The immunobiology of periodontal disease continues to evolve, resulting in a number of foundational changes to the view of this disease across scientific disciplines. The first level of this evolution was fundamentally based upon the extraordinary microbiologic studies that provided a solid framework for understanding the basic stimuli for the disease (48), as seen in its recent incarnation of the Human Microbiome Project (292). The second evolving level was directly linked to the continued progress in parsing out the complexity of the human inflammatory, innate and adaptive immune responses that paralleled these microbiological findings. This molecular knowledge has created a schematic of the host-bacterial interactions that likely occur in the periodontium, as the host responds to the resident microbiota, both commensals and pathogens, with the outcomes being a maintenance of homeostasis or select individuals succumbing to a disease process. These findings were interpreted to indicate that the disease process in the periodontium was ‘collateral damage’ from the normal host response repertoire attempting to manage the chronic septic environment and resulting challenge to host tissues. This review attempts to enlighten the interdisciplinary research community that is documenting the complexity of periodontal disease on the historical evolution of the field of periodontal immunology, and the ongoing research emphasis that is ‘drilling down’ ever more deeply into the molecular workings of the cells and tissues to explain the variation in disease initiation, progression, and resolution across the population.
‘The Way We Were’
Taking the community back about 40 years in the field of periodontology, our view of the biology of the disease was focused on two primary issues: (i) the identification and study of selected bacteria that had been cultured from the oral cavity and seemed to increase in periodontal lesions, albeit with a recognition by the ‘bacteriologists’ that the majority of species in the subgingival plaque were unculturable; and (ii) the incorporation of the rapidly emerging findings describing the complexity of the host response to infection, including molecular studies of inflammatory cells and biomolecules, emerging concepts of innate immunity and a more detailed description of the molecular processes needed for adaptive immunity, including the recently delineated mucosal (secretory) immune system.
Resistance to infection
Based on the hypothesis that accumulation and emergence of bacterial species in the gingival sulcus could act as a trigger for periodontal disease, studies were conducted that attempted to delineate how the host could resist the direct deleterious effects of this microbial challenge. The immune responses of mammals and the pathogenic/virulence capabilities of microorganisms have evolved together, each producing selective pressures on the other. Two primary attributes that are required by a pathogen for the production of disease are: (i) the ability to metabolize and multiply in or upon host tissues; and (ii) the ability resist host-defense mechanisms for a period of time sufficient to reach the numbers required to produce overt disease. Many bacteria produce infection by multiplying primarily outside phagocytic cells and, generally, when they are ingested they are readily killed by the phagocytes. These microorganisms produce infection and disease only under two general circumstances: (i) when the bacteria possess a structure or a mechanism that prevents them from being readily phagocytosed, and/or (ii) when impairment exists within the host intracellular killing mechanisms. These types of bacteria may cause surface infections (mucosal surfaces), systemic infections or local infections. Colonization and infection of a mucous membrane is very dependent on the adhesive properties of the bacteria. The oral cavity represents the entry portal for a wide array of microbial challenges, both transient and more permanent. The more permanent challenges are represented by the substantial bacterial colonization that exists in the oral cavity. This environment presents a variety of niches, including the oral mucosa, the tongue, the tooth surface and the gingival sulcus, for the establishment of unique microbial ecologies. Many members of the complex oral microbiota maintain a symbiotic relationship with the host; however, select individual species are clearly pathogens within this environment (169). In order for the host to maintain homeostasis within the oral cavity, various immune-response systems contribute to controlling the microbial colonization. These systems represent three inter-related entities – the salivary, the systemic (serum) and the gingival tissue immune systems – all of which have been evaluated for their contribution to resistance to periodontal infections.
The gingival tissues and gingival crevicular fluid have been shown to contain a complex array of immune components that not only bathe the gingival sulcus, but are also deposited into the oral cavity (77). Gingival crevicular fluid was shown to be derived from gingival capillary beds (serum components) and from both resident and emigrating inflammatory cells. This fluid contains a range of innate, inflammatory and adaptive immune molecules and cells that are presumably enhanced to contribute to the interaction of host and bacteria in this ecological niche. The oral epithelium represents the only site in the body in which the epithelial barrier is deliberately breached by hard tissues (i.e. cementum and enamel), which must be sealed from the external milieu. Hence, even within healthy gingival tissue, there is a fluid transudate that flows from the site of this seal, presumably as a mechanical factor to minimize bacterial accumulation. This fluid also contains a variety of macromolecular components that are derived from serum and from the interstitium of the gingiva. Thus, in addition the mechanical cleansing action of the fluid flow, these innate and acquired immune molecules may contribute to the healthy homeostasis. As inflammation of the gingiva increases, the transudate changes to an inflammatory exudate, which contains higher levels of serum-derived molecules, vascular-derived cellular components of inflammation and locally derived molecules from the gingival tissues. As the macromolecules derived from serum and from gingival tissues are structurally identical, it has been difficult to determine accurately the contribution of each to the exudate. However, there are clearly unique molecules that are produced in the local tissues.
Innate responses and inflammation
The environment contains a large variety of infectious microbial agents, including bacteria, viruses, fungi and parasites. Most infections in normal individuals are of limited duration and leave little permanent damage as a result of the ability of the individual’s immune system to combat infectious agents. Innate immunity acts as a first line of defense against infections, and most potential pathogens are eliminated before they establish an overt infection. However, during this era, emphasis in innate immunity targeted the ubiquitous nonspecific activities comprising an innate physiologic process called inflammation. The literature focused on the inflammatory response providing an orderly sequence of coordinated events devised to protect the host from infections and to minimize damage to host tissues. In periodontal health, a transudate fluid, reflecting the passage of serum or other body fluids through basement membranes, bathes the gingival sulcus. The fluid is characterized as having a low protein content and few cells. The vascular phase of inflammation affects the microcirculation, resulting in increased leakage of fluid from gingival capillary beds and the influx of polymorphonuclear leukocytes, creating a cell-rich inflammatory exudate. Production of this inflammatory exudate is a hallmark of the acute inflammatory response to provide a primary nonspecific defense. Members of this family of host-response cells have been shown to contain a wide array of granule products, including histamine, leukotrienes, heparin, serotonin and hyaluronic acid, as well as antimicrobial enzymes and factors. The studies further depicted that if these first defenses were unsuccessful, a chronic inflammatory response would ensue, involving many cells and biomolecules of the adaptive immune system. During this chronic inflammatory phase, enhanced tissue destruction was noted, even in the presence of activation of more specific reactions to numerous oral bacteria, including those considered pathogens, in this early stage of targeting likely etiologic agents. From these findings of a very active immune response accompanying progressive tissue destruction, a conundrum has resulted regarding the value of specific immunity to these chronic oral infections.
