Abstract
Purpose
To evaluate the effect of cryopreservation and thawing of ovarian tissue from oncological patients opting for fertility preservation on ovarian tissue viability.
Methods
In this prospective cohort study, the ovarian tissue viability before and after cryopreservation and thawing was measured for 25 newly diagnosed oncological patients who had their ovarian tissue cryopreserved. Outcome measures were follicle integrity (histology), follicle viability (Calcein viability assay), steroid hormone production (estradiol and progesterone production in vitro) and overall tissue viability (glucose uptake in vitro). This study was conducted at a Cryobank for storage of ovarian tissue in a university hospital.
Results
Cryopreserved/thawed ovarian tissue showed a decreased glucose uptake when compared to tissue that had not been cryopreserved. In addition, a diminished E2 and P4 production was observed after cryopreservation and thawing, despite the fact that numbers of viable follicles as determined by the Calcein viability assay were comparable. Histological examination revealed a higher percentage of degenerated follicles after cryopreservation and thawing.
Conclusions
Ovarian tissue cryopreservation and thawing impairs the viability of ovarian tissue in oncological patients opting for fertility preservation.
Keywords: Ovarian tissue, Fertility preservation, Cancer, Tissue damage, Follicles
Introduction
Ovarian tissue can be harvested for the purpose of fertility preservation and cryopreserved before the onset of gonadotoxic therapy. When a patient is cured from her (oncological) disease but has developed ovarian insufficiency, the tissue can be thawed and autotransplanted in an attempt to restore fertility. Thus far, at least 30 live births have been reported following cryopreservation and autotransplantation of ovarian tissue.[1] Various methods for ovarian tissue cryopreservation and thawing [2-9] are being used worldwide. Although the efficacy of cryopreservation of ovarian tissue may thus differ from one center to another, information regarding these differences is scarce. [10, 11] This is remarkable given the fact that the use of cryopreservation of ovarian tissue has increased considerably in the past decade [5, 12-22] with tissue from at least 2,500 patients now being stored at a limited number of highly experienced centers [23].
Obviously, pregnancies and live births [24, 25] as well as living follicles identified after xenotransplantation [26] indicate that there must be (partial) follicle survival after cryopreservation/thawing according to the protocols of experienced centers. Nevertheless, it remains unknown from such data whether there is any room to improve these frequently used protocols in order to obtain higher pregnancy rates. Furthermore, patients cannot be counseled about the technique’s success chances based on this information. Although there are studies that quantify the impact of slow-freezing on human ovarian tissue viability, these studies do not per definition focus on the protocols that are currently being used by the major centers. [27-38] Moreover, most of these studies did not take the viability of the stromal cell compartment into account. [27-38] This compartment, however, is essential for post-autotransplantation neovascularization, follicle survival, and life span of the ovarian graft [39] and is considered to be more sensitive to ischemic and cryoinjury than primordial follicles [8, 40].
The aim of our current study was to assess the efficacy of the cryopreservation and thawing protocols from one of the largest centers for ovarian tissue cryopreservation in the world, the Cryobank Bonn (Academic Hospital Bonn, Germany), with respect to the survival of follicles as well as cortical stromal tissue. By overnight transport, this Cryobank – where ovarian tissue is stored from more than 1,080 patients – receives tissue from hospitals throughout Germany. Orthotopic autotransplantation of ovarian tissue in 30 patients who had their tissue cryopreserved in Bonn has resulted in three live births and one additional pregnancy that has ended in a spontaneous abortion, providing proof of concept for the methods used at this facility.[2] In the current study we provide a frame of reference regarding the impact of cryopreservation-thawing on ovarian tissue viability and aim to facilitate future efforts to optimize cryopreservation and thawing methods that are currently being used worldwide.
Materials and methods
Study design, patients and study approval
Following informed consent and approval of the institutional ethics committee, up to 10 % of the ovarian tissue from each patient who had her tissue cryopreserved at the Cryobank Bonn before the start of gonadotoxic cancer treatment from January through November 2012 was available for this study. As we required twelve 3-mm cortex biopsies per patient for our experiments, only those patients for whom twelve biopsies required less than 10 % of the tissue were included in this prospective cohort study. For each patient, we investigated ovarian tissue viability directly after the tissue’s arrival at the Cryobank, as well as after cryopreservation and thawing using four measurements (Fig. 1). Subsequently, results obtained before and after cryopreservation were compared.
Fig. 1.
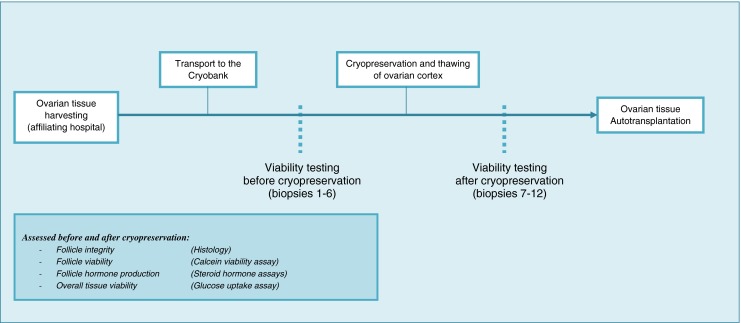
Study design. The chain of events preceding autotransplantation of ovarian tissue according to protocols of the Cryobank Bonn. In a prospective cohort study, the viability of follicular and stromal cell compartments of the ovarian cortex was assessed directly after the tissue’s arrival at the Cryobank as well as following cryopreservation and thawing for the same patients
Study procedures
Ovarian tissue harvesting, transport, and preparation
For each patient, (part of) one ovary was laparoscopically dissected at one of the Cryobank’s affiliating hospitals and transferred into a tube with cold Custodiol (Dr. Franz Köhler Chemie GmbH, Bensheim, Germany). This tube was placed in a transport box (DeltaT, Giessen, Germany) containing 6 cool packs (4 °C) and a temperature sensor for overnight road transport (±22 h) to the central facility at the Cryobank Bonn. Immediately after arrival, the medulla was removed and cortex strips (10x5x1 mm) were prepared for clinical purposes on a culture dish placed at a precooled surface (0–4 °C) using precision forceps and scalpels. Of the remaining cortical tissue, 12 biopsies were obtained for each patient using a 3-mm diameter biopsy punch (pfm medical ag, Cologne, Germany). Of these 3-mm biopsies, six were cryopreserved and stored in MVE Vapor phase storage tanks (MTG, Technology for life, Bruckberg, Germany) for a period varying from 24 h to 7 months and six were directly used as fresh control tissue.
