Abstract
The analytical resolution of individual chromosome peaks in the flow karyotype of cell lines is dependent on sample preparation and the detection sensitivity of the flow cytometer. We have investigated the effect of laser power on the resolution of chromosome peaks in cell lines with complex karyotypes. Chromosomes were prepared from a human gastric cancer cell line and a cell line from a patient with an abnormal phenotype using a modified polyamine isolation buffer. The stained chromosome suspensions were analyzed on a MoFlo sorter (Beckman Coulter) equipped with two water-cooled lasers (Coherent). A bivariate flow karyotype was obtained from each of the cell lines at various laser power settings and compared to a karyotype generated using laser power settings of 300 mW. The best separation of chromosome peaks was obtained with laser powers of 300 mW. This study demonstrates the requirement for high-laser powers for the accurate detection and purification of chromosomes, particularly from complex karyotypes, using a conventional flow cytometer.
Keywords: flow karyotype, resolution, chromosomes, laser power, photo-saturation
Chromosome rearrangement is a common observation in individuals with an abnormal phenotype as well as in cancer cells. Many cancers display multiple structurally rearranged chromosomes giving rise to complex karyotypes. In recent years, there has been an increased interest in the identification of candidate disease causing genes disrupted or fused at rearrangement breakpoints (1-5). Such rearranged chromosomes can be detected and purified using flow cytometry (6-9). Conventionally, flow karyotype analysis has required the use of high-powered water-cooled lasers although there has been some success in the analysis of a cell line with a simple karyotype using low powered air-cooled lasers (10).
In this study, we investigate the effect of laser power on the resolution of the bivariate flow karyotype using a conventional flow cytometer with chromosomes prepared from human cell lines with complex structural rearrangements.
Materials and Methods
Cell Culture
Chromosomes were prepared from human lymphoblastoid cells B19580 t(11;15); which carried a copy each of derivative chromosome 11 (126 Mega base pairs in size) and derivative chromosome 15 (108 Mega base pairs in size), and a gastric cancer line YCC2 (ascites derived from the stomach area).
Lymphoblastoid and gastric cancer cell lines were cultured in RPMI medium (Gibco) and MEM medium (with Earle’s salts and 25 mM HEPES, Sigma) respectively and supplemented with 10% fetal bovine serum (Invitrogen) and antibiotics (Penicillin and streptomycin, Sigma). The cell lines were treated with demecolcine (0.1 μg/ml) for 6 h after subculturing for 24 h.
Chromosome Preparation and Staining
Hoechst (HO) 33258 (Sigma) and Chromomycin A3 (Sigma) were used to stain the prepared chromosomes as described previously (11). The stained chromosome suspensions were treated overnight to a final concentration of 10 mM of sodium citrate and 25 mM of sodium sulphite before flow analysis.
Laser Excitation Power
Two water-cooled argon ion lasers (Coherent, Innova 300 series) were used as the excitation light sources. One of the lasers was tuned to emit multiline UV (330–360 nm) and the other to emit light at 458 nm. The power of both lasers was kept stable using a light control feedback system (Coherent).
A flow karyotype of the human lymphoblastoid cell line B19580 t(11;15) was generated with both lasers tuned to power settings of 25, 50, and 300 mW. The flow karyotype of the gastric cancer cell line was measured with both lasers tuned to a power setting of 300 mW and at a combination setting of 60 mW for multi-line UV and 40 mW for 458 nm to mimic the power levels available from solid state lasers.
Flow Cytometric Analysis
The stained chromosome suspensions were analyzed on a flow cytometer (MoFlo®, Beckman Coulter) as described previously (11). A total of 50,000–100,000 events were acquired for all cell lines and displayed on a bivariate data plot of HO versus Chromomycin fluorescence. Data collected from the experiments were analyzed using Summit (analysis software from Beckman Coulter). The coefficient of variance (CV) of the chromosome peaks was measured by drawing gates around the chromosomes of interest on the bivariate flow karyogram and calculating the half-max CVs of the gated regions univariately for HO and Chromomycin fluorescence.