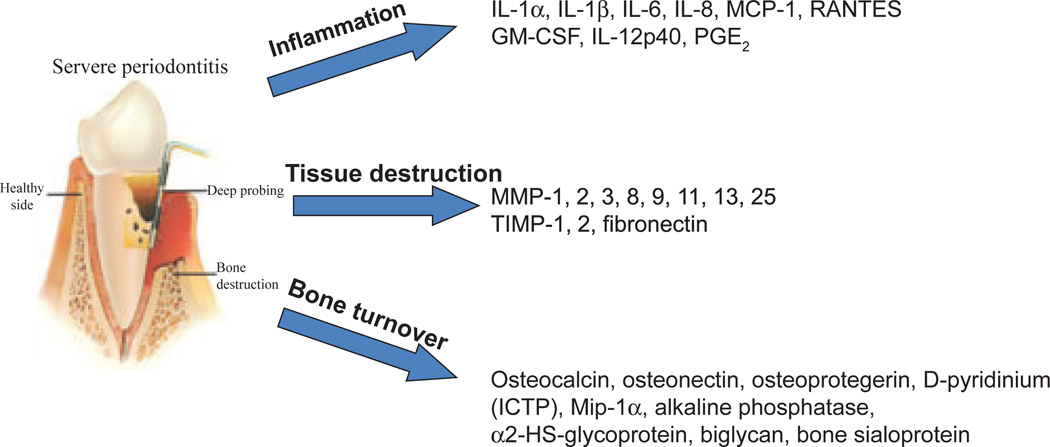
Cytokines and select inflammatory mediators play crucial roles in the maintenance of tissue homeostasis, a process that requires a delicate balance between anabolic and catabolic activities. Research findings on inflammatory mediators in gingival crevicular fluid have observed a range of molecules that are elevated with gingivitis and various forms of periodontitis. These include prostaglandin E2 and other eicosanoids (194), interleukin-1alpha and interleukin-1beta (250), interleukin-8 (24), interleukin-6 (56), interleukin-10 (280), transforming growth factor-beta (344), monocyte chemoattractant protein-1 (110) and tumor necrosis factor-alpha (278), as examples. Additionally, various investigations have demonstrated elevated levels of a range of tissue/cell-specific biomolecules in periodontitis, including aspartate aminotransferase, alkaline phosphatase and myeloperoxidase (252), an array of acute-phase proteins (e.g. α2-macroglobulin, α1-antitrypsin, transferrin, C-reactive protein and plasminogen activator inhibitor) (80), various complement components (12, 13), enzymes such as cathepsins (193) and matrix metalloproteinases (3, 94) and antimicrobial molecules [e.g. calprotectin (171) and antimicrobial peptides (65)]. During this research period, the paradigm evolved that while the range of responses of host cells in the periodontium to the microbial challenge is an important response to this septic environment, there was little doubt that excessive and/or continuous production of these mediators in inflamed periodontal tissues was considered to be responsible for the progression of periodontitis (350). Finally, in this era of studies of inflammatory responses in periodontitis, particularly related to patients with aggressive periodontitis (e.g. localized juvenile periodontitis and early-onset periodontitis) were the findings of alterations in neutrophil functions, including, chemotaxis, phagocytosis, superoxide production and bactericidal mechanisms (285).
Nevertheless, these studies generally emphasized descriptions of the cells and components that were present in the gingival tissues during periodontal health and gingival diseases. The studies attempted to delineate changes in these parameters related to progressing disease and resulting from treatment. Moreover, the findings began to elucidate potential mechanisms of the tissue destruction and cells that could be involved in defining resistance or susceptibility to progressing periodontitis. However, this knowledge base represents ‘The Way We Were’ and further elaboration of the dynamics of this environment is clearly required to understand the breadth and the variability of this disease in the population.
‘Act of Valor’
Cell-mediated immunity includes a range of actions based upon the requirement for viable cells to be present to affect the immune outcomes. Historically, the field of cellular immunity evolved to support the notion that a primary cell type in this immunity pathway – the T-lymphocyte – was associated with two distinct types of immunologic functions: effector and regulatory. The effector functions included activities such as killing of virally infected cells and tumors. In contrast, the regulatory functions served to amplify or suppress the immune response through cytokines and/or receptor–ligand interactions of costimulatory molecules acting on other effector lymphocytes, including B- and T-cells. Hence, it was suggested that unique subsets of helper and cytotoxic T-cells were involved in: (i) modulating immune responses; (ii) cooperating with B-cells in the induction of antibody synthesis; (iii) stimulating the release of cytokines for cellular communication to activate phagocytic cells; and (iv) aiding in the elimination of many intracellular and viral pathogens. Most circulating T-cells express a combination of cluster determinant (CD) markers, including CD2, CD3, CD4 (helper T-cells) or CD8 (cytotoxic T-cells), and a T-cell antigen receptor (303). In addition to T-lymphocytes, the second primary cell type involved in activating the adaptive immune response is the monocyte/macrophage, which is responsible for antigen recognition, immune stimulation and the tissue consequences that follow immune stimulation. Macrophages functionally overlap as effector cells in both natural and adaptive immune responses, whereby they can be involved in inflammatory responses and antigen presentation, and, once activated by cytokines, demonstrate nonspecific cytotoxicity to altered cells by both direct contact and by cytolytic factors.