Cryopreservation
The Cryobank’s slow-freezing protocol was modified from a procedure published by Gosden and Isachenko.[41, 42]. Nunc CryoTubes (Sigma-Aldrich, St. Louis, MO, USA) were filled with 1.7 ml Leibovitz’s L-15 GlutaMAX cryomedium (Gibco, Carisbad, CA, USA), 10 % CryoSure-DMSO (Wak-Chemie Medical GmbH, Steinbach, Germany), and 10 % serum substitute supplement (SSS; Irvine Scientific, Santa Ana, CA, USA) and precooled to 2 °C. After placing six ovarian biopsies in the CryoTubes, the tubes were cryopreserved in a programmed freezer (IceCube 14S-A, SY-LAB, Neupurkersdorf, Austria). CryoTubes were pre-incubated at 2 °C for 40 min, and then cooled at 2 °C/min until automatic seeding at−6 °C. After successful ice nucleation, CryoTubes were further cooled (0.3 °C/min up to−40 °C; 10 °C/min up to−140 °C) and stored at−150 °C in MVE Vapor phase storage tanks (MTG, Technology for life, Bruckberg, Germany).
Thawing
After removal from the liquid nitrogen, CryoTubes were kept at room temperature for 30 s and then placed in a 37 °C water bath for 2 min. Using a continuous dilution protocol modified from Isachenko, [43] ovarian cortex strips were transferred to a sterile container with 10 ml Dulbecco’s Phosphate Buffered Saline (DPBS; Gibco), 0.75 M sucrose (MP Biomedicals, Eschwege, Germany), 10 % Fetal Bovine Serum (FBS; PAA Laboratories GmbH, Cölbe, Germany), and 0.1 mg/ml Pen/Strep (Lonza, Basel, Schwitzerland). After incubation under continuous agitation (15 min), a solution of 50 ml DPBS/10 % FBS/0.1 mg/ml Pen/Strep was added with 100 ml/h using a perfusion device. Subsequently, the cortex strips were transferred to 5 ml pre-warmed (37 °C) HEPES-buffered culture medium (Gamete, Cook Medical Europe LTD, Limerick, Ireland) and incubated under continuous agitation for 15 min. This step was repeated once using fresh Gamete medium, after which the tissue was washed three times (5 min) in the tissue culture medium.
Outcome measures
Before and after cryopreservation and thawing (Fig. 1), the viability of the ovarian cortex was assessed using four parameters focused at follicle viability and integrity as well as the overall viability of the cortex, including the stromal tissue.
Follicle viability and integrity
Histology
To assess follicle integrity, one 3-mm cortex biopsy per patient was fixed in Bouin’s solution (Sigma-Aldrich), dehydrated and embedded in paraffin wax for the fresh and cropreserved-thawed situation. Eight-μm sections were stained with haematoxylin and eosin and evaluated using light microscopy. Follicles were categorized into morphologically normal or degenerated follicles at primordial, primary, secondary, pre-antral or antral stages according to criteria predefined by Gougeon et al.. [44] Follicles were considered to be degenerated when pyknotic nuclei, cytoplasm shrinkage, or disorganized granulosa cells were observed. [44] Two 8-μm sections, together covering 14 mm2, were evaluated and all visible follicles were scored.
Calcein viability assay
The viable follicle count was determined using a Calcein AM follicle viability staining. This staining was performed at day 0 (the day the fresh tissue arrived in Bonn or the day of tissue thawing) and at day 7 of tissue culture, as described in the next paragraph (“Steroid hormone production”). Calcein AM is a non-fluorescent cell-permeant compound that can be hydrolysed by intracellular esterases, producing the strongly fluorescent Calcein. One 3-mm cortex biopsy (7 mm3) was incubated in a 0.2 % Calcein AM (Promega, Mannheim, Germany)/DPBS solution supplemented with 1 mg/ml Collagenase type 1A (Sigma-Aldrich) at 37 °C in humidified air with 5 % CO2 for 2 h. During this incubation, the suspension was resuspended by pipetting every 30 min. The reaction was terminated by adding an equal volume of DPBS, after which all viable follicles that could be observed in the suspension using fluorescence microscopy at 495 nm were counted.
