Abstract
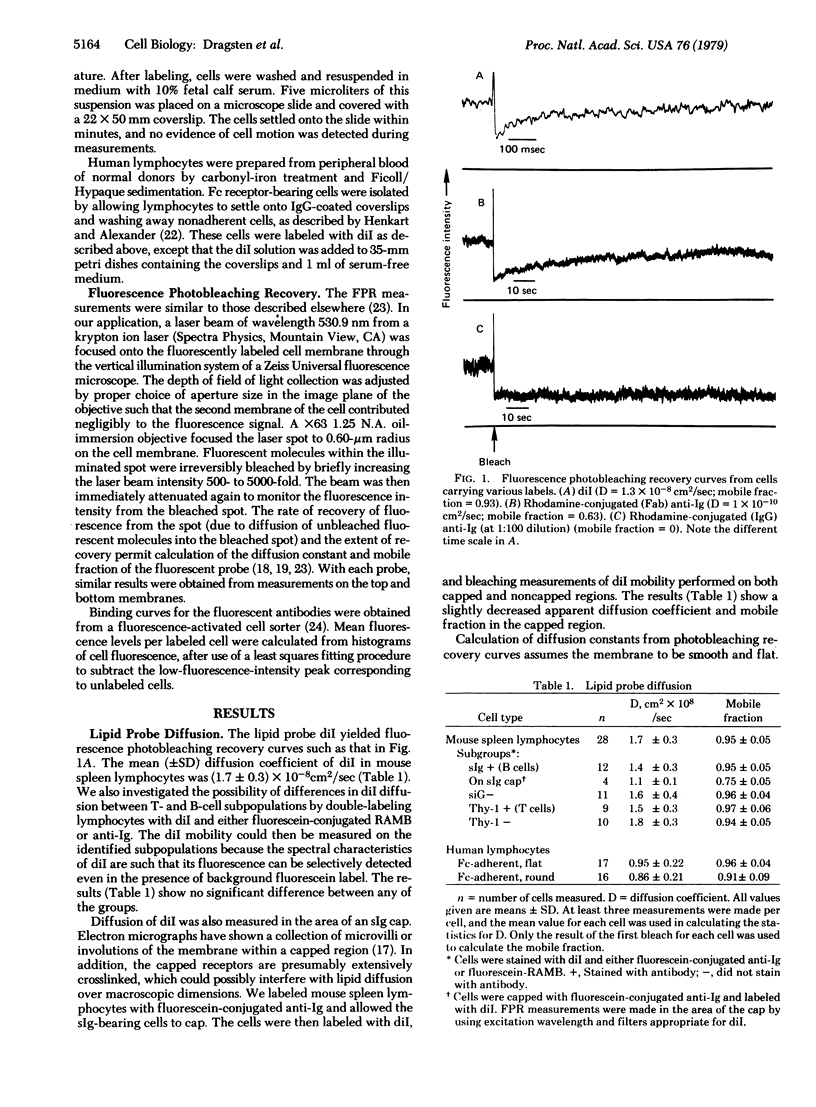
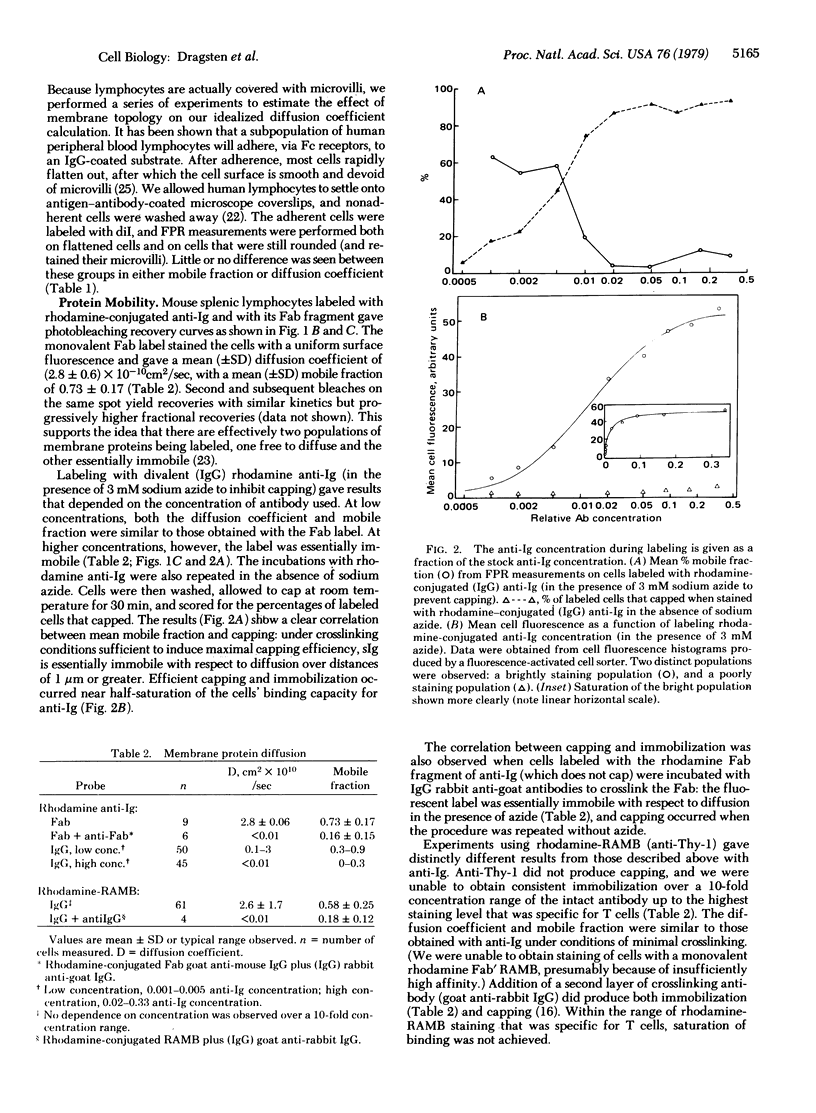
Fluorescence photobleaching recovery was used to measure the lateral diffusion coefficient and mobile fraction of surface immunoglobulin (sIg), Thy-1 antigen, and a lipid probe in the plasma membrane of mouse lymphocytes. The lipid probe (3,3′-dioctadecylindocarbocyanine) had a mean (±SD) diffusion coefficient of (1.7 ± 0.3) × 10-8 cm2/sec, with essentially all of the probe mobile in the membrane. We detected little or no effect on the diffusion of this probe due to the presence of microvilli. Its diffusion was slightly restricted in capped regions. No differences in lipid probe mobility were detected between T and B cells. Fifty to 90% of the detectable sIg and Thy-1 antigen was free to move in the plane of the membrane with diffusion coefficients of ≈3 × 10-10 cm2/sec; the remainder was immobile. Crosslinking of sIg with anti-Ig antibodies (in the presence of azide to inhibit capping) completely immobilized sIg at high concentrations but failed to do so at low concentrations. Thy-1 antigen could not be immobilized with an IgG rabbit anti-mouse brain reagent without an additional layer of crosslinking antibody. In parallel labelings (in the absence of azide), capping of sIg and Thy-1 antigen was observed only under crosslinking conditions sufficient to immobilize the membrane antigen. Sodium azide, colchicine, and cytochalasin B had no measurable effect on lipid probe, sIg, or Thy-1 diffusion.
Keywords: receptor mobility, fluorescence photobleaching, capping
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E., Henkart P. The adherence of human Fc receptor-bearing lymphocytes to antigen-antibody complexes. II. Morphologic alterations induced by the substrate. J Exp Med. 1976 Feb 1;143(2):329–347. doi: 10.1084/jem.143.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Jovin T. M. Automated cell sorting with flow systems. Annu Rev Biophys Bioeng. 1978;7:527–558. doi: 10.1146/annurev.bb.07.060178.002523. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Schlessinger J., Koppel D. E., Axelrod D., Webb W. W. Measurement of lateral transport on cell surfaces. Prog Clin Biol Res. 1976;9:137–147. [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Henkart P., Alexander E. The adherence of human Fc receptor bearing lymphocytes to immobilized antigen-antibody complexes. I. Specificity and use as preparative separation technique. J Immunol Methods. 1978;20:155–171. doi: 10.1016/0022-1759(78)90253-3. [DOI] [PubMed] [Google Scholar]