Substantial literature has been provided detailing the characteristics of the cell-mediated adaptive immune responses across gingival health towards various forms of periodontal disease. Gingival health is histologically a balance between the existing subgingival microbiota and host resistance factors. Thus, there is some minimal inflammation with an associated flow of fluid into the healthy sulcus and the existence of some inflammatory cells in the tissues. In this state, the gingival tissues contain low numbers of lymphocytes, primarily T-cells that were suggested to be critical to maintaining a homeostasis between the host periodontal tissues and the bacterial plaque. Thus, even in clinically healthy sites, it appears that the host controls the local response capabilities and provides some intrinsic control of the inflammatory response (78).
Gingivitis has primarily been described as a response to the bacteria present in accumulated plaque. Beyond the acute response of neutrophils, there is some lymphocytic infiltrate containing T-cells; however, advanced and more chronic gingivitis can contain plasma cells. These local tissue responses are not associated with bacteria in tissues, but occur in response to products that appear to traverse the gingival epithelium that has lost some of its innate protective barrier functions. These observations were recapitulated in experimental gingivitis, where the inflammatory lesion remains dominated by lymphocytes. In general, systemic T-cell responses in gingivitis patients are slightly elevated compared with healthy subjects. These responses were noted to occur to some members of the microbiota that appear to increase in the supragingival and subgingival plaque associated with gingivitis. The results suggest that antigens from this bacterial accumulation have greater access to the systemic circulation, presumably through a break in the integrity of the epithelial barrier of innate defense.
Aggressive periodontitis has had numerous names over the decades, including ‘early-onset periodontitis’, ‘localized and generalized juvenile periodontitis’ and ‘rapidly progressive periodontitis’. Irrespective of what they have been called, these diseases appear to occur in a ‘high-risk’ group of individuals (26), and the hallmark of these diseases is a periodontal destruction that initiates at an early age, is rapidly progressive and is often not commensurate with the level of local inciting factors. The T-cells identified in periodontal lesions have been shown to have a decreased CD4/CD8 ratio when compared with normal gingival tissues or peripheral blood from the same patients, suggesting an altered immunoregulation that may contribute to periodontal pathology (61, 176, 298, 327), and the hall-mark of these diseases is a periodontal destruction that initiates at an early age, appears as rapid progression and is often not commensurate with the level of local inciting factors. However, there were reports of variable, and sometimes abnormal, circulating CD4/CD8 ratios in patients with aggressive periodontitis.
Extensive studies examining the characteristics of the inflammatory infiltrate in chronic (adult) periodontitis have been performed. In patients with aggressive periodontitis, the CD4/CD8 ratios were reduced in lesions from adults with chronic periodontitis when compared with homologous peripheral blood and normal/chronic gingivitis tissues (66, 89). The macrophage to T-cell ratio was also increased in lesions from adults with chronic periodontitis. In addition, these investigations suggested that the T-helper cells in diseased chronic adult periodontitis tissues expressed both cell-activation and memory-formation markers (33, 66). Taubman et al. (326) showed that CD8+ cells were enriched in diseased tissues and this, coupled with an alteration in the phenotype of CD4+ cells, suggested a role of these cells in regulating periodontal disease progression. Subsequent studies demonstrated that the majority of the T-cells were T-cell antigen receptor-alpha/beta positive, with lower numbers of T-cell antigen receptor-alpha/beta-positive cells restricted to the lamina propria of periodontitis lesions and differing from the levels in tissue from healthy sites (210).
Gingival tissue specimens were also characterized for T-cell antigen receptor-Vbeta usage. The results indicated that the T-cell repertoire in the gingival tissues differed significantly from that in peripheral blood, with a skewing of the Vbeta usage among both naïve and activated/memory T-cells (22, 357). These studies were extended to examine T-cell clones, and demonstrated that microorganisms (e.g. Porphyromonas gingivalis) preferentially induced a subset of Vbeta on CD4 and CD8 T-cells and that the activation appeared to be antigen specific (149). These studies also led to more detailed examination of the cytokine profile of these cloned T-cells when challenged with specific antigens. The results showed skewed cytokine profiles of T-cells derived from patients with adult periodontitis compared with T-cells from healthy or gingivitis patients.
However, it appears that characterization of these cytokines/mediators supports the local nature of host–parasite interactions in periodontitis and an emphasis, at the T-cell level, of unique features in this microenvironment that result in altered T-cell distribution and likely functions that are critical to the maintenance of periodontal health. These more specialized defenders emigrate into the infected/-inflamed tissues to maintain or re-establish homeostasis in this septic environment, as ‘An Act of Valor’.