Steroid hormone production
To assess the follicles’ developmental potential, we measured in vitro produced estradiol (E2) and progesterone (P4) in a 7-day culture. Four cortex biopsies were cultured separately in a 24-well plate (TPP, Trasadingen, Switzerland) at 37 °C in humidified air with 5 % CO2. Each well contained 2 ml of Dulbecco’s Modified Eagle Medium (DMEM), High Glucose (4.5 g/L) supplemented with L-Glutamine (PAA Laboratories), 10 % FBS and 0.1 % Pen/Strep (GIBCO; 10.000 units penicillin/ml and 10.000 μg streptomycin/ml). No gonadotrophins were added to the culture medium. For day 0 measurements, unconditioned medium was collected approximately 4 h after warming in the cell culture incubator. Conditioned supernatant was collected at day 4, and replaced by 2 ml of fresh medium, which was collected at day 7. E2 and P4 concentrations of the day 0 (control), and day 4 and 7 conditioned medium samples were determined by established chemiluminescent-microparticle-immunoassays using an Architect i2000 system (Abbott Diagnostics, IL, USA).[45] The limits of sensitivity and interassay coefficients of variation for these assays were 10 pg/ml and <7.4 % for E2 and 0.1 ng/ml and <6.2 % for P4. The total amount of E2 and P4 (picograms) produced during the day 0–4 and day 4–7 culture periods was calculated per hour of culture for each series of four biopsies. The E2 and P4 production of each biopsy were corrected for the weight of the biopsy that had produced the hormones.
Overall tissue viability
Glucose uptake assay
The tissue’s overall viability was examined by measuring its glucose uptake from the culture medium (per mg tissue per hour) during the same 7-day culture as described above for the steroid hormone assays. As stromal cells cover large parts of the ovarian cortex, this assay, modified from Gerritse et al.,[46] is thought to give a good reflection of the viability of this cell type. Glucose concentrations in the culture medium (day 0, 4, 7) were measured using an Architect analyzer. At the end of the culture, biopsies were weighed (mg) and the amount of glucose consumed (nanomoles) per mg tissue per hour was calculated for day 0–4 and day 4–7 for each biopsy.
Although glucose uptake has earlier been correlated to the extent of tissue damage sustained by bovine ovarian cortex, [46] we first confirmed this in a control experiment for the human situation. In this experiment, series of four cryopreserved-thawed biopsies that were cultured for 7 days were exposed to either room temperature for one night, or 50 ºC for 20 min, or snap-freezing without using a cryoprotectant. In addition a control experiment using medium only as well as a control experiment using cryopreserved-thawed biopsies that were not exposed to damaging conditions, were performed.
Data-analysis
Data-analysis was performed using IBM SPSS Statistics version 21 (IBM corporation, New York, USA). For all tests, differences with a p-value of 0.05 or less were considered to be statistically significant. E2 and P4 production (per mg tissue per hour), glucose uptake (nmol per mg tissue per hour), and the viable follicle counts were compared before versus after cryopreservation and thawing using related-samples Wilcoxon Signed Rank tests. Data were presented as median and interquartile range (IQR). To address the effect of a patient’s age or preoperative AMH level on cryodamage, we performed ANCOVA analyses with the differences in glucose uptake, follicle viability count and steroid hormone production between the fresh versus the cryopreserved/thawed situation as dependent variable. ANCOVA analyses were also performed to assess the influence of the duration of cryopreservation, i.e. the length of the interval between the moment of cryopreservation and thawing.
Results
Patient characteristics
All 25 study participants (Table 1) had an indication for gonadotoxic cancer therapy and had their ovarian tissue cryopreserved for reasons of fertility preservation. None of the participants had received prior gonadotoxic treatment.
Table 1.
Patient characteristics, histology and follicle viability. Characteristics of patients at the time of ovarian tissue cryopreservation and results of the Calcein follicle viability staining and histology (‘-‘no follicles were observed in the histologically evaluated biopsy). N = patient number. HL = Hodgkin’s lymphoma. NHL = Non-Hodgkin’s lymphoma. Transport temp = Temperature measured in the transport box at arrival in Bonn (°C). (D) = Degenerated follicle as observed during histological examination. IQR = Interquartile range
| N | Age | Cancer type | AMH level (μg/L) | Transport Temp (ºC) | Viable follicle count (7 mm3 cortex tissue) | Histology (14 mm2 cortex tissue) | ||
|---|---|---|---|---|---|---|---|---|
