Abstract
AIM: To establish a visceral pain model via colorectal distension (CRD) and to evaluate the efficiency of behavioral responses of CRD by measuring the score of abdominal withdrawal reflex (AWR) in rats.
METHODS: Thirty-eight male SD rats weighing 180-240g were used to establish the visceral pain model. The rat was inserted intra-anally with a 7 cm long flexible latex balloon under ether anesthesia, and colorectal distensions by inflating the balloon with air were made 30 min after recovering from the anesthesia. Five AWR scores (AWR0 to AWR4) were used to assess the intensity of noxious visceral stimuli. It was regarded as the threshold of the minimal pressure (kPa) for abdominal flatting was induced by colorectal distension.
RESULTS: A vigorous AWR to distension of the descending colon and rectum was found in 100% of the awake rats tested. The higher the pressure of distension, the higher the score of AWR. The distension pressures of 0, 2.00, 3.33, 5.33 and 8.00 kPa produced different AWR scores (P < 0.05). The pain threshold of AWR was constant for up to 80 min after the initial windup (first 1-3 distensions), the mean threshold was 3.69 ± 0.35 kPa. Systemic administration of morphine sulfate elevated the threshold of visceral pain in a dose-dependent and naloxone reversible manner.
CONCLUSION: Scoring the AWR during colorectal distensions can assess the intensity of noxious visceral stimulus. Flatting of abdomen (AWR 3) to CRD as the visceral pain threshold is clear, constant and reliable. This pain model and its behavioral assessment are good for research on visceral pain and analgesics.
Keywords: Visceral pain, Colorectal distension, Rat behavior
INTRODUCTION
Visceral pain is produced by diseases[1]. It has not yet been studied more than the somatic pain due primarily to the lack of a reliable, stable model of visceral pain. The mechanisms of acute and chronic visceral pain have been extensively studied since the reports from Ness et al in 1998[2-4]. But implantation of the electrode in the abdominal muscle to record the electric muscle graph is complicated, and disturbs the movement of the awake experimental animals and increases the somatic hypersensitivity[5]. The practicability of the model is therefore limited. Al-Chaer et al[5] have established the abdominal withdrawal reflex (AWR) scores in a model of chronic visceral hypersensitivity in rats. We used AWR scores of the acute colorectal distension to establish a visceral pain model in rats. The minimal pressure for flatting of abdomen induced by CRD was regarded as the threshold for the assessment of the reliability and stability of the model and its behavioral reaction.
MATERIALS AND METHODS
Experiment animals
Male Sprague-Dawley (depurator grade) rats (n = 38) weighing 180-240g were obtained from the Experiment Animal Center of Soochow University.
Establishment of CRD
The colorectal distension balloon was made from a latex glove finger attached to an 8# rubber urethral catheter (7 cm in length and 2.5 cm in diameter).
Rats were randomly grouped for the experiment. Rats were deprived of food but had free access to water 24 h before the experiment. The prepared colorectal distension balloon covered with lubricant was totally inserted into the descending colon and rectum under ether anesthesia. Rats were put into a 20 cm×15 cm×15 cm glass box for the experiments 30 min after they were fully recovered from the anesthesia. The tube of balloon was connected via a Y connector to a air pump and a sphygmomanometer. The balloon that was inserted into the rat coloratura was inflated with air at a speed of 0.133 kPa/s(1 kPa = 7.5 mmHg), and the pressure inside the balloon was continuously monitored. The distension duration and intervals between two distensions were selected.
Behavioral study
Five abdominal withdrawal reflex (AWR) scores (AWR0 to AWR4) were used to assess the intensity of noxious visceral stimuli: AWR0: remarkable behavior changes; AWR1: immobility of the rat body or occasionally clinches of the head; AWR2: mild abdominal muscle contraction; AWR3: lifting the abdomen off the box platform or flatting of abdomen; AWR4: body arching or lifting pelvic structures off the platform[5]. The cut-off pressure to avoid the rectum injury was set at 13.33 kPa.
The pain threshold was defined as the minimal pressure (kPa) inside the balloon when the rat showed flatting of abdomen (AWR 3) during the colorectal distension (CRD).