‘Lost in Translation’
Acquired (or adaptive) immunity comprises the coordinate activation and function of cells and various molecules that facilitates the ability of the body to recognize and discriminate foreign-ness (i.e. non-self). Specific immune responses and specific immunity are usually developed following recovery from an infectious disease. The paradigm for this arm of the immune response was that the primary infection resulted in a state of decreased susceptibility to a subsequent attack by the same microorganism. The hallmark of humoral immunity is the production of antibodies, which are proteins produced by B-lymphocytes that are uniquely constructed to interact with the immune-stimulating antigens. The type of protective acquired immunity differs with different infectious agents. In general, protection mediated by humoral antibody (immunoglobulin) is effective against agents that exist extracellularly. In contrast, intracellular parasitic infections are principally resolved by cell-mediated immune reactions. B-cells are derived from the bone marrow, which remains the major repository for B-lymphocyte stem cells throughout life. Mature B-lymphocytes are the products of lymphoid stem cells that undergo a sequence of differentiation under the influence of a special microenvironment. In mammals the differentiation occurs first in the fetal liver and subsequently in the bone marrow. The B-cell development can be divided into two stages: antigen dependent; and antigen independent. B-lymphocytes have been classically defined by the presence or absence of membrane-bound immunoglobulin on the outer surface of their membrane. While mature B-cells predominately express membrane-bound immunoglobulin on their cell membrane, plasma (or memory) cells predominately produce secretory immunoglobulin. One of the most fascinating aspects of B-Iymphocytes is their heterogeneity; they differ in terms of the specificity of their antibody-combining sites and hence antigenic specificity. Immunoglobulins (antibodies) are glycoproteins representative of the adaptive immune system and are present in the serum and fluids of all mammals. These molecules bind specifically to foreign antigens, such as bacteria, viruses, parasites and toxins. Five distinct classes of immunoglobulins – IgG, IgA, IgM, IgD and IgE – have been identified in most higher mammals. The constant regions of the human heavy chains also define four distinct subclasses of IgG (IgG1, IgG2, IgG3 and IgG4) and two subclasses of IgA in humans. Additionally, a unique structural variant of IgA exists in exocrine secretions, which is a dimer of IgA with an attached joining (J) chain and a secretory component. While there are hundreds of gene segments that recombine with one another to generate the variable regions of antibodies, relatively few gene segments encode the constant regions, which define the heavy chains for the major classes or isotypes of immunoglobulin (IgM, IgD, IgG1, IgG2, IgA, IgE, etc.) and the light chain types (κ or λ). Plasma cells can produce and release thousands of molecules per second, and the C-terminal portion of such secreted immunoglobulins is different from the membrane form of immunoglobulin made by B-cells.
A substantial amount of literature has detailed the characteristics of the humoral adaptive immune response across gingival health towards various forms of periodontal disease. Immunoglobulins of all isotypes are generally present at low levels in gingival crevicular fluid from healthy gingival sites, minimizing the potential for various hypersensitivity reactions that could contribute to local tissue destruction. There is also a wealth of literature supporting the existence of local specific antibody production by plasma cells present in inflamed tissues of the periodontal pocket because levels of local antibody can be significantly greater than those in the serum (77, 195). Cross-sectional studies have suggested that those gingival crevicular fluid samples with elevated antibody frequently harbor homologous bacteria, as well as suggesting that a combination of the antigen and the host-response is frequently associated with progressing disease (81). After treatment, these local antibody levels decreased with pocket depth, attachment level stabilized and inflammation resolved (156). The presence of all subclasses of IgG were identified in gingival crevicular fluid from patients with aggressive periodontitis, with the IgG1 and/or IgG4 levels in gingival crevicular fluid often elevated relative to the levels in serum (77, 271), and generally exhibited some specificity for Aggregatibacter actinomycetemcomitans. Elevated IgG levels were also reported in gingival crevicular fluid from patients with chronic adult periodontitis (196, 307). Furthermore, certain subclasses of IgG appeared to be specifically elevated in gingival crevicular fluid in active sites of patients with chronic adult periodontitis (271, 353). We and others have found a significant frequency of gingival crevicular fluid samples with elevated antibody to P. gingivalis in periodontitis sites of patients with chronic adult periodontitis compared with healthy sites (18, 86, 223, 319). The results support a unique local response in individual sites within certain patients, and these results were interpreted to be consistent with some contribution of antibody to progression or resolution of the disease, apparent as subclass responses at sites of infection and disease.
An additional area of research findings in periodontitis was the detection and characterization of serum antibodies to oral bacteria (87, 130, 177, 226). The data demonstrated that antibodies to most oral bacteria were detectable in healthy subjects and were generally directed towards the microorganisms that colonize the supragingival and subgingival plaque in health (i.e. oral streptococci and Actinomyces). Serum antibodies to these types of bacteria were also found in gingivitis patients. Bimstein & Ebersole (26) reported serum antibody-response patterns in children and young adults to a group of oral microorganisms. The antibody levels exhibited an age-related increase to most microorganisms. The antibody levels in both the children and adults were dramatically different to numerous of the proposed periodontopathogens compared with those levels detected in periodontitis patients.
The humoral immune response in patients with aggressive periodontitis, particularly in those with localized disease, is consistent in populations across the globe. These patients, as well as many with more generalized disease, appear to have a common feature of infection with A. actinomycetemcomitans and a robust local and systemic antibody response to the oral pathogen (76, 85, 130, 269). These studies showed significant elevations in serum IgG and in specific antibody of all four IgG subclasses, IgG1-4, which have been shown to be elicited selectively by certain types of antigens and to have diverse functions (82, 203, 213). Longitudinal investigations of host immune responses in aggressive periodontitis have examine the dynamics of systemic antibody responses to A. actinomycetemcomitans. These results suggested that A. actinomycetemcomitans infections relate to aggressive (early onset, localized juvenile) periodontitis with accompanying antibody responses that reflect periods of active disease. The dynamics of A. actinomycetemcomitans infection and the level and specificity of systemic antibody responses to this pathogen supported an important contribution of the immune response to managing this infection. However, the attributes of the relationship that exists between A. actinomycetemcomitans and infected hosts, which results in a stable commensal relationship, or more frequently develops into a pathologic host–parasite interaction, remain to be defined.
Elevated systemic antibody levels have routinely been noted in patients with chronic adult periodontitis (83, 87, 177, 255). The specificity of this antibody is most frequently directed towards P. gingivalis. It also appears quite clear that P. gingivalis is highly correlated with a subset of patients with both aggressive and chronic periodontitis (112, 305, 324). Longitudinal studies expanded on these findings and supported that P. gingivalis responses are unique in certain individuals and that these antibody levels are modulated with episodes of disease activity and treatment (15, 88, 236, 239, 283). Many of these studies also demonstrated that while P. gingivalis appears to play a prominent role in chronic periodontitis in adults, it is clear that other putative periodontopathogens comprise a distinct proportion of the population.
