Abstract
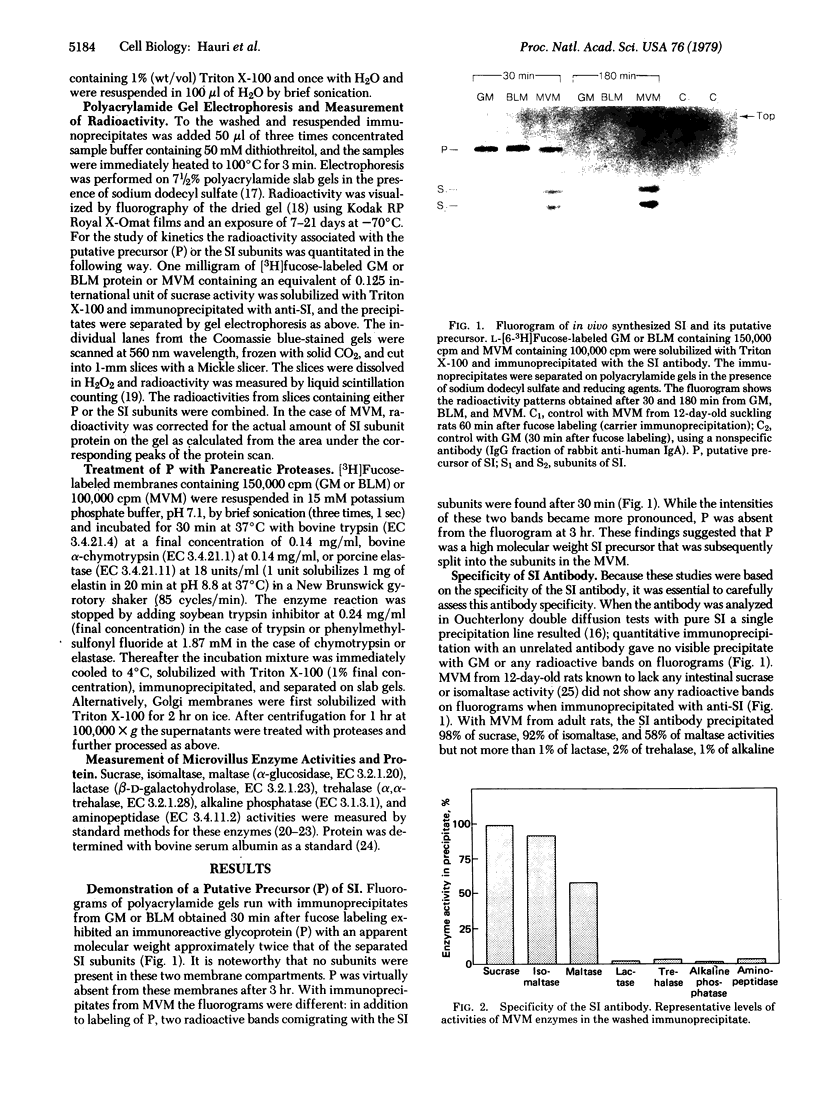
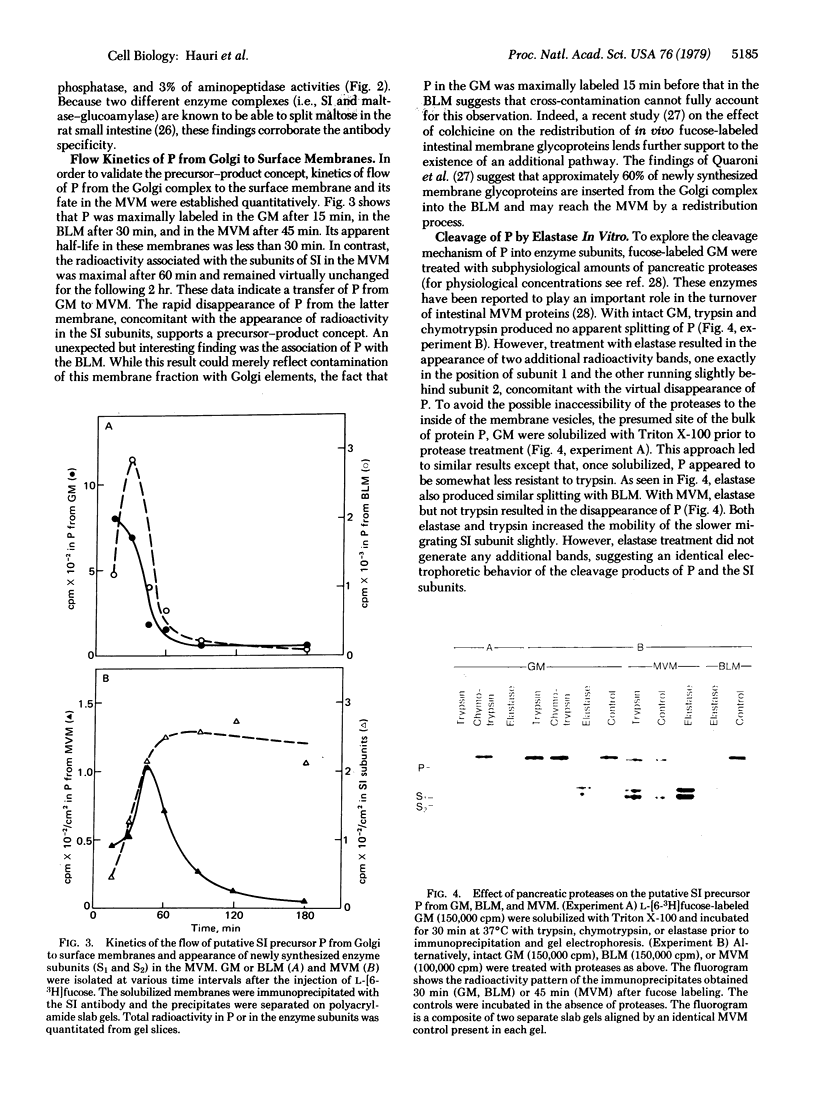
The biosynthesis in vivo of rat intestinal sucrase—isomaltase [a complex of sucrose α-glucohydrolase, EC 3.2.1.48, and oligo-1,6-glucosidase (dextrin 6-α-D-glucanohydrolase), EC 3.2.1.10] has been studied by following the incorporation of L-[6-3H]fucose into the enzyme with time. Immunoprecipitation of sucrase—isomaltase from Triton-X-100-solubilized Golgi or basolateral membranes and subsequent polyacrylamide gel electrophoresis revealed the presence of an immunoreactive glycoprotein with an apparent molecular weight approximately twice that of the separated sucrase—isomaltase subunits, but no active subunits were found in these membranes. This glycoprotein was also found in the microvillus membrane in addition to the subunits of sucrase—isomaltase. Kinetic studies showed a maximal labeling of this glycoprotein in Golgi membranes at 15 min, in basolateral membranes at 30 min, and in microvillus membranes at 45 min and a half-life of less than 30 min in each membrane. However, the radioactivity of the sucrase—isomaltase subunits in the microvillus membrane reached a plateau after 60 min. These data suggest that sucrase—isomaltase is synthesized as a one-chain polypeptide precursor that is split into the subunits after its transfer to the microvillus membrane. Elastase (EC 3.4.21.11), but not trypsin (EC 3.4.21.4) or α-chymotrypsin (EC 3.4.21.1), split the putative precursor into two polypeptides that had electrophoretic behaviors similar to those of the active enzyme subunits. These studies suggest that pancreatic proteases may play an important role in the late posttranslational processing of sucrase—isomaltase in vivo.