| Before cryopreservation | After cryopreservation | Before cryopreservation | After cryopreservation | |||||
| 1 | 24 | Rectal | 1,6 | 5 | 65 | 62 | 2 primordial (1 D) | 1 primordial |
| 2 | 31 | Breast | 0,6 | 4,5 | 47 | 39 | 1 primordial | - |
| 3 | 29 | Cervix | no sample | 4 | 47 | 28 | - | 4 primordial |
| 4 | 21 | HL | no sample | 4 | 173 | 291 | 11 primordial, 4 primary, 6 secondary | 1 primordial (D), 8 primary, |
| 5 | 33 | HL | 1,9 | 6 | 102 | 113 | 7 primordial (1 D) | 2 primordial (D), 1 primary (D) |
| 6 | 39 | Breast | 1,6 | 4 | 32 | 24 | 5 primordial (2 D) | - |
| 7 | 21 | HL | 2,9 | 6,5 | 248 | 310 | 31 primordial, 2 primary | - |
| 8 | 20 | HL | 3,6 | 5,5 | 231 | 183 | 48 primordial, 4 primary | - |
| 9 | 27 | HL | 2,1 | 5,5 | 128 | 133 | - | - |
| 10 | 23 | Chondrosarcoma | 1,8 | 5,5 | 128 | 46 | - | - |
| 11 | 33 | Breast | 1,8 | 4,5 | 93 | 135 | 6 primordial, 6 primary, 1 secondary | 18 primordial, 1 primary |
| 12 | 26 | Cervix | no sample | 6,5 | 74 | 24 | - | - |
| 13 | 29 | Breast | 2,1 | 4,5 | 83 | 220 | 3 primordial | 3 primordial, 1 antral |
| 14 | 21 | NHL | 1,1 | 5 | 291 | 91 | 19 primordial, 5 primary | - |
| 15 | 35 | Breast | 0,6 | 7 | 38 | 40 | − | 1 secondary |
| 16 | 31 | Breast | no sample | 6 | 286 | 350 | − | − |
| 17 | 21 | HL | 2,5 | 5,5 | 400 | 233 | − | − |
| 18 | 31 | Breast | 1,4 | 2 | 53 | 160 | − | − |
| 19 | 24 | HL | 1,8 | 6,5 | 129 | 150 | − | 5 primordial (2 D) |
| 20 | 36 | Breast | 1,9 | 10 | 18 | 27 | − | − |
| 21 | 20 | B-cell lymphoma | 2,5 | 5,5 | 180 | 200 | − | − |
| 22 | 35 | Breast | 1,9 | 5,5 | 10 | 13 | 1 primordial (D) | − |
| 23 | 31 | Breast | 0,8 | 5,0 | 34 | 46 | − | − |
| 24 | 34 | Breast | 2,9 | 5,5 | 56 | 76 | − | 2 primordial (1 D) |
| 25 | 18 | Breast | 2,1 | 6,0 | 258 | 93 | − | 9 primordial (6 D) |
| Median (IQR) | Total | |||||||
| 29 (21–33) | 5.5 (4.5–6.0) | 93 (47–206) | 93 (40–192) | 162 follicles of which 5 degenerated (97% intact) primordial follicles: 112 (5 degenerated; 96% intact) |
57 follicles of which 13 degenerated (77% intact) primordial follicles: 45 (12 degenerated; 73% intact) |
|||
Follicle viability and integrity
Histology and Calcein viability assay
When looking at the entire study population, a larger percentage of degenerated (primordial) follicles was observed in the cryopreserved-thawed tissue when compared to the fresh controls (Table 1). Most follicles were in the primordial or primary stage, although a small proportion of follicles in more advanced stages were observed. A relatively large number of histological sections suffered from a low number, or even a complete absence of follicles, presumably due to the fact that we only used a single 3-mm biopsy because of a paucity of cortical tissue available for research purposes. After proteolytic digestion of biopsies for the Calcein viability assay, more follicles could be evaluated. With this assay, a similar number of viable follicles was observed before (median: 93; IQR 47–206) and after (median: 93; IQR 40–192) cryopreservation (p = 0.647). At culture day 7, similar follicle counts were obtained when compared with day 0. In the fresh tissue, a median number of 85 follicles (IQR 39–165) was observed, whereas a median number of 91 follicles (IQR 29–156) was observed after cryopreservation and thawing (p = 0.747). For some individual patients, however, remarkable differences in follicle counts before and after cryopreservation were found (Table 1). Furthermore, cortical pieces were heterogeneous with respect to follicle density as some patients had fewer and others had considerably more follicles in cryopreserved/thawed tissue when compared to fresh.
Steroid hormone production
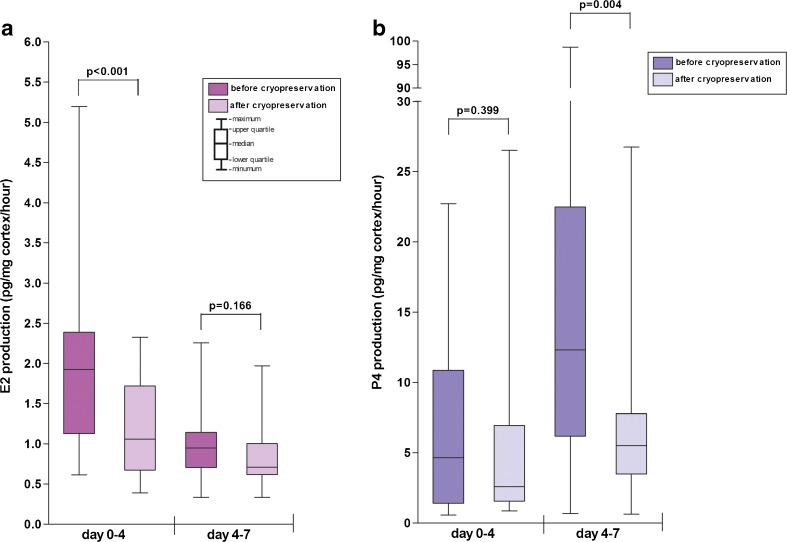
In the unconditioned medium, before starting the cultures, E2 and P4 were below detection level. No significant difference was observed in the weight of biopsies that were cultured before versus after cryopreservation (data not shown). During the first four days of culture, the median E2 production was reduced with 45 % after cryopreserved-thawed biopsies when compared to the fresh biopsies after correction for biopsy weight (p < 0.001; fig. 2a). With prolonged culture, the E2 production in both cultures diminished. There was no statistically significant difference in the median E2 production at culture day 7 (p = 0.166; fig. 2a). The production of P4 increased during the cultures, with a significantly higher P4 production at the end of the culture in the fresh biopsies when compared to the cultures of cryopreserved-thawed biopsies. After correction for biopsy weight (fig. 2b), the level of P4 before cryopreservation was similar to the level after cryopreservation and thawing during day 0–4 (p = 0.339). For day 4–7 a median reduction of the tissue’s P4 production of 55 % (p = 0.004) was observed.