Finally, these observations were extended by many investigators to describe the specificity of these antibodies for individual antigenic components of oral bacteria, including outer membrane proteins, the leukotoxin of A. actinomycetemcomitans, and lipopolysaccharide, serotypic determinants and fimbriae of P. gingivalis, among others (35, 99, 226, 253, 346, 351, 352). The level and antigenic specificity of the antibody were reported to be related to the severity/extent of disease, disease activity and response to therapy in various patient populations.
Questions have arisen as to why there exists a coincidence of chronic infection with accompanying periods of exacerbated disease and an active, often substantial, local and systemic host antibody response in periodontitis. The data generated over many years support the concept that the quality of the humoral immune response to suspected periodontopathogens should have an effect on the etiology of periodontitis. Studies examining functional capabilities of the antibody suggested that this antibody should provide some protective ability; however, the detailed antigenic specificity and mode of action of this antibody may be crucial for truly effective protection. Support for this concept is linked to the use of vaccines against oral pathogens in both nonhuman primates (79, 235, 261, 262, 264) and now commercially in companion animals (241, 270) that appear to provide a level of protection. These studies indicated that serum and local antibody produced during infection could successfully lower the specific infection or minimize emergence of the pathogens towards a threshold that is necessary for destructive processes. Thus, the available evidence reinforced the hypothesis that these antibody molecules produced in response to infection modify the host–parasite association, although the variations in outcomes across investigations indicated a more complicated relationship between the antibody and the oral infections of periodontitis. This conundrum may be related to expression of antigenic drift and diversity in these oral opportunistic pathogens that result from host immune pressures, resulting in a selective advantage to remain in chronic colonization of the subgingival ecology. Alternatively, the antibody may not function efficiently within the context of the complex multispecies biofilms that inhabit the sulcus, and may protect these pathogens from optimally effective host responses (see ‘A Bug’s Life’ section). At present, with our current level of knowledge, the true significance and function of this antibody remains to be elucidated, and languishes somewhat ‘Lost in Translation’.
‘The Neverending Story’
Interleukin 1 was discovered by Gery et al. in 1972 (115) and was described as a lymphocyte-activating factor based on its mitogenic activity on lymphocytes. However, by 1985 it was discovered that preparations of interleukin-1 actually consisted of two distinct proteins, now called interleukin-1alpha and interleukin-1beta, with somewhat different, but often overlapping, functions (152). Of interest in this continually evolving field is that over the years, interleukin-1 has also been known as lymphocyte-activating factor, fibroblast-activating factor, B-cell-activating factor, leukocyte endogenous mediator, epidermal cell-derived thymocyte-activating factor, serum amyloid A inducer of hepatocyte-stimulating factor, catabolin, hemopoetin-1, endogenous pyrogen, osteoclast-activating factor and proteolysis-inducing factor. This facet of cytokine/chemokine biology, and the capacity of this area of host–response science to parse out a more well-defined molecular basis for macro-clinical immune activities and symptoms, continue to expand. Beyond this initial interleukin, we now have defined interleukins through to interleukin-33. These biomolecules have a range of overlapping functions to help engage and control immune and inflammatory responses. Interleukins act as differentiation and maturation factors for cells involved in immune responses, as well as function to communicate with nonimmune cells (e.g. epithelial cells, osteoblasts and osteoclasts). They represent warning signals for innate and immune responses, and through these activities are critical chemoattractants, leading cells to migrate into areas of challenge to the host. A number of the secreted proteins have direct antimicrobial properties. Finally, representatives of this family of host-resistance factors are crucial for engaging the systemic acute-phase response through triggering hepatocytes to produce an array of protective molecules in the vasculature.
The literature is replete with primary and review articles that target individual molecules or relate an array of these effector molecules to susceptibility or resistance to disease(s). Some particularly interesting articles related to oral and systemic health include tumor necrosis factor-alpha, which is produced by activated macrophages and other cells and has a broad spectrum of biological actions on many different immune and nonimmune target cells. Tumor necrosis factor-alpha is an important inflammatory mediator and accounts for the activity of cachectin, a lethally toxic, cytokine-mediated wasting (cachexia) factor, the induction of fever and stimulation of several of the acute-phase reactants (23). Interleukin-6 is clearly an interleukin that mediates communication between a large number of cell types by playing a role in the proliferation and differentiation of B-lymphocytes, plasmacytomas and hybridomas, hematopoietic progenitors, hepatocytes and T-lymphocytes (4). Other activities overlap those of interleukin-1 and tumor necrosis factor-alpha, and thus interleukin-6 is considered a major immune and inflammatory mediator. Interleukin-8 is the best-characterized member of the chemokine family, is produced following activation of cells with bacterial lipopolysaccharide and selected cytokines, and is the primary molecule to activate and chemoattract neutrophils (178). It is recognized that the control of the inflammatory and immune responses is a critical factor in the value of these systems; thus, molecules such as transforming growth factor-beta and interleukin-10 have been described as anti-inflammatory cytokines (150, 220). Finally, this communication system is directly linked to redirecting the character of the immune response, such that levels of interleukin-10 and interleukin-12, secreted by dendritic cells, in the microenvironment of T-cells have the capacity to enhance T-helper 1 or T-helper 2 cell responses (211). A common feature of all of these cytokines and chemokines is the existence of specific receptors for the molecules that are distributed qualitatively and quantitatively across a range of specific target cells.
Most importantly, this evolving area of biology as ‘The Neverending Story’ continues to create a fingerprint on the science of periodontology to aid in diagnosis/risk assessment, as well as to define mechanisms of disease progression or individual resistance to periodontitis in greater detail.