Keywords: fucose labeling, putative enzyme precursor, membrane flow, late posttranslational processing, elastase
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Tedesco F. J. The possible role of pancreatic proteases in the turnover of intestinal brush border proteins. Biochim Biophys Acta. 1975 Aug 5;401(1):28–40. doi: 10.1016/0005-2736(75)90338-7. [DOI] [PubMed] [Google Scholar]
- Asp N. G., Dahlqvist A. Human small intestine -galactosidases: specific assay of three different enzymes. Anal Biochem. 1972 Jun;47(2):527–538. doi: 10.1016/0003-2697(72)90147-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Frank G., Brunner J., Hauser H., Wacker H., Semenza G., Zuber H. The hydrophobic anchor of small-intestinal sucrase--isomaltase: N-terminal sequence of isomaltase subunit. FEBS Lett. 1978 Dec 1;96(1):183–188. doi: 10.1016/0014-5793(78)81090-4. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Morré D. J., Deumling B., Cheetham R. D., Kartenbeck J., Jarasch E. D., Zentgraf H. W. Synthesis and turnover of membrane proteins in rat liver: an examination of the membrane flow hypothesis. Z Naturforsch B. 1971 Oct;26(10):1031–1039. doi: 10.1515/znb-1971-1016. [DOI] [PubMed] [Google Scholar]
- Freedman R. A., Weiser M. M., Isselbacher K. J. Calcium translocation by Golgi and lateral-basal membrane vesicles from rat intestine: decrease in vitamin D-deficient rats. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3612–3616. doi: 10.1073/pnas.74.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Green J. R. The identification of rat intestinal membrane enzymes after electrophoresis on polyacrylamide gels containing sodium dodecyl sulphate. Biochem J. 1978 Jul 15;174(1):61–66. doi: 10.1042/bj1740061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Freiburghaus A., Grenier J. F., Hadorn B. Biosynthesis of brush border glycoproteins by human small intestinal mucosa in organ culture. Biochim Biophys Acta. 1977 Jun 16;467(3):327–339. doi: 10.1016/0005-2736(77)90310-8. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Irving R. A., Toneguzzo F., Rhee S. H., Hofmann T., Ghosh H. P. Synthesis and assembly of membrane glycoproteins: presence of leader peptide in nonglycosylated precursor of membrane glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):570–574. doi: 10.1073/pnas.76.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leapman S. B., Deutsch A. A., Grand R. J., Folkman J. Transplantation of fetal intestine: survival and function in a subcutaneous location in adult animals. Ann Surg. 1974 Jan;179(1):109–114. doi: 10.1097/00000658-197401000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Morré D. J., Kartenbeck J., Franke W. W. Membrane flow and intercoversions among endomembranes. Biochim Biophys Acta. 1979 Apr 23;559(1):71–52. doi: 10.1016/0304-4157(79)90008-x. [DOI] [PubMed] [Google Scholar]
- Oda K., Ikehara Y., Kato K. Isolation of Golgi fractions from colchicine-treated rat liver. II. Electrophoretic characterization. Biochim Biophys Acta. 1979 Apr 4;552(2):225–237. doi: 10.1016/0005-2736(79)90279-7. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Kirsch K., Weiser M. M. Synthesis of membrane glycoproteins in rat small-intestinal villus cells. Effect of colchicine on the redistribution of L-[1,5,6-3H]fucose-labelled membrane glycoproteins among Golgi, lateral basal and microvillus membranes. Biochem J. 1979 Jul 15;182(1):213–221. doi: 10.1042/bj1820213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- Roncari G., Zuber H. Thermophilic aminopeptidases from Bacillus stearothermophilus. I. Isolation, specificity, and general properties of the thermostable aminopeptidase I. Int J Protein Res. 1969;1(1):45–61. doi: 10.1111/j.1399-3011.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Weiser M. M., Neumeier M. M., Quaroni A., Kirsch K. Synthesis of plasmalemmal glycoproteins in intestinal epithelial cells. Separation of Golgi membranes from villus and crypt cell surface membranes; glycosyltransferase activity of surface membrane. J Cell Biol. 1978 Jun;77(3):722–734. doi: 10.1083/jcb.77.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]