Pharmacology study
To observe the effect of morphine on the abdominal withdrawal reflex, 24 rats were divided into 4 groups (n = 6 in each group and giving morphine s.c. 1 mg/kg, 2 mg/kg, 4 mg/kg and 8 mg/kg, respectively). Each received colorectal distension once per 5 min. The mean score of the 4th and 5th distension was regarded as the basic value. The pain thresholds at 10, 15, 20 min after morphine administration were recorded. The maximal possible effect (MPE) was used to compare the effect of morphine[6]. Percent maximal possible effect (%MPE) = (post drug value - baseline value)/(cutoff value - baseline value) × 100%[7].
Statistical analysis
Data were presented as mean ± SD. The statistical significance tested by analysis of variance (P < 0.05) and post hoc. LSD (with comparable variance) or Tamhane’s T2 (with incomparable variance) method was selected. All the analyses were performed using SPSS 10.0 software.
RESULTS
Stepping increased pressure of colorectal distension
The scores of abdominal withdrawal reflex were recorded in 8 rats receiving the colorectal distension every 4 min with stepping increased pressures of 0, 2.00, 3.33, 5.33, 8.00 kPa respectively and lasted for 30 s. The mean value of 3 times of abdominal withdrawal reflex was adopted. All the rats showed the abdominal withdrawal reflex to the colorectal distension (Figure 1). The higher the pressure, the higher scores of the abdominal withdrawal reflex. There were statistically significant differences among the five pressure grades.
Figure 1.
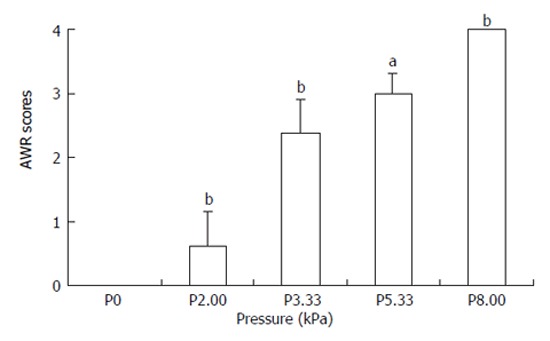
AWR scores under stepping increased pressure of colorectal distension aP < 0.05 , bP < 0.01 vs the basal pressure.
Repeated observation of pain thresholds after CRD at different times
The pain threshold was measured in 6 rats receiving a colorectal distension per 4 min. The results showed (Figure 2) that the threshold was higher at the first distention and then declined. It became stable after 3 trials. The mean pain threshold was 3.69 ± 0.35 kPa. The pain thresholds were measured on d 5 and 10 after the procedure in the same rats, and the mean thresholds were 3.59 ± 0.22 kPa and 3.63 ± 0.31 kPa with no significant difference among them.
Figure 2.
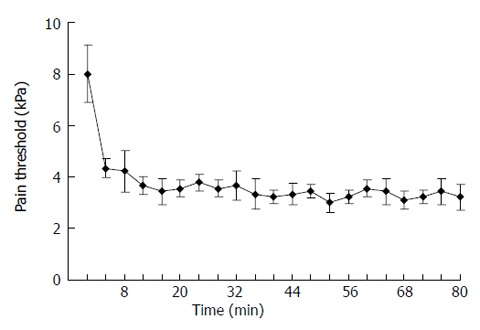
Repeated observations of pain thresholds after CRD at different times. The threshold was higher at the first distention, and then declined. It became stable after 3 trials. The mean pain threshold was 3.69 ± 0.35 kPa.
Effect of mophine-naloxone on AWR to CRD
Subcutaneous administration of morphine increased the pain threshold in a dose-dependent manner. The pain thresholds of the rats that received respectively, 2 mg/kg, 4 mg/kg, 8 mg/kg of morphine were significantly higher compared to the basal values (P < 0.01). The duration of antinociceptive effect produced by 8 mg/kg morphine lasted for more than 2.5 h (150.45 ± 22.50 min, Figure 3). Naloxone (0.5 mg/kg, i.p.) 20 min after 4 mg/kg morphine administration increased the movement of rats and the analgesic effect of morphine was totally antagonized. Five min after administration of naloxone, the pain threshold decreased to the basal level.