‘For Whom The Bell TOLL[s]’
A major area of research emphasis over the last decade has been related to the dramatic progress in understanding the crucial role of innate immunity in maintaining homeostasis of the host, whose surfaces are dominated by a microbiota that comprises a concentration of cells ten times greater than the number of mammalian cells in the body. A common feature of the innate response is the capacity of host cells to detect pathogen-associated molecular patterns or microbial-associated molecular patterns as alarm triggers of host responses. The pathogen-associated molecular patterns/microbial-associated molecular patterns are recognized by a range of cell-surface receptors that engage general molecular patterns of the microbial structures (e.g. lipopolysaccharide, CpG in DNA, peptidoglycan, etc.), and are best represented by the toll-like receptors. Engagement of the toll-like receptors results in specific intracellular signal pathways that interface with surface-associated adaptor molecules, such as myeloid differentiation primary response protein MyD88 and TIR-domain-containing adapter-inducing interferonbeta/translocating chain-associated membrane protein. These adaptor molecules trigger various MAPKs – serine/threonine-specific kinases – involved in directing cellular responses to various stimuli, including as mitogens, heat shock and proinflammatory cytokines. The MAPK pathways subsequently activate nuclear factor of kappa light polypeptide gene enhancer in B-cells and other nuclear transcription factors, resulting in the regulation of cell proliferation and differentiation, gene expression, cell survival and apoptosis, as examples. Importantly, related to understanding the immunobiology of periodontitis, these signal pathways are critical for eliciting the production of a wide array of cytokines and chemokines in host cell communication and defense. Evidence suggests that variation in the structural characteristics of microbial-associated molecular patterns (e.g. lipopolysaccharide from Escherichia coli and from P. gingivalis) could determine the activation of different toll-like receptors (16). Moreover, the levels of transcription factor activation (e.g. nuclear factor of kappa light polypeptide gene enhancer in B-cells) and cytokine production appear to change depending on the toll-like receptor activated (29). Ongoing studies will continue to delineate the role of toll-like receptors in local responses to commensal and pathogenic bacteria in the periodontium with a goal of elucidating more critically how the innate immune system manages the oral microbial burden to maintain homeostasis.
‘Good Will Hunting’
Mucosal tissues are colonized by an extremely dense and diverse microbiota of commensal bacteria, and occasionally interact with pathogenic microorganisms. These sites continuously sample foreign material via various cell types, including dendritic cells, which are innate immune cells within the skin and mucosa (including oral and gingival epithelium) that respond rapidly to infection, carrying crucial information on the infection to lymph nodes to trigger an immune response. Dendritic cells are effective at antigen processing and presentation, and are particularly adept at stimulating naïve T-cells to control the quality of T-effector cells (55, 118, 182, 201). Dendritic cells also play a critical role in innate immunity, responding to microbial challenge and producing elevated levels of interleukin-12 and interferons for host innate defenses (71, 100, 108, 113, 172, 173, 238, 245, 274).
Immature dendritic cell subpopulations differ in their repertoire of pattern recognition receptors (21, 136),, which recognize distinct classes of pathogen-associated or microbial-associated molecular patterns, including a range of bacterial, viral and fungal pathogens, through engagement of lipopolysaccharide, lipoteichoic acid and nucleic acid (e.g. CpG, DNA, double-stranded RNA) ligands (27, 167, 189, 355), enabling avid uptake of these foreign materials by the immature dendritic cells (199). This recognition of microbial components by immature dendritic cells triggers the production of selected cytokines, interleukin-1beta and tumor necrosis factor-alpha, that enhance the maturation of immature dendritic cells, resulting in the up-regulation of major histocompatibility complex class I and class II molecules, costimulatory molecules (CD40, CD80 and CD86) and various adhesion molecules (intercellular adhesion molecule 1 and very late antigen 4) (2, 155, 161, 208, 228). The immature dendritic cells require a maturation stimulus to irreversibly differentiate into active, T-cell-stimulatory, mature dendritic cells. Immature dendritic cells have several features that allow them to capture antigen: (i) phagocytosis (for the uptake of microorganisms) (188); (ii) macropinocytosis (the formation of large pinocytic vesicles to sample extracellular material) (12, 197); and (iii) active endocytosis mediated through receptors (e.g. macrophage mannose receptor and Fc receptors) (42, 155, 187, 355). The captured antigens enter the dendritic cell’s endocytic pathway where they interact with large amounts of major histocompatibility complex class II–peptide complexes in compartments that are abundant in immature dendritic cells (38, 44, 155, 279). During maturation of dendritic cells, these compartments convert to nonlysosomal vesicles and discharge their major histocompatibility complex–peptide complexes to decorate the dendritic cell surface (38, 44, 273, 279). Once primed, the dendritic cells migrate to secondary lymphoid compartments to present the complexes of antigen–peptide–major histocompatibility complex to naïve CD4+/CD8+ T-cells. Dendritic cell maturation is typically triggered by the interaction of products of microbial or viral pathogens (lipopolysaccharide, lipoteichoic acid, CpG and dsRNA) with pattern recognition receptors, such as toll-like receptors, enabling the uptake of antigenic components by immature dendritic cells (27, 43, 136, 138, 161, 166, 167, 240, 256). Following maturation of the dendritic cells with a hallmark of nuclear factor of kappa light polypeptide gene enhancer in B-cells activation contributing to effective antigen presentation to the adaptive immune system (49, 138, 162, 165, 345), the cells produce interleukin-6 and interleukin-12, enabling communication with both B- and T-cells as the major effector cell for antigen processing and presentation in adaptive immunity (114, 129, 132, 143, 165, 186). These cells express both major histocompatibility complex class I and class II cell-surface proteins, and present them to T-cells, allowing the dendritic cells to contribute towards directing the effector-cell outcomes of antigen contact.
