doi: 10.1016/s1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto S, Amo A, Hama S, Ito K, Nakaoka Y, Morimoto Y. Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod. 2013;28:1528–1535. doi: 10.1093/humrep/det059. [DOI] [PubMed] [Google Scholar]
- 16.Rehman KS, Bukulmez O, Langley M, Carr BR, Nackley AC, Doody KM, et al. Late stages of embryo progression are a much better predictor of clinical pregnancy than early cleavage in intracytoplasmic sperm injection and in vitro fertilization cycles with blastocyst-stage transfer. Fertil Steril. 2007;87:1041–1052. doi: 10.1016/j.fertnstert.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Shiku H, Shiraishi T, Ohya H, Matsue T, Abe H, Hoshi H, et al. Oxygen consumption of single bovine embryos probed by scanning electrochemical microscopy. Anal Chem. 2001;73:3751–3758. doi: 10.1021/ac010339j. [DOI] [PubMed] [Google Scholar]
- 18.Checiu I, Checiu M. There are no in vivo pulsations of mouse blastocysts. Rom J Morphol Embryol. 1996;42:147–154. [PubMed] [Google Scholar]
- 19.Lin SP, Lee RK, Tsai YJ. In vivo hatching phenomenon of mouse blastocysts during implantation. J Assist Reprod Genet. 2001;18:341–345. doi: 10.1023/A:1016640923269. [DOI] [PMC free article] [PubMed] [Google Scholar]