- Isersky C., Taurog J. D., Poy G., Metzger H. Triggering of cultured neoplastic mast cells by antibodies to the receptor for IgE. J Immunol. 1978 Aug;121(2):549–558. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. IgE and reaginic hypersensitivity. Ann N Y Acad Sci. 1971 Dec 31;190:443–456. doi: 10.1111/j.1749-6632.1971.tb13554.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Chang T. H., Taggart M., Ishizaka K. Histamine release from rat mast cells by antibodies against rat basophilic leukemia cell membrane. J Immunol. 1977 Nov;119(5):1589–1596. [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Johnson M., Edidin M. Lateral diffusion in plasma membrane of mouse egg is restricted after fertilisation. Nature. 1978 Mar 30;272(5652):448–450. doi: 10.1038/272448a0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K., Filier J. S., Jarrett D. B. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest. 1977 Nov;60(5):1094–1106. doi: 10.1172/JCI108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Arbeit R. D., Dickler H. B., Henkart P. A. Inhibition of lymphocyte mitogenesis by immobilized antigen-antibody complexes. J Exp Med. 1975 Oct 1;142(4):814–826. doi: 10.1084/jem.142.4.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Sieckmann D. G., Asofsky R., Mosier D. E., Zitron I. M., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. I. Parameters of the proliferative response. J Exp Med. 1978 Mar 1;147(3):814–829. doi: 10.1084/jem.147.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Moorhead J. W., Claman H. N. Anti-immunoglobulin stimulation of murine lymphocytes. I. Age dependency of the proliferative response. J Immunol. 1976 Jun;116(6):1656–1661. [PubMed] [Google Scholar]
- Wolf D. E., Schlessinger J., Elson E. L., Webb W. W., Blumenthal R., Henkart P. Diffusion and patching of macromolecules on planar lipid bilayer membranes. Biochemistry. 1977 Jul 26;16(15):3476–3483. doi: 10.1021/bi00634a029. [DOI] [PubMed] [Google Scholar]