Figure 3.
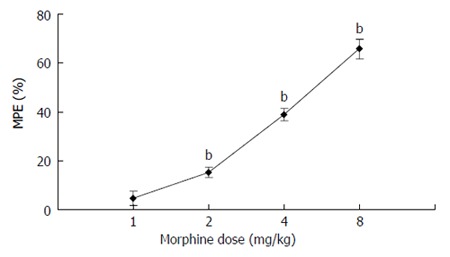
Effect of morphine on AWR to CRD. The pain thresholds of the rats that received 2 mg/kg, 4 mg/kg, 8 mg/kg of morphine respectively were significantly higher than the basal values (P < 0.01). bP < 0.01 vs the basal values.
Anatomic observation
After the distension experiment, rats (n = 6) with the balloon remained in the coloratura and the pressure inside the balloon kept at 8.00 kPa (AWR 4) were anesthetized with chloral hydrate. Shaped like column, the balloon occupying the whole descending colon and rectum was found in the left hypochondria region after opening the abdomen. The distended balloon was 8.94±1.20 cm in length and 1.20 ± 0.21 cm in diameter. Inflating the balloon at a speed of 0.133 kPa per second, the pressure causing rupture of the colon was 19.80 ± 1.38 kPa. Although the length of the whole colon in rats was 16.90 ± 2.52 cm, the site of the rupture was always in the descending colon.
DISCUSSION
Visceral noxious stimulus similar to the somatic noxious stimulus contributes to the false effective responses in animals, including agony behavior, bray, and response of the circulation and respiration system, muscle contraction, escaping, etc. The ideal visceral noxious stimulus with less effect on surrounding tissues should be controllable and repeatable[8,9]. The chemical[10], electrical stimuli[11]on the visceral afferent fibers are the most commonly used methods in research of visceral pain, but the responses to chemical or electrical stimuli are instable and nonspecific[2].
The quantified EMG of abdominal muscles[2], cardiovascular response[2], latency of step-down[12] and abdominal musculature contractions[6] have been adopted for assessment to the stimulus and response intensity, yet these indexes are easily interfered with multi-factor. Behaviors such as immobility of rat body, anus distension, abdominal muscle contraction, back arching, testis lifting, hind leg spreading with gradually increasing the pressure in colorectalregion have been found in rats. If the pressure is persistent such behaviors can last for a longer time. Therefore, the responses to colorectal distension meet the nociceptive pain standards in animal experiment. CRD seems to mimic the pathological mechanisms of pain in humans[9].
The abdominal withdrawal reflex (AWR) is a semi-quantitative, autonomic motor reflex that is similar to visceromotor response (VMR). The reflex arc of abdominal withdrawal reflex involves the supraspinal mechanisms. The scoring of AWR is based on the enhancement of the abdominal muscle contraction, which is free of the direct abdominal muscle stimulation by the electrodes, so the somatic hypersensitivity during the study of visceral pain is eliminated[5]. In the present study, all the rats showed the abdominal withdrawal reflex to the colorectal distention, suggesting that the AWR scores reflect the visceral pain to some extent.
Since the observation of rat body or head movement (AWR 1) and the initiation of abdominal muscle constriction (AWR 2) are difficult, we consider that AWR 3 (lifting the abdomen off the box platform or flatting of abdomen) and AWR4 are more reliable responses of the animals to the visceral pain. The animal’s performance of flatting of abdomen (AWR 3) is most clear and exact in response to CRD. If the distention pressure in the balloon does not change, the performance of flatting of abdomen is stable within a few minutes and no significant variations in pain threshold among individuals are noticed. Therefore, the minimal pressure (mmHg) inside the balloon is the most eligible index of the pain threshold when the rat shows flatting of abdomen(AWR 3)during CRD.
In this study, morphine (sc) inhibited the responses to CRD in a dose-dependent manner and this inhibitory effect of morphine could be antagonized by naloxone, demonstrating the stability and reliability of the visceral pain model produced by CRD in rats.