The regulatory role of dendritic cells is of particular importance at mucosal surfaces as they are in constant and intimate association with external antigenic stimuli. The dendritic cells are clearly major antigen-presenting cells that capture diverse antigens throughout the body, process them and through various surface ligands and cell-communication factors enter into a cognate interaction with T-cells to trigger adaptive immune responses. While there is solid evidence supporting that dendritic cells have the ability to take up a broad and diverse array of antigens, experimental details documenting this generally present data of how the dendritic cells present antigens from individual bacterial species (129, 138, 141, 225, 317, 342, 356) or isolated antigens (5, 58, 155, 237). The adaptive immune response depends upon the responding subpopulation of dendritic cells (218, 219), the types of microorganisms and the local microenvironment (17). The signaling pathways activated through the receptors can lead to different response patterns (e.g. T-helper 1 vs. T-helper 2 responses).
Over the last decade both immature dendritic cells and mature dendritic cells have been identified in the periodontium, with data providing phenotypic descriptions of these cells, describing changes in these cell populations with progressing periodontal disease and demonstrating in vitro that these dendritic cells can function in antigen-presentation critical in controlling the adaptive antibody-response patterns in periodontal disease to individual bacteria (47, 55, 159, 160, 172, 173, 216, 321). The dendritic cells in chronic periodontitis have been reported to be abundant in the lamina propria of the pocket epithelium, demonstrated by elevations in major histocompatibility complex class II antigens, as well as in costimulatory molecules. In addition, cytokine profiles of in vitro-generated dendritic cells suggested that the oral pathogen, P. gingivalis, appeared to drive the dendritic cells towards a T-helper 2 anaphase-promoting complex/cyclosome activity (160), and only fimbriated P. gingivalis strains could interact and activate dendritic cells to mature and upregulate costimulatory molecules (158). Besides, P. gingivalis lipopolysaccharide can inhibit dendritic cell maturation, as well as chemokine production, suggesting tolerogenic properties for the anaphasepromoting complex/cyclosomes (47, 164). Extending the observations of these studies by Gemmell and colleagues documented multiple anaphase-promoting complex/cyclosome subpopulations in healthy and diseased human gingival tissues and demonstrated that gingival tissue dendritic cell-infiltrating subpopulations change in response to treatment (62, 111, 184). Numerous biomarkers of innate immunity (e.g. lipopolysaccharide-binding protein, CD14 and toll-like receptors) are observed in gingival tissues, irrespective of the health of the tissues, although changes in toll-like receptor 2/toll-like receptor 4 appear in diseased gingiva (272). Combined, these results support the likely role of dendritic cells in diseased tissues and suggest that the development and function of these anaphase-promoting complex/cyclosomes may actually differ between the forms of periodontal disease (54).
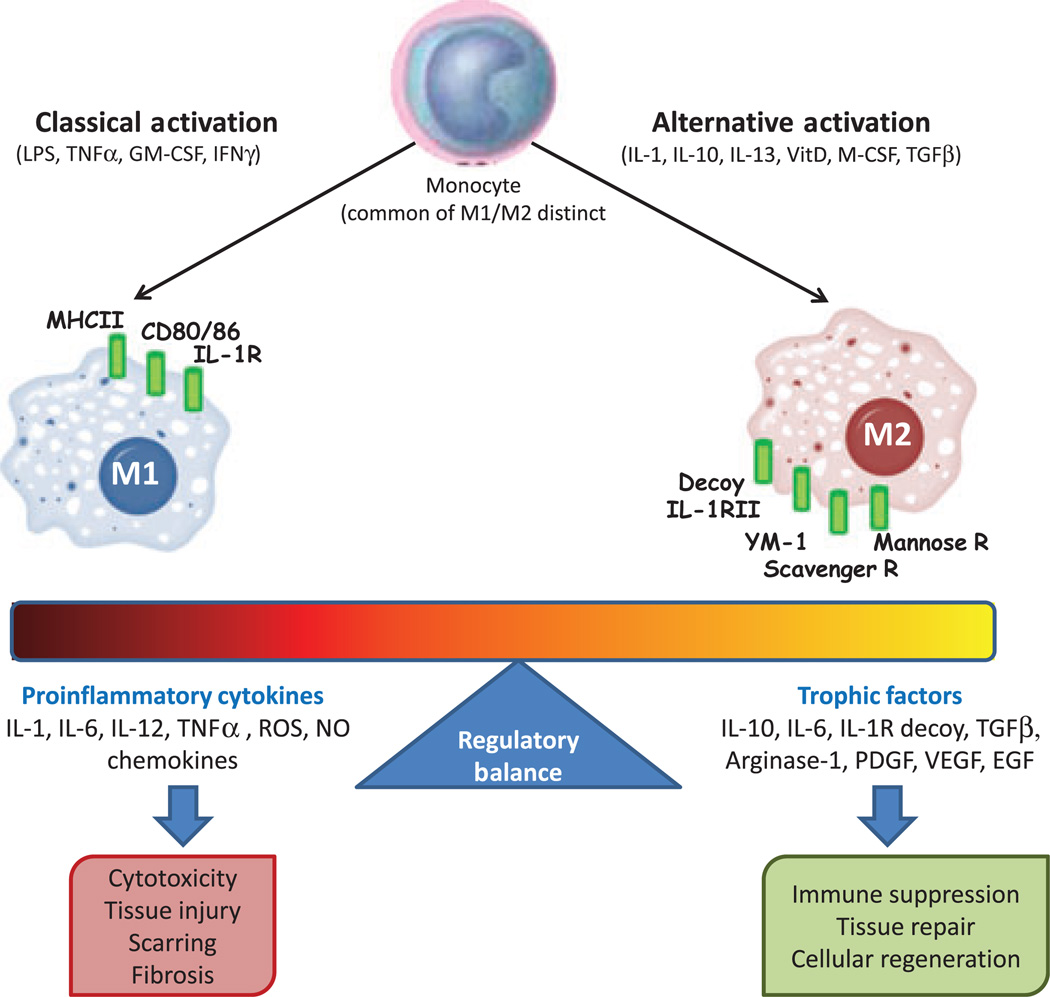
Of interest in this area of understanding host responses in the periodontium is the relatively recent classification of tissue macrophages into three major categories based on their functions (37, 229, 299). ‘Classical’ macrophage activation, which occurs during T-helper 1 immune responses, is involved in cellular immunity against intracellular pathogens resulting from the production of pro-inflammatory molecules and nitric oxide (Fig. 1). Macrophages can also develop a ‘deactivated’ phenotype in association with interleukin-10 and transforming growth factor-beta signaling. ‘Alternative’ macrophage activation occurs by interleukin-4 and interleukin-13 signaling through the specific interleukin-4 receptor during T-helper 2 immune responses (98, 291). New surface phenotypic markers discriminate the M1/M2 cells, including expression of mannose and scavenger receptors and chitinase-like molecules (YM-1) on M2. Alternative activation of the macrophages results in the downstream activation of arginase-1 that is considered to be crucial for their functional activities in wound healing (244). The M2 cells are also critical for immunomodulation, immunosuppression and (potentially) immunopathology in infectious diseases, as well as in noninfectious diseases (e.g. insulin resistance) and tissue repair. However, little is known regarding how these somewhat distinctive macrophage types transition to dendritic cells and whether these differences in functional features of the macrophages are carried forward to dendritic cell functions.
