Abstract
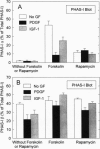
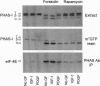
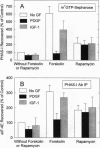
Incubating rat aortic smooth muscle cells with either platelet-derived growth factor BB (PDGF) or insulin-like growth factor I (IGF-I) increased the phosphorylation of PHAS-I, an inhibitor of the mRNA cap binding protein, eukaryotic initiation factor (eIF) 4E. Phosphorylation of PHAS-I promoted dissociation of the PHAS-I-eIF-4E complex, an effect that could partly explain the stimulation of protein synthesis by the two growth factors. Increasing cAMP with forskolin decreased PHAS-I phosphorylation and markedly increased the amount of eIF-4E bound to PHAS-I, effects consistent with an action of cAMP to inhibit protein synthesis. Both PDGF and IGF-I activated p70S6K, but only PDGF increased mitogen-activated protein kinase activity. Forskolin decreased by 50% the effect of PDGF on increasing p70S6K, and forskolin abolished the effect of IGF-I on the kinase. The effects of PDGF and IGF-I on increasing PHAS-I phosphorylation, on dissociating the PHAS-I-eIF-4E complex, and on increasing p70S6K were abolished by rapamycin. The results indicate that IGF-I and PDGF increase PHAS-I phosphorylation in smooth muscle cells by the same rapamycin-sensitive pathway that leads to activation of p70S6K.
Full text
PDF


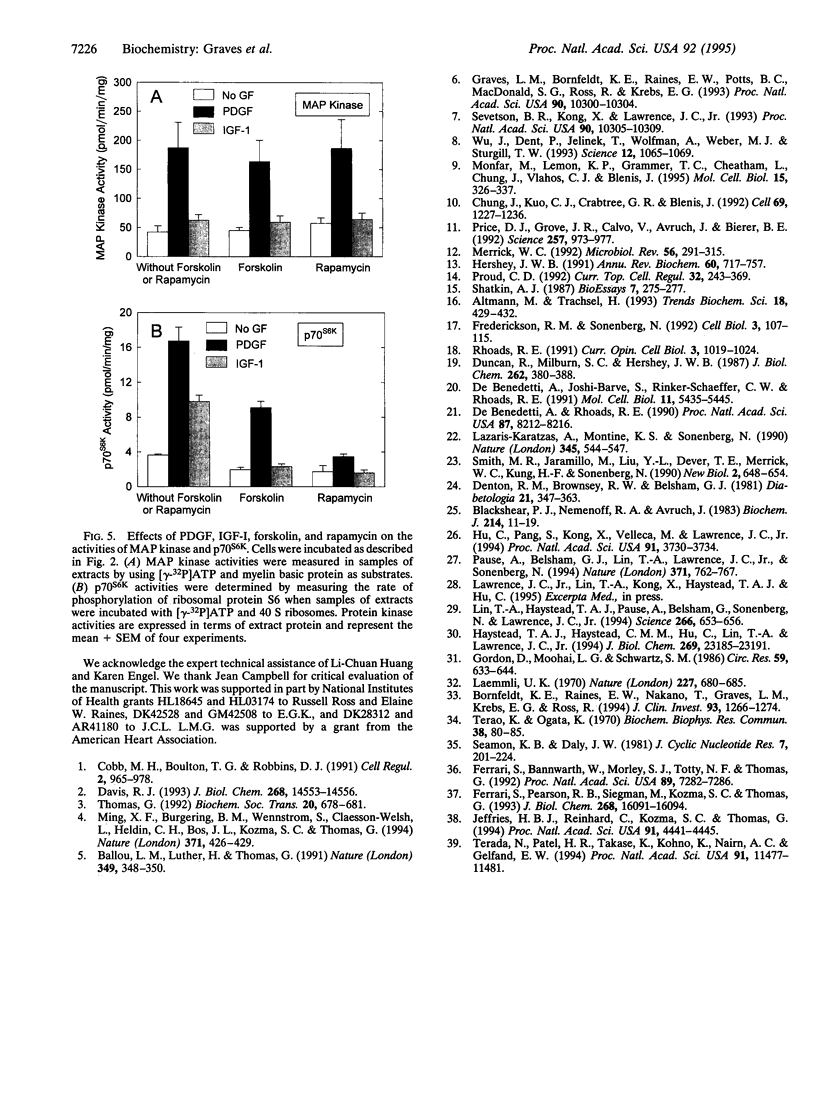
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann M., Trachsel H. Regulation of translation initiation and modulation of cellular physiology. Trends Biochem Sci. 1993 Nov;18(11):429–432. doi: 10.1016/0968-0004(93)90143-b. [DOI] [PubMed] [Google Scholar]
- Ballou L. M., Luther H., Thomas G. MAP2 kinase and 70K S6 kinase lie on distinct signalling pathways. Nature. 1991 Jan 24;349(6307):348–350. doi: 10.1038/349348a0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Insulin and growth factors stimulate the phosphorylation of a Mr-22000 protein in 3T3-L1 adipocytes. Biochem J. 1983 Jul 15;214(1):11–19. doi: 10.1042/bj2140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Raines E. W., Nakano T., Graves L. M., Krebs E. G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994 Mar;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- De Benedetti A., Joshi-Barve S., Rinker-Schaeffer C., Rhoads R. E. Expression of antisense RNA against initiation factor eIF-4E mRNA in HeLa cells results in lengthened cell division times, diminished translation rates, and reduced levels of both eIF-4E and the p220 component of eIF-4F. Mol Cell Biol. 1991 Nov;11(11):5435–5445. doi: 10.1128/mcb.11.11.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Rhoads R. E. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8212–8216. doi: 10.1073/pnas.87.21.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Ferrari S., Bannwarth W., Morley S. J., Totty N. F., Thomas G. Activation of p70s6k is associated with phosphorylation of four clustered sites displaying Ser/Thr-Pro motifs. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7282–7286. doi: 10.1073/pnas.89.15.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Pearson R. B., Siegmann M., Kozma S. C., Thomas G. The immunosuppressant rapamycin induces inactivation of p70s6k through dephosphorylation of a novel set of sites. J Biol Chem. 1993 Aug 5;268(22):16091–16094. [PubMed] [Google Scholar]
- Frederickson R. M., Sonenberg N. Signal transduction and regulation of translation initiation. Semin Cell Biol. 1992 Apr;3(2):107–115. doi: 10.1016/s1043-4682(10)80020-0. [DOI] [PubMed] [Google Scholar]