Results
Flow Karyotype Resolution of B19580 t(11;15) and Gastric Cancer Cell Line, YCC2
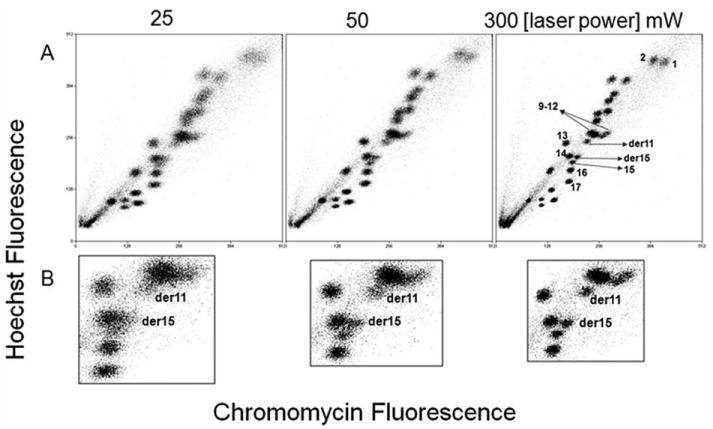
The effect of the intensity of laser power on the resolution of the flow karyotype of a human lymphoblastoid cell line B19580 t(11;15) is shown in Figure 1 and Table 1. The resolution of the flow karyotype was observed to improve as the intensity of the laser power was increased from 25 to 300 mW (Fig. 1A). The best apparent resolution was observed at a laser power setting of 300 mW. These observations are detailed in the zoomed-in data of the translocated chromosomes of the cell line shown in Figure 1B. The translocated chromosome clusters labeled as derivative 11 and 15, ~8 Mega base pairs difference in size from their unrearranged counterparts, are clearly resolved from the neighboring peaks. However, these chromosome peaks became more diffuse and merged with surrounding clusters as the laser power was reduced to 50 mW and 25 mW (Fig. 1B). The CVs for HO and Chromomycin (CA3) fluorescence for derivatives 11 and 15 are shown in Table 1. The lowest CVs for HO fluorescence were observed at a laser power setting of 300 mW and represented 20% and 15.6% improvements for derivatives 11 and 15 respectively over the CVs obtained at a laser power setting of 25 mW. Similarly, the lowest CVs for CA3 fluorescence were observed at a laser power setting of 300 mW and represented 13.2% and 20.5% improvements for derivatives 11 and 15 respectively over the CVs obtained at a laser power setting of 25 mW.
Figure 1. Bivariate flow karyotype of chromosomes from a human lymphoblastoid cell line B19580 t(11;15).
The resolution of the flow karyotype is measured at laser power settings varied from 25 mW to 300 mW. Complete flow karyotypes are shown in panel A. Panel (B) shows the zoomed-in data of the region around the chromosome 9—12 cluster that carries the derivative chromosomes.
Table 1. Coefficient of variation of chromosome peaks of derivative 11 and 15 from B19580t (11;15) lymphoblastoid cell line.
| der 11 |
der 15 |
|||
|---|---|---|---|---|
| COEFFICIENT OF VARIATION |
COEFFICIENT OF VARIATION |
|||
| LASER POWER (mW) | HOECHST (% DECREASE IN CV OVER LASER POWER AT 25 mW) | CA3 (% DECREASE IN CV OVER LASER POWER AT 25 mW) | HOECHST (% DECREASE IN CV OVER LASER POWER AT 25 mW) | CA3 (% DECREASE IN CV OVER LASER POWER AT 25 mW) |
| 25 | 2.94 | 3.19 | 1.73 | 3.12 |
| 50 | 2.78 (−5.4%) | 2.96 (−7.2%) | 1.53 (−11.6%) | 2.85 (−8.7%) |
| 300 | 2.35 (−20%) | 2.77 (−13.2%) | 1.46 (−15.6%) | 2.48 (−20.5%) |
Chromosomes were prepared using a polyamine isolation method and analyzed with laser power intensities varied from 25 mW to 300 mW.
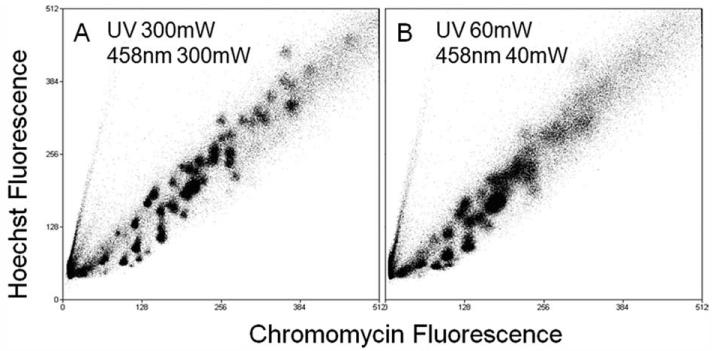
The effect of the intensity of laser power on the resolution of the complex flow karyotype of a gastric cancer cell line is shown in Figure 2. It is clear that a great improvement of chromosome resolution in the flow karyotype was produced at a laser power setting of 300 mW and revealed a total of 40 chromosome clusters with 36 of them distinctively resolved from their neighbors (Fig. 2A). In contrast, at a combinational laser power setting at 60 mW of multi-line UV and 40 mW of 458 nm, almost half of the chromosome clusters were not resolved (Fig. 2B).
Figure 2. Bivariate flow karyotype of chromosomes from a human gastric cell line, YCC2.
The flow karyotypes were produced using the standard laser power setting of 300 mW (A) and a combination setting of 60 mW of multiline UV and 40 mW of 458 nm (B).
