Abstract
Epidemiological evidence supports a protective effect of physical activity for breast cancer in older women, but the mechanisms are not well understood. We used 18-month-old BALB/c mice injected in the mammary fat pad with syngeneic 4T1 tumor cells as a model of invasive breast cancer. During the tumor progression phase, there was a significant decrease in labeling for F4/80, a marker for mouse macrophages, and CD34, a marker for vascular endothelial cells, in primary tumors from mice that ran higher average distances compared to mice that ran lower average distances (p≤0.05). These observations suggest that immunohistochemistry can be used to monitor stromal cell populations in tumors from old mice under exercise conditions.
Keywords: breast cancer, exercise, aging, tumor stromal cells, tumor microenvironment, immunohistochemistry
Stromal cells are important systems that support tumor cell growth and metastasis (1). We used 18-month-old BALB/cBy mice (obtained from the National Institute on Aging) orthotopically implanted with 4T1 breast cancer cells as a model to study the effect of exercise on stromal cell populations. The 4T1 breast cancer cell line in mice is highly invasive, with the ability to metastasize to lungs, brain, liver, bone, lymph nodes, and blood, similar to triple negative invasive breast cancer in women (2).
Mice were randomly selected, with 15 mice in the running group. Runners were given access to free running wheels in individual cages as previously described (3). We monitored the running activity continuously for 60 days. 4T1 cells (ATCC, Manassas, VA), cultured under standard laboratory conditions, were injected at a concentration of 1×104 cells into the 4th mammary fat pad of each mouse. Mice were allowed to run another 30 days when the experiment ended. Running wheels (measuring 15.5 cm by diameter) transmitted electronic signals wirelessly to a monitoring hub (Med Associates ENV-044, Vermont) and raw data were exported to Microsoft Excel for processing. The distance ran by each mouse was calculated as (3.14×15.5 cm×number of revolutions)/(100 cm per m×1,000 m per km). Mice were housed in a standard rodent room within a specific pathogen-free (SPF) barrier facility with 12-hour light and dark cycles. Ambient temperature in the room was kept at 70–74°F. Mice were fed standard rodent chow (5,053; Picolab, Richmond, IN) and water ad libitum. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Washington, Seattle.
At 30 days post-tumor cell injection, mice were euthanized by CO2 asphyxiation. Carcasses and mammary tumors were weighed to obtain tumor burden, defined as tumor weight normalized to carcass weight. The breast tumor from each mouse was fixed in 10% neutral-buffered formalin. Immunohistochemistry was performed as previously described using paraffin-embedded tumor sections (4). Primary antibodies to specific markers were incubated at 1:50 for 1 hour. Sections were blocked in 15% goat/5% mouse serum. BGA rat secondary antibodies (Jackson ImmunoResearch Labs Inc., West Grove, PA, USA) were used and incubated at 1:200 for 30 min and slides then counterstained with hematoxylin. Digital images were captured using a Nikon Eclipse E400 bright field microscope (Nikon Corporation, Tokyo, Japan) attached with a Nikon Coolpix 995, digital camera (Nikon Corporation, Tokyo, Japan; 3.3 megapixels, resolution 2,048×1,536 pixels). Five random images at 60× magnification were captured per tumor sample under blinded conditions and saved in JPG (Joint Photographic Experts Group) format. The number of labeled cells was also determined blindly and independently by two co-authors of this paper (CPB and WL) by counting positive cells in a grid of eight squares encompassing the entire plane of view at 20× magnification per slide using five random field views. The labeling index was calculated as the average percentage of stained cells relative to unstained cells. Student's t-test was used to detect significant differences between groups.