Fig. 1.
Phenotypes of macrophages with varied stimuli and functional capacity that affect the characteristics of host responses. EGF, epidermal growth factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFNγ, interferon gamma; IL, interleukin; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; MHC, major histocompatibility complex; NO, nitric oxide; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; TGFβ, transforming growth factor-beta; TNFα, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor; VitD, vitamin D3; YM-1, eosinophil chemotactic factor-L.
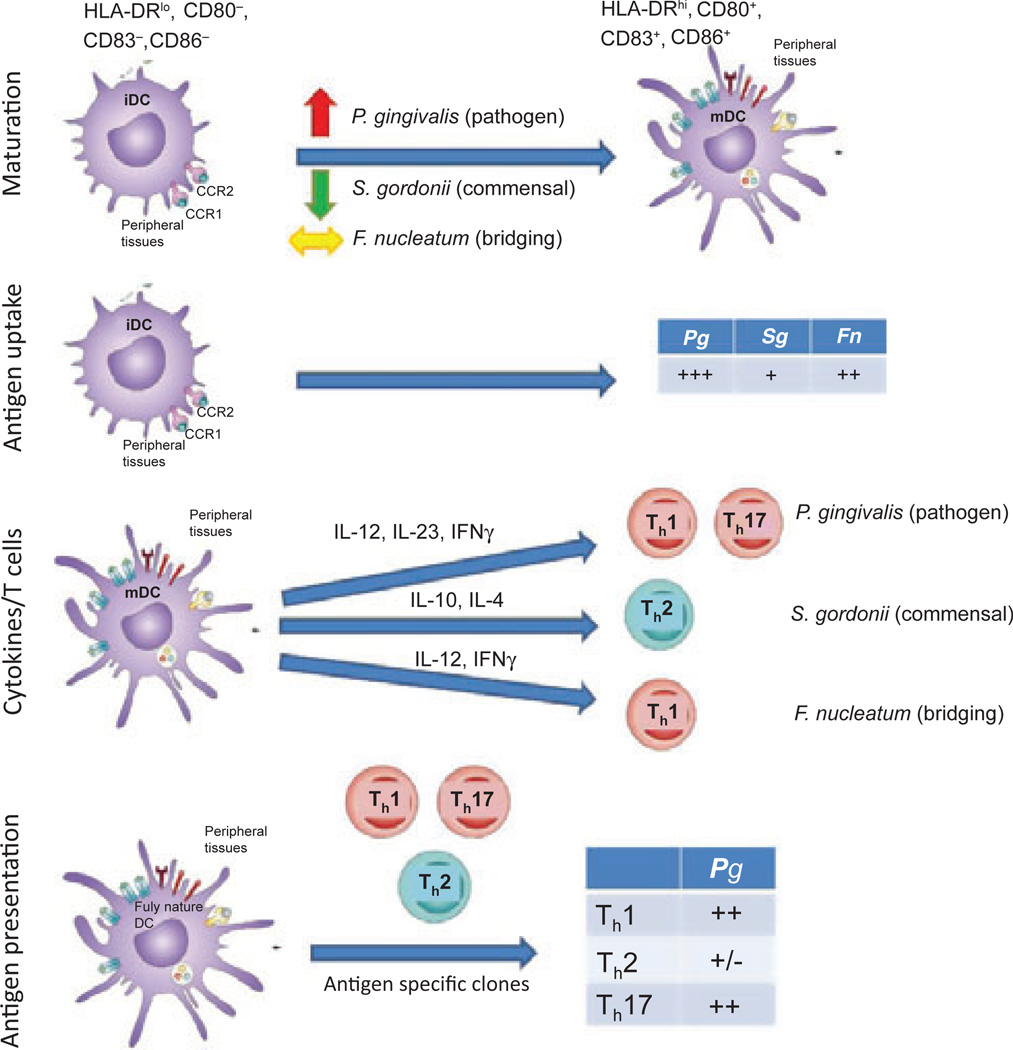
The ability to discriminate harmful pathogenic microbes from commensal species is a critical property of the immune system at mucosal sites for maintaining health and presumably is dependent upon the regulated function of dendritic cells. Nevertheless, the literature provides minimal information on the differences between immature and mature dendritic cell processes in response to commensal vs. pathogenic microorganisms. Some emerging concepts include that mechanisms of action at mucosal surfaces include ignorance of commensal microbial-associated molecular patterns, compartmentalized toll-like receptor expression and commensal-driven attenuation of proinflammatory signaling (267). In this regard, intestinal epithelial cells have been shown to be ‘tolerant’ of commensal microbial-associated molecular patterns through additional mechanisms that regulate binding of microbial-associated molecular patterns and signaling of pattern recognition receptors (304