Discussion and Conclusions
While flow cytometric analysis of chromosomes using low power air-cooled lasers has been reported previously using a cell line with a relatively simple karyotype (10), we show that such laser powers are inadequate for the study of cells with complex chromosome rearrangements. Because of cost restraints, most standard flow sorters and analysers are equipped with low-powered lasers unsuitable for the analysis of cells with complex flow karyotypes. To obtain flow karyotype data with sufficient resolution, higher laser powers are required.
The highest laser power settings of 300 mW used in this study, and which we use routinely for chromosome analysis, are sufficient to produce a degree of, if not complete, photosaturation of the fluorochromes, where the relationship between fluorescence intensity and excitation power is no longer linear but approaches a plateau (data not shown). This approach generates a highly resolved flow karyotype that is less sensitive to illumination differences as chromosomes transit the laser illumination (12). We suggest that it is this factor that leads to the improved resolution of the flow karyotypes of both cell lines used in this study and such high-laser powers are necessary to obtain a well resolved flow karyotype for high resolution bivariate chromosome analysis. With the advent of second generation sequencing, flow sorting of chromosomes is entering another new phase of clinical utility allowing purification of specific chromosomes of interest for complete sequencing. High-resolution flow karyotyping and sorting is essential for this purpose to maximize the relevance of the sequence produced and minimize the costs.
Acknowledgments
The authors thank Dr. Simon Holden (Guy’s Hospital, United Kingdom) and Dr. Oi Lian Kon (National Cancer Centre Singapore) who kindly provided the cell lines used in this study.
Grant sponsor: Wellcome Trust; Grant number: WT077008
Literature Cited
- 1.Marx J. Medicine. Fused genes may help explain the origins of prostate cancer. Science. 2005;310:603. doi: 10.1126/science.310.5748.603a. [DOI] [PubMed] [Google Scholar]
- 2.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 3.Yin CC, Cortes J, Barkoh B, Hayes K, Kantarjian H, Jones D. t(3;21)(q26;q22) in myeloid leukemia: An aggressive syndrome of blast transformation associated with hydroxyurea or antimetabolite therapy. Cancer. 2006;106:1730–1738. doi: 10.1002/cncr.21797. [DOI] [PubMed] [Google Scholar]
- 4.Gasparini P, Sozzi G, Pierotti MA. The role of chromosomal alterations in human cancer development. J Cell Biochem. 2007;102:320–331. doi: 10.1002/jcb.21481. [DOI] [PubMed] [Google Scholar]
- 5.Burrow AA, Williams LE, Pierce LC, Wang YH. Over half of breakpoints in gene pairs involved in cancer-specific recurrent translocations are mapped to human chromosomal fragile sites. BMC Genomics. 2009;10:59. doi: 10.1186/1471-2164-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray JW, Trask B, van den Engh G, Silva A, Lozes C, Grell S, Schonberg S, Yu LC, Golbus MS. Application of flow karyotyping in prenatal detection of chromosome aberrations. Am J Hum Genet. 1988;42:49–59. [PMC free article] [PubMed] [Google Scholar]
- 7.Lebo RV, Golbus MS, Cheung MC. Detecting abnormal human chromosome constitutions by dual laser flow cytogenetics. Am J Med Genet. 1986;25:519–529. doi: 10.1002/ajmg.1320250314. [DOI] [PubMed] [Google Scholar]
- 8.Cram LS, Bartholdi MF, Ray FA, Travis GL, Jett JH, Kraemer PM. Quantitation of one aspect of karyotype instability associated with neoplastic transformation in Chinese hamster cells. Prog Nucleic Acid Res Mol Biol. 1983;29:39–42. doi: 10.1016/s0079-6603(08)60428-6. [DOI] [PubMed] [Google Scholar]
- 9.Howarth KD, Blood KA, Ng BL, Beavis JC, Chua Y, Cooke SL, Raby S, Ichimura K, Collins VP, Carter NP, et al. Array painting reveals a high frequency of balanced translocations in breast cancer cell lines that break in cancer-relevant genes. Oncogene. 2008;27:3345–3359. doi: 10.1038/sj.onc.1210993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey T, Houck DW, Shenker BJ, Hoffman RA. Bivariate flow karyotyping with air-cooled lasers. Cytometry. 1994;16:169–174. doi: 10.1002/cyto.990160211. [DOI] [PubMed] [Google Scholar]
- 11.Ng BL, Carter NP. Factors affecting flow karyotype resolution. Cytometry A. 2006;69:1028–1036. doi: 10.1002/cyto.a.20330. [DOI] [PubMed] [Google Scholar]
- 12.Bartholdi MF, Sinclair DC, Cram LS. Chromosome analysis by high illumination flow cytometry. Cytometry. 1983;3:395–401. doi: 10.1002/cyto.990030602. [DOI] [PubMed] [Google Scholar]