Ness and Gebhart[2] reported that the VMR threshold is 22.4 ± 0.9 mmHg(2.99 ± 0.12 kPa), which is similar to the values of the AWR 2 in the present study. The pain threshold in our study was 3.69 ± 0.35 mmHg (AWR3), which was much lower than that reported by A1-Chaer
et al[5] [53.5 ± 2.8 mmHg(7.13 ± 0.37 kPa)]. This difference may be due to the different species of the animals and the length of the balloon used. The relationship between the length of balloon and the threshold of distention pressure suggests a possible mechanism of “spatial summation” in visceral pain[9].
In the present study, the visceral pain threshold at the first distention was higher, which might be attributed to the “windup” effect induced by the initial noxious stimuli on visceral fibers[2]. We found that the threshold after the initial 2-3 trials became more stable in the actual visceral pain experiment. It is better to wait 1 or 2 min before the next CRD is given. The feces in the coloratura interfered with the insertion of the balloon and affected the pressure in the colorectum, thus the animals should be deprived of food intake before experiment. The pressure causing the rupture of colon was 16.67 to 21.33 kPa, averaged 19.80±1.38 kPa. To avoid the injury of the colon during the CRD, the cut-off pressure should be 13.33 mmHg when a 7 cm long balloon is used.
In conclusion, using colorectal distension to establish a model of visceral pain in rats is simple and reliable. The scores of abdominal withdrawal reflex (AWR) to CRD are good for quantitative behavior assessment. This model can be used in experiments with free movement of animals. It provides a good animal model for study of the mechanism of visceral pain and the effects of different drugs on visceral pain.
Footnotes
Supported by Natural Science Foundation of Jiangsu Province, NO.BK2005033; Medical Foundation of Department of Health, Jiangsu Province, No. H200325; Natural Science Foundation of Department of Education, Jiangsu Province, No. 04kJB320127; and Medical Foundation of Department of Health, Zhejiang Province, No. 2004A084
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
References
- 1.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 2.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 3.Saito K, Kanazawa M, Fukudo S. Colorectal distention induces hippocampal noradrenaline release in rat: an in vivo microdialysis study. Brain Res. 2002;947:146–149. doi: 10.1016/s0006-8993(02)03007-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhai QZ, Traub RJ. The NMDA receptor antagonist MK-801 attenuates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain. 1999;83:321–329. doi: 10.1016/s0304-3959(99)00116-5. [DOI] [PubMed] [Google Scholar]
- 5.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 6.Imamachi N, Saito Y, Hara K, Sakura S, Kosaka Y. The non-NMDA glutamate receptor antagonist CNQX augments lidocaine antinociception through a spinal action in rats. Anesth Analg. 1999;89:416–421. doi: 10.1097/00000539-199908000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt TK, Mousa SA, Brack A, Schmidt DK, Rittner HL, Welte M, Schafer M, Stein C. Modulation of peripheral endogenous opioid analgesia by central afferent blockade. Anesthesiology. 2003;98:195–202. doi: 10.1097/00000542-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Ness TJ. Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain. 2000;87:83–88. doi: 10.1016/S0304-3959(00)00272-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang HY, Huang TK. Experiment study of the visceral pain. Jiepouxue Zazhi. 1995;18:282–287. [Google Scholar]
- 10.Ghia JE, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. The effect of a chromogranin A-derived peptide (CgA4-16) in the writhing nociceptive response induced by acetic acid in rats. Life Sci. 2004;75:1787–1799. doi: 10.1016/j.lfs.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Laird JM, de la Rubia PG, Cervero F. Excitability changes of somatic and viscero-somatic nociceptive reflexes in the decerebrate-spinal rabbit: role of NMDA receptors. J Physiol. 1995;489(Pt 2):545–555. doi: 10.1113/jphysiol.1995.sp021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ness TJ, Randich A, Gebhart GF. Further behavioral evidence that colorectal distension is a ‘noxious’ visceral stimulus in rats. Neurosci Lett. 1991;131:113–116. doi: 10.1016/0304-3940(91)90349-x. [DOI] [PubMed] [Google Scholar]