Fig. 2.
Steroid hormone production by ovarian cortex. Boxplots displaying the ovarian tissue’s E2 and P4 production before and after cryopreservation and thawing. Data are either presented per mg cortical tissue (figure a and c) or per follicle (figure b and d)
Overall tissue viability
Our control experiments revealed that the glucose uptake assay has the capacity to indicate various extents of tissue damage in human ovarian tissue. Four cryopreserved-thawed biopsies that were cultured for 7 days had a mean glucose uptake of 16.9 nmol glucose per milligram tissue per hour (SD 5.2), biopsies that were exposed either to room temperature for one night, or 50 ºC for 20 min, or snap-frozen without using a cryoprotectant had a glucose uptake of 2.9 nmol/mg/h (SD 2.1), 15.8 nmol/mg/h (SD 5.1) and a non-detectable glucose uptake respectively. Medium only showed stable glucose concentrations during the incubation period (day 0: mean: 23.2 mmol/L, SD 0.5; day 4: 24.5 mmol/L, SD 0.7; day 7: 24.3 mmol/L; SD 0.2).
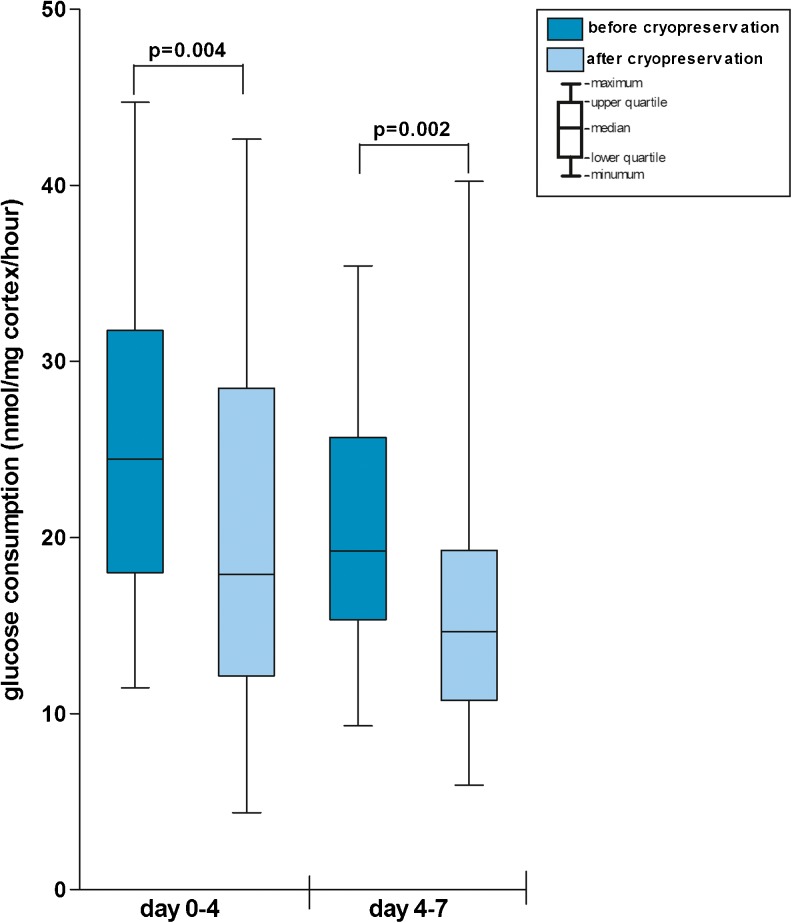
At day 0, a median glucose concentration in the medium of 23.5 nmol/L was observed (range 21.9–25.0 nmol/L). Cryopreserved and thawed ovarian tissue from the patients in our study cohort showed a lower glucose uptake than tissue that had not been cryopreserved, indicating a reduced viability of the stromal cell compartment (Fig. 3 ). During the first culture period (day 0–4) a reduction in the median glucose uptake per mg per hour of 27 % was observed after cryopreservation and thawing when compared to the tissue that had not been cryopreserved (p = 0.004). For the second culture period (day 4–7) this reduction in median glucose uptake was 24 % (p = 0.002).
Fig. 3.
Glucose uptake by ovarian cortex. Boxplot displaying the ovarian tissue’s glucose uptake before and after cryopreservation and thawing per milligram tissue per hour of culture
Effects of age, AMH, and duration of cryopreservation on cryodamage
A higher age and lower AMH-level were statistically significantly associated with a lower number of viable follicles observed with the Calcein AM viability assay for fresh as well as cryopreserved/thawed tissue. Despite this, the effect of cryopreservation and thawing on the tissue and follicle viability was comparable for younger and older patients. The differences in the glucose uptake, steroid hormone production, and number of viable follicles measured for the fresh versus cryopreserved/thawed tissue were not statistically significantly influenced by age, AMH, or the length of the interval between cryopreservation and thawing (data not shown).
Discussion
This prospective cohort study indicates that the viability of young oncological patients’ ovarian tissue is impaired as a result of the cryopreservation and thawing process when using protocols from the Cryobank Bonn. Although various large centers for cryopreservation of ovarian tissue exist, [12, 13, 15, 17, 18, 21, 24] to the best of our knowledge, cohort studies in which the impact of currently used cryopreservation/thawing protocols on both follicle and stromal cell survival in young cancer patients is measured have not been published before. Earlier studies considering the impact of slow freezing techniques other than evaluated here, generally focused on follicle viability only [27-38] and often described patient populations without an indication for fertility preservation (e.g. patients applying for a sterilization, cystectomy, or caesarean section) [8, 27-29, 32, 34, 35, 38].
