Abstract
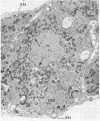
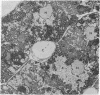
A knowledge of the biological characteristics of carcinogen-induced hyperplastic nodules of rat liver may be important in the understanding of cancer development. Although its biological role remains to be elucidated, the level of microsomal epoxide hydrase (epoxide hydrolase, EC 3.3.2.3) is 5- to 7-fold greater in hyperplastic nodules nodules induced by feeding the hepatocarcinogen 2-acetylaminofluorene than in liver of control rats. After removal of the carcinogen from the diet, the high level of the enzyme is maintained in those nodules that persist and in the hepatocellular carcinomas that subsequently develop. The availability of antibody to the epoxide hydrase made it possible to use electron microscopic immunocytochemistry to localize this enzyme in the cells of hyperplastic nodules. The immunocytochemical procedure provides direct visual evidence for the presence of this enzyme in smooth endoplasmic reticulum and also in rough endoplasmic reticulum (including the nuclear envelope) of the nodule's parenchymal cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W., Lu A. Y., Thomas P. E., Ryan D., Kizer D. E., Griffin M. J. Identification of epoxide hydrase as the preneoplastic antigen in rat liver hyperplastic nodules. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3240–3243. doi: 10.1073/pnas.75.7.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W., Thomas P. E., Korzeniowski D., Seifried H., Jerina D. M., Lu A. Y. Liver microsomal epoxide hydrase: activation, inhibition, and immunological properties of the purified enzyme. Mol Pharmacol. 1978 Nov;14(6):1107–1120. [PubMed] [Google Scholar]
- Lin J. C., Hiasa Y., Farber E. Preneoplastic antigen as a marker for endoplasmic reticulum of putative premalignant hepatocytes during liver carcinogenesis. Cancer Res. 1977 Jul;37(7 Pt 1):1972–1981. [PubMed] [Google Scholar]
- Lu A. Y., Ryan D., Jerina D. M., Daly J. W., Levin W. Liver microsomal expoxide hydrase. Solubilization, purification, and characterization. J Biol Chem. 1975 Oct 25;250(20):8283–8288. [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Molin S. O., Nygren H., Hansson H. A. Binding of glutaraldehyde reacted peroxidase to cell surfaces--a source of error in immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):325–326. doi: 10.1177/26.4.96176. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Edelstein D. Reversal of orotic acid-induced fatty liver in rats by clofibrate. Lab Invest. 1977 Feb;36(2):215–231. [PubMed] [Google Scholar]
- Okita K., Kligman L. H., Farber E. A new common marker for premalignant and malignant hepatocytes induced in the rat by chemical carcinogens. J Natl Cancer Inst. 1975 Jan;54(1):199–202. doi: 10.1093/jnci/54.1.199. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. N., Cameron R. G., Farber E., Griffin M. J., Joly J. G., Murray R. K. Multiplicity of induction patterns of rat liver microsomal mono-oxygenases and other polypeptides produced by administration of various xenobiotics. Biochem J. 1979 Aug 15;182(2):317–327. doi: 10.1042/bj1820317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire R. A., Levitt M. H. Report of a workshop on classification of specific hepatocellular lesions in rats. Cancer Res. 1975 Nov;35(11 Pt 1):3214–3223. [PubMed] [Google Scholar]
- Stout D. L., Becker F. F. Alteration of the ability of liver microsomes to activate N-2-fluorenylacetamide to a mutagen of Salmonella typhimurium during hepatocarcinogenesis. Cancer Res. 1978 Aug;38(8):2274–2278. [PubMed] [Google Scholar]