- Gordon D., Mohai L. G., Schwartz S. M. Induction of polyploidy in cultures of neonatal rat aortic smooth muscle cells. Circ Res. 1986 Dec;59(6):633–644. doi: 10.1161/01.res.59.6.633. [DOI] [PubMed] [Google Scholar]
- Graves L. M., Bornfeldt K. E., Raines E. W., Potts B. C., Macdonald S. G., Ross R., Krebs E. G. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Haystead C. M., Hu C., Lin T. A., Lawrence J. C., Jr Phosphorylation of PHAS-I by mitogen-activated protein (MAP) kinase. Identification of a site phosphorylated by MAP kinase in vitro and in response to insulin in rat adipocytes. J Biol Chem. 1994 Sep 16;269(37):23185–23191. [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hu C., Pang S., Kong X., Velleca M., Lawrence J. C., Jr Molecular cloning and tissue distribution of PHAS-I, an intracellular target for insulin and growth factors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3730–3734. doi: 10.1073/pnas.91.9.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies H. B., Reinhard C., Kozma S. C., Thomas G. Rapamycin selectively represses translation of the "polypyrimidine tract" mRNA family. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Lin T. A., Kong X., Haystead T. A., Pause A., Belsham G., Sonenberg N., Lawrence J. C., Jr PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994 Oct 28;266(5185):653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X. F., Burgering B. M., Wennström S., Claesson-Welsh L., Heldin C. H., Bos J. L., Kozma S. C., Thomas G. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature. 1994 Sep 29;371(6496):426–429. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- Monfar M., Lemon K. P., Grammer T. C., Cheatham L., Chung J., Vlahos C. J., Blenis J. Activation of pp70/85 S6 kinases in interleukin-2-responsive lymphoid cells is mediated by phosphatidylinositol 3-kinase and inhibited by cyclic AMP. Mol Cell Biol. 1995 Jan;15(1):326–337. doi: 10.1128/mcb.15.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994 Oct 27;371(6500):762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Proud C. G. Protein phosphorylation in translational control. Curr Top Cell Regul. 1992;32:243–369. doi: 10.1016/b978-0-12-152832-4.50008-2. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E. Protein synthesis, cell growth and oncogenesis. Curr Opin Cell Biol. 1991 Dec;3(6):1019–1024. doi: 10.1016/0955-0674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Sevetson B. R., Kong X., Lawrence J. C., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. mRNA caps--old and newer hats. Bioessays. 1987 Dec;7(6):275–277. doi: 10.1002/bies.950070611. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Jaramillo M., Liu Y. L., Dever T. E., Merrick W. C., Kung H. F., Sonenberg N. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990 Jul;2(7):648–654. [PubMed] [Google Scholar]
- Terada N., Patel H. R., Takase K., Kohno K., Nairn A. C., Gelfand E. W. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao K., Ogata K. Preparation and some properties of active subunits from rat liver ribosomes. Biochem Biophys Res Commun. 1970 Jan 6;38(1):80–85. doi: 10.1016/0006-291x(70)91086-7. [DOI] [PubMed] [Google Scholar]
- Thomas G. The mitogen-activated p70s6k. Biochem Soc Trans. 1992 Aug;20(3):678–681. doi: 10.1042/bst0200678. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]