Although we observed a similar number of viable follicles with intracellular enzyme activity (Calcein viability assay) before and after cryopreservation/thawing in this cohort of 25 young cancer patients, ovarian tissue damage after cryopreservation was indicated by a decreased percentage of normal, intact follicles of 77 % after versus 97 % before cryopreservation (histology)). The high percentage of normal follicles in the fresh tissue suggests that the transport of ovarian tissue did not have a deleterious effect on the follicles. The observed percentages of tissue viability impairment as a result of cryopreservation and thawing are roughly consistent with the 70–80 % follicle survival reported earlier for various slow-freezing protocols. [8, 27, 29, 30, 35, 38, 39] In addition, we observed a 24–27 % reduction in overall tissue viability using the glucose uptake assay. This decreased glucose uptake indicates a reduced metabolism of the tissue during culture as a result of cryodamage. As the ovarian cortex tissue mainly exists of stromal cells, the glucose uptake assay is thought to predominantly reflect the viability of this compartment. However, the decline in the median glucose uptake per milligram tissue per hour observed in our study (24–27 %) was considerably higher than the increase in apoptosis in stromal cells (11 %) observed in a single prior study analyzing ovarian tissue from women with benign cysts. [8] It is known from situations of trauma, hemorrhagic shock, organ transplantation, and autoimmune diseases that damage sustained by ischemic insult may lead to so-called ischemia/reperfusion-associated tissue damage as soon as this tissue is neovascularized. [47] After neovascularization, the ischemic tissue activates the immune system that may lead to further tissue injury. [47-49] For this reason it is essential that during optimization of cryopreservation/thawing protocols not only the viability of the follicles should be taken into account, but also the viability of the stromal cell compartment.
Various techniques to examine ovarian tissue viability have been reported in the literature, [8, 28, 34, 50-53] but a golden standard does not exist. With respect to the result from our current study, one should bear in mind that only small biopsies were available for histology and the Calcein assay, meaning that some sample bias with respect to the density of follicles in the tissue did occurr. Furthermore, the numbers of follicles that could be histologically evaluated was limited. Although steroid hormone production has earlier been associated with ovarian tissue damage. [30, 37, 54-56], The clinical relevance of measuring steroid hormone production is severely restricted. Namely, as developing follicles produce larger amounts of steroid hormones than primordial and primary follicles, [57] whereas one specifically aims to preserve the more numerous small follicles that have the ability to mature after autotransplantation. From steroid hormone measurements in vitro, it remains unknown which part of the reduction in steroid hormone production after cryopreservation (varying from 11 to 55 %) could be attributed to the expected loss of antral follicles during cryopreservation, and which part reflects the loss of small follicles. E2 levels are expected to rise as a result of granulosa cell proliferation, whereas P4 is produced after luteinization of these cells in a more advanced stage of folliculogenesis.[57, 58] Possibly, this was the reason why P4 levels rose in the second culture period, after the fourth day of culture. As alternative methods to assess the viability of ovarian tissue, measuring the tissue’s uptake of bromodeoxyridine during culture has been proposed.[59] To assess follicle viability, follicle isolation and 3D culture with evaluation of the follicle structures and diameter have been suggested [59].
In conclusion, the current study indicates that cryopreservation/thawing of ovarian tissue from cancer patients following protocols from one of the largest centers for ovarian tissue cryopreservation in the world (Cryobank Bonn), significantly impairs the viability of the tissue. Although these findings cannot be extrapolated to other protocols, various other clinics have comparable freezing protocols to the DMSO-based protocol that was evaluated in the current paper.[60, 6, 13, 61] Nevertheless, ethylene glycol is also used as a cryoprotectant in a major center reporting live births.[26] With respect to thawing, a variety of protocols has been reported. [61, 60, 26] Consistently with a small number of earlier studies, the current study showed a reduction in stromal cell viability as a result of cryopreservation and thawing. For this reason, it is advisable to – apart from assessing follicle viability – include measurements on stromal cell viability in future studies evaluating cryopreservation and thawing procedures. To assess follicle survival after cryopreservation and thawing, a viability assay and histology are considered useful, whereas the relevance of steroid hormone assays is restricted. By optimizing cryopreservation as well as thawing procedures, the viability of ovarian tissue after cryopreservation and thawing - and conceivably the clinical outcome - may be improved in the near future. To investigate whether cryodamage results from cryopreservation and/or thawing, the viability of ovarian tissue from the same patients should ideally be investigated after various combinations of cryopreservation and thawing protocols. This information is especially relevant as tissue has been cryopreserved for a large number of patients who may consider thawing and autotransplantation of their tissue in the future.[23] Furthermore, the effects of ovarian tissue transport on the tissue’s condition, which could not be measured in the current study, should be further investigated. Since fertility preservation by means of cryopreservation of ovarian tissue is no longer considered experimental by some [24] and is applied on an increasing scale, efforts to optimize the laboratory phases have become imperative. The data presented in the current study can be used as a frame of reference for the evaluation and modification of currently used protocols.
Acknowledgments
Unconditional funding was received from the Nijmegen Centre of Evidence Based Practice (NCEBP), Oranjestipendium foundation, and Merck Serono.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule Efficacy of ovarian tissue cryopreservation.
