Abstract
When murine erythroleukemia (MEL) cells are induced with hemin, they carry out several early functions of the erythroid program. However, they do not become committed to terminal differentiation nor do they become benzidine positive. This is in contrast to MEL cells induced with dimethyl sulfoxide (Me2SO) which undergo a more complete program of erythroid differentiation. In order to determine the relationship between commitment and various events in the erythroid program, we compared the induction of MEL cells with hemin and with Me2SO. The amount of globin mRNA accumulated in inducing MEL cells and the rate of its synthesis and turnover were quantitated. Although MEL cells induced with hemin accumulated significantly less globin mRNA than did cells induced with Me2SO, the rate of synthesis of globin mRNA was the same in fully induced cells, irrespective of inducer. Therefore, there is no evidence that induction with hemin produces an early program that is different or altered from that which is part of Me2SO induction. MEL cells induced with Me2SO specifically destabilize their globin mRNA after 4 days of induction. This raises the question of whether this destabilization of globin mRNA is an independently programmed late event, as suggested by the time of its occurrence, or, alternatively, whether it might be the inevitable consequence of an early event(s). For instance, destabilization might be linked to the synthesis or translation of globin mRNA. Because MEL cells induced with hemin do not destabilize their globin mRNA, we have concluded that this turnover of globin mRNA is a late event, occurring only in a committed cell population.
Keywords: erythroid differentiation, Friend cells, dimethyl sulfoxide induction
Full text
PDF
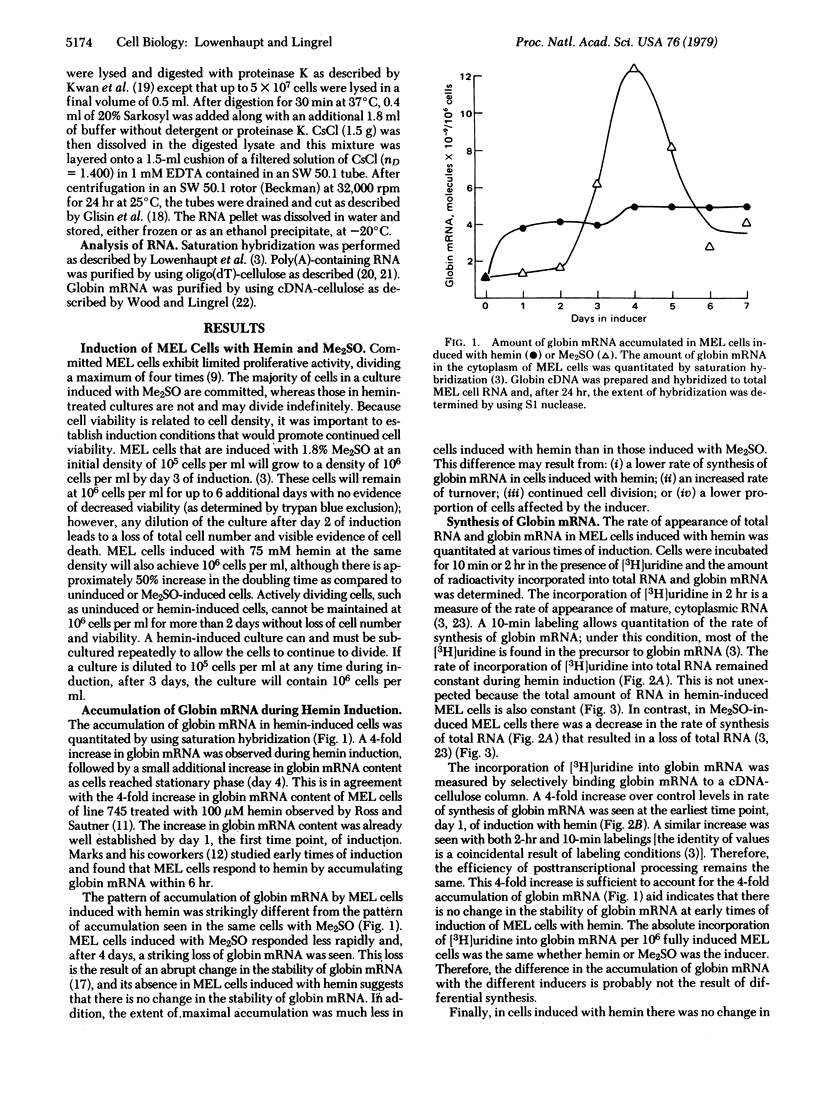

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Voloch Z., Bastos R., Levy S. Biosynthesis and stability of globin mRNA in cultured erythroleukemic Friend cells. Cell. 1976 Aug;8(4):495–503. doi: 10.1016/0092-8674(76)90217-8. [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Theoretical analysis of a model for globin messenger RNA accumulation during erythropoiesis. J Mol Biol. 1977 Feb 25;110(2):205–218. doi: 10.1016/s0022-2836(77)80069-7. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Wuu K. D., Noyes A. N., Young R., Scher W., Friend C., Preisler H. D., Bank A. Hemoglobin biosynthesis in murine virus-induced leukemic cells in vitro: structure and amounts of globin chains produced. Blood. 1972 Dec;40(6):823–835. [PubMed] [Google Scholar]
- Burka E. R. Hemin: an inhibitor of erythroid cell ribonuclease. Science. 1968 Dec 13;162(3859):1287–1287. doi: 10.1126/science.162.3859.1287. [DOI] [PubMed] [Google Scholar]