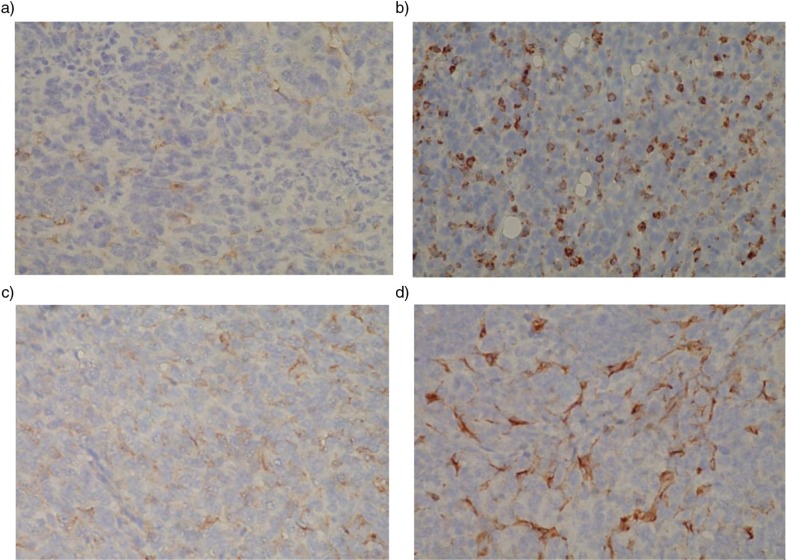
During the tumor progression phase of 4T1 breast cancer in old BALB/c mice, we observed significant differences in labeling for F4/80, a marker for mouse macrophages, and CD34, a marker for vascular endothelial cells, in primary tumors from mice that ran higher average distances compared to mice that ran lower average distances, p≤0.05, N=2, from four mice (Table 1). It can be seen that macrophages are much less frequent in a tumor from a long-distance runner (Fig. 1a) compared to a tumor from a short-distance runner (Fig. 1b). The decrease in CD34 labeling in a long-distance runner (Fig. 1c) compared to a short-distance runner (Fig. 1d) suggests that increased running distance was also associated with decreased vascularization. There were no significant correlations between running distance and labeling for cell proliferation (Ki-67) or apoptosis (Caspase 3) in primary tumors (Table 1), suggesting that exercise training might be targeting stromal cell populations. Even though pre-tumor running was associated with an anti-tumor effect in a distance dependent manner (5), no correlation was seen between tumor burden and running distance during the tumor progression period. It is possible that the rapid and aggressive tumor growth may have overwhelmed any anti-tumor effects by suppressed stromal cells. However, there were no significant correlations between distance ran before tumor injection and labeling for F4/80 and CD34 as well as for Ki67 and Caspase 3 in primary tumors collected at the termination of the study (data not shown) suggesting other unknown mechanisms are likely responsible for the antitumor effects of pre-tumor running.
Table 1.
Stromal cell populations are decreased in tumors from mice that ran further distances after 4T1 tumor cell injection, that is, during the tumor progression phase
| Percent labeling | ||||
|---|---|---|---|---|
| Average daily distance ran (km) | F4/80 | CD34 | Caspase 3 | Ki67 |
| 1.6 | 41±5* | 48±8* | 24±9 | 33±7 |
| 4.8 | 19±6* | 25±3* | 18±7 | 42±10 |
During this time, short-distance runners ran an average of 1.6 km/day while long-distance runners ran an average of 4.7 km/day. Labeling for F4/80, a marker for macrophages, and CD34, a marker for vascular endothelial cells, was decreased in primary tumors from long-distance runners compared to short-distance runners (N=4, *p≤0.05). The number of labeled cells was determined in a blinded manner, and independently by two co-authors of this paper (CPB and WL). Positive cells were counted in a grid of eight squares that encompassed the entire plane of view, at 20× magnification per slide using five different field views. Labeling was calculated as the average percentage of positive cells, relative to non-stained cells. No statistical differences between groups were observed for Caspase 3, a marker for apoptosis, or Ki67, a marker for cell proliferation.
Fig. 1.
Tumors from long-distance runners had decreased labeling for macrophages and vascular endothelial cells compared to short-distance runners. (a) and (b) Reddish brown stains show greatly reduced F4/80 positive macrophages in tumor tissue from a long-distance runner, but increased cell numbers in tumor tissue from a short-distance runner, respectively. (c) and (d) Reddish brown stain shows greatly reduced CD34 positive vascular endothelial cells in tumor tissue from a long-distance runner, but an extensive increase in these cells in tumor tissue from a short-distance runner, respectively.
These observations suggest that immunohistochemistry can be used to monitor stromal cell populations in tumors from old mice under exercise conditions, and further support the concept of using old mice for modeling in preclinical cancer studies.
Acknowledgements
This work was supported by R21 CA 140916, NIH/NCI and P01 AG01751 and P30 AG013280, NIH/NIA.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Montel V, Mose ES, Tarin D. Tumor–stromal interactions reciprocally modulate gene expression patterns during carcinogenesis and metastasis. Int J Cancer. 2006;119:251–63. doi: 10.1002/ijc.21757. [DOI] [PubMed] [Google Scholar]
- 2.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protocol Immunol. 2000:20.2.1–20.2.16. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 3.Goh J, Tsai JM, Bammler TK, Farin FM, Endicott E, Ladiges WC. Exercise training in transgenic mice is associated with an early attenuation of mammary tumor growth in a dose-dependent manner. PLoS One. 2013;8(11):80123. doi: 10.1371/journal.pone.0080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatemie S, Goh J, Pettan-Brewer C, Ladiges W. Breast tumors in PyMT transgenic mice expressing mitochondrial catalase have decreased labeling for macrophages and endothelial cells. Pathobiol Aging Age Relat Dis. 2012;2 doi: 10.3402/pba.v2i0.17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh J, Endicott E, Ladiges WC. Pre-tumor exercise decreases breast cancer in old mice in a distance-dependent manner. Am J Cancer Res. 2014;4(4):378–84. [PMC free article] [PubMed] [Google Scholar]