The first two authors should be regarded as joint first authors.
References
- 1.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–49. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 2.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97(2):387–90. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 4.Gracia CR, Chang J, Kondapalli L, Prewitt M, Carlson CA, Mattei P, et al. Ovarian tissue cryopreservation for fertility preservation in cancer patients: successful establishment and feasibility of a multidisciplinary collaboration. J Assist Reprod Genet. 2012;29(6):495–502. doi: 10.1007/s10815-012-9753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93(3):762–8. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. doi: 10.1016/S0140-6736(11)61781-9. [DOI] [PubMed] [Google Scholar]
- 7.Radford JA, Lieberman BA, Brison DR, Smith AR, Critchlow JD, Russell SA, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin’s lymphoma. Lancet. 2001;357(9263):1172–5. doi: 10.1016/S0140-6736(00)04335-X. [DOI] [PubMed] [Google Scholar]
- 8.Sanfilippo S, Canis M, Romero S, Sion B, Dechelotte P, Pouly JL, et al. Quality and functionality of human ovarian tissue after cryopreservation using an original slow freezing procedure. J Assist Reprod Genet. 2013;30(1):25–34. doi: 10.1007/s10815-012-9917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt KL, Ernst E, Byskov AG, Nyboe Andersen A, Yding Andersen C. Survival of primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod. 2003;18(12):2654–9. doi: 10.1093/humrep/deg500. [DOI] [PubMed] [Google Scholar]
- 10.Kolp LA, Hubayter Z. Autotransplantation of cryopreserved ovarian tissue: a procedure with promise, risks, and a need for a registry. Fertil Steril. 2011;95(6):1879–86. doi: 10.1016/j.fertnstert.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Bedaiwy MA, El-Nashar SA, El Saman AM, Evers JL, Sandadi S, Desai N, et al. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod. 2008;23(12):2709–17. doi: 10.1093/humrep/den301. [DOI] [PubMed] [Google Scholar]
- 12.Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22(2):162–71. doi: 10.1016/j.rbmo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez M, Novella-Maestre E, Teruel J, Ortiz E, Pellicer A. The Valencia Programme for Fertility Preservation. Clin Transl Oncol. 2008;10(7):433–8. doi: 10.1007/s12094-008-0227-4. [DOI] [PubMed] [Google Scholar]
- 14.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–5. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16(6):617–30. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 16.Feigin E, Abir R, Fisch B, Kravarusic D, Steinberg R, Nitke S, et al. Laparoscopic ovarian tissue preservation in young patients at risk for ovarian failure as a result of chemotherapy/irradiation for primary malignancy. J Pediatr Surg. 2007;42(5):862–4. doi: 10.1016/j.jpedsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Poirot CJ, Martelli H, Genestie C, Golmard JL, Valteau-Couanet D, Helardot P, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49(1):74–8. doi: 10.1002/pbc.21027. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reprod (Cambridge, England) 2008;136(6):681–9. doi: 10.1530/REP-08-0097. [DOI] [PubMed] [Google Scholar]
- 19.Revel A, Revel-Vilk S, Aizenman E, Porat-Katz A, Safran A, Ben-Meir A, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92(2):458–63. doi: 10.1016/j.fertnstert.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Borgstrom B, Hreinsson J, Rasmussen C, Sheikhi M, Fried G, Keros V, et al. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab. 2009;94(1):74–80. doi: 10.1210/jc.2008-0708. [DOI] [PubMed] [Google Scholar]
- 21.Meirow D, Baum M, Yaron R, Levron J, Hardan I, Schiff E, et al. Ovarian tissue cryopreservation in hematologic malignancy: ten years’ experience. Leuk Lymphoma. 2007;48(8):1569–76. doi: 10.1080/10428190701471957. [DOI] [PubMed] [Google Scholar]
- 22.Lawrenz B, Jauckus J, Kupka MS, Strowitzki T, von Wolff M. Fertility preservation in >1,000 patients: patient’s characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet. 2011;283(3):651–6. doi: 10.1007/s00404-010-1772-y. [DOI] [PubMed] [Google Scholar]
- 23.Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update. 2013;19(5):483–506. doi: 10.1093/humupd/dmt020. [DOI] [PubMed] [Google Scholar]
- 24.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Callejo J, Salvador C, Gonzalez-Nunez S, Almeida L, Rodriguez L, Marques L, et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 2013;6(1):33. doi: 10.1186/1757-2215-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt KL, Yding Andersen C, Starup J, Loft A, Byskov AG, Nyboe AA. Orthotopic autotransplantation of cryopreserved ovarian tissue to a woman cured of cancer - follicular growth, steroid production and oocyte retrieval. Reprod Biomed Online. 2004;8(4):448–53. doi: 10.1016/S1472-6483(10)60929-7. [DOI] [PubMed] [Google Scholar]
- 27.Campos JR, Rosa-e-Silva JC, Carvalho BR, Vireque AA, Silva-de-Sa MF, Rosa-e-Silva AC. Cryopreservation time does not decrease follicular viability in ovarian tissue frozen for fertility preservation. Clinics (Sao Paulo) 2011;66(12):2093–7. doi: 10.1590/S1807-59322011001200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod. 2010;25(10):2559–68. doi: 10.1093/humrep/deq192. [DOI] [PubMed] [Google Scholar]
- 29.Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod. 2003;18(11):2420–8. doi: 10.1093/humrep/deg439. [DOI] [PubMed] [Google Scholar]
- 30.Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reprod (Cambridge, England) 2009;138(2):319–27. doi: 10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- 31.Maltaris T, Dragonas C, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Simple prediction of the survival of follicles in cryopreserved human ovarian tissue. J Reprod Dev. 2006;52(4):577–82. doi: 10.1262/jrd.18012. [DOI] [PubMed] [Google Scholar]
- 32.Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67(3):481–6. doi: 10.1016/S0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 33.Rimon E, Cohen T, Dantes A, Hirsh L, Amit A, Lessing JB, et al. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol. 2005;27(2):345–53. doi: 10.3892/ijo.27.2.345. [DOI] [PubMed] [Google Scholar]
- 34.Sanfilippo S, Canis M, Ouchchane L, Botchorishvili R, Artonne C, Janny L, et al. Viability assessment of fresh and frozen/thawed isolated human follicles: reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1) J Assist Reprod Genet. 2011;28(12):1151–6. doi: 10.1007/s10815-011-9649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Dechelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20(7):1786–92. doi: 10.1093/humrep/dei002. [DOI] [PubMed] [Google Scholar]
- 36.Fabbri R, Pasquinelli G, Keane D, Magnani V, Paradisi R, Venturoli S. Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reprod Biomed Online. 2010;21(6):819–28. doi: 10.1016/j.rbmo.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Isachenko V, Isachenko E, Reinsberg J, Montag M, Braun F, van der Ven H. Cryopreservation of human ovarian tissue: effect of spontaneous and initiated ice formation. Reprod Biomed Online. 2008;16(3):336–45. doi: 10.1016/S1472-6483(10)60593-7. [DOI] [PubMed] [Google Scholar]
- 38.Marsella T, Sena P, Xella S, La Marca A, Giulini S, De Pol A, et al. Human ovarian tissue cryopreservation: effect of sucrose concentration on morphological features after thawing. Reprod Biomed Online. 2008;16(2):257–67. doi: 10.1016/S1472-6483(10)60583-4. [DOI] [PubMed] [Google Scholar]
- 39.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–65. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82(3):679–85. doi: 10.1016/j.fertnstert.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196° C. Hum Reprod. 1994;9(4):597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 42.Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007;55(3):261–8. doi: 10.1016/j.cryobiol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Isachenko V, Montag M, Isachenko E, van der Ven K, Dorn C, Roesing B, et al. Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reprod Biomed Online. 2006;13(2):228–34. doi: 10.1016/S1472-6483(10)60620-7. [DOI] [PubMed] [Google Scholar]
- 44.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–7. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 45.Liebenthron J, Koster M, Drengner C, Reinsberg J, van der Ven H, Montag M. The impact of culture conditions on early follicle recruitment and growth from human ovarian cortex biopsies in vitro. Fertil Steril. 2013;100(2):483–91. doi: 10.1016/j.fertnstert.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 46.Gerritse R, Beerendonk CC, Westphal JR, Bastings L, Braat DD, Peek R. Glucose/lactate metabolism of cryopreserved intact bovine ovaries as a novel quantitative marker to assess tissue cryodamage. Reprod Biomed Online. 2011;23(6):755–64. doi: 10.1016/j.rbmo.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141(1):3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130(1):41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev. 2012;23(3):69–84. doi: 10.1016/j.cytogfr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98(5):1291–8. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 51.Fabbri R, Venturoli S, D’Errico A, Iannascoli C, Gabusi E, Valeri B, et al. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89(2):259–66. doi: 10.1016/S0090-8258(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 52.Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87(5):1200–7. doi: 10.1016/j.fertnstert.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 53.Milenkovic M, Diaz-Garcia C, Wallin A, Brannstrom M. Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification. Fertil Steril. 2012;97(5):1176–82. doi: 10.1016/j.fertnstert.2012.01.123. [DOI] [PubMed] [Google Scholar]
- 54.Isachenko E, Isachenko V, Nawroth F, Rahimi G, Weiss JM. Effect of long-term exposure at suprazero temperatures on activity and viability of human ovarian cortex. Fertil Steril. 2009;91(4 Suppl):1556–9. doi: 10.1016/j.fertnstert.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 55.Bridges PJ, Brusie MA, Fortune JE. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest Anim Endocrinol. 2005;29(3):508–22. doi: 10.1016/j.domaniend.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 56.Gerritse R, Peek R, Sweep F, Thomas C, Braat D, Kremer J, et al. In vitro 17β-oestradiol release as a marker for follicular survival in cryopreserved intact bovine ovaries. Cryo Letters. 2010;31(4):318–28. [PubMed] [Google Scholar]
- 57.Fritz MA, Speroff L. Current concepts of the endocrine characteristics of normal menstrual function: the key to diagnosis and management of menstrual disorders. Clin Obstet Gynecol. 1983;26(3):647–89. doi: 10.1097/00003081-198309000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Lund SA, Murdoch J, Van Kirk EA, Murdoch WJ. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: relevance to luteal function. Biol Reprod. 1999;61(2):388–92. doi: 10.1095/biolreprod61.2.388. [DOI] [PubMed] [Google Scholar]
- 59.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;28(5):1267–79. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril. 2007;87(2):418 e7- e15. doi: 10.1016/j.fertnstert.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 61.Luyckx V, Scalercio S, Jadoul P, Amorim CA, Soares M, Donnez J, et al. Evaluation of cryopreserved ovarian tissue from prepubertal patients after long-term xenografting and exogenous stimulation. Fertil Steril. 2013;100(5):1350–7. doi: 10.1016/j.fertnstert.2013.07.202. [DOI] [PubMed] [Google Scholar]