- Eisen H., Nasi S., Georgopoulos C. P., Arndt-Jovin D., Ostertag W. Surface changes in differentiating Friend erythroleukemic cells in culture. Cell. 1977 Apr;10(4):689–695. doi: 10.1016/0092-8674(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Farkas W., Marks P. A. Partial purification and properties of a ribonuclease from rabbit reticulocytes. J Biol Chem. 1968 Dec 25;243(24):6464–6473. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Furusawa M., Sugano H. Erythrocyte membrane-specific antigens in Friend virus-induced leukemia cells. Bibl Haematol. 1973;39:955–967. doi: 10.1159/000427928. [DOI] [PubMed] [Google Scholar]
- Keppel F., Allet B., Eisen H. Appearance of a chromatin protein during the erythroid differentiation of Friend virus-transformed cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):653–656. doi: 10.1073/pnas.74.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. P., Wood T. G., Lingrel J. B. Purification of a putative precursor of globin messenger RNA from mouse nucleated erythroid cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):178–182. doi: 10.1073/pnas.74.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Trent C., Lingrel J. B. Mechanisms for accumulation of globin mRNA during dimethyl sulfoxide induction of murine erythroleukemia cells: synthesis of precursors and mature mRNA. Dev Biol. 1978 Apr;63(2):441–454. doi: 10.1016/0012-1606(78)90148-3. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Nudel U., Salmon J., Fibach E., Terada M., Rifkind R., Marks P. A., Bank A. Accumulation of alpha- and beta-globin messenger RNAs in mouse erythroleukemia cells. Cell. 1977 Oct;12(2):463–469. doi: 10.1016/0092-8674(77)90122-2. [DOI] [PubMed] [Google Scholar]
- Puckett L., Darnell J. E. Essential factors in the kinetic analysis of RNA synthesis in HeLa cells. J Cell Physiol. 1977 Mar;90(3):521–534. doi: 10.1002/jcp.1040900315. [DOI] [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Rovera G., Abramczuk J., Surrey S. The effect of hemin on the expression of beta globin genes in friend cells. FEBS Lett. 1977 Sep 15;81(2):366–370. doi: 10.1016/0014-5793(77)80556-5. [DOI] [PubMed] [Google Scholar]
- Rovera G., Vartikar J., Connolly G. R., Magarian C., Dolby T. W. Hemin controls the expression of the beta minor globin gene in Friend erythroleukemic cells at the pretranslational level. J Biol Chem. 1978 Nov 10;253(21):7588–7590. [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherton C. C., Kabat D. Changes in RNA and protein metabolism preceding onset of hemoglobin synthesis in cultured Friend leukemia cells. Dev Biol. 1976 Jan;48(1):118–131. doi: 10.1016/0012-1606(76)90051-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Wood T. G., Lingrel J. B. Purification of biologically active globin mRNA using cDNA-cellulose affinity chromatography. J Biol Chem. 1977 Jan 25;252(2):457–463. [PubMed] [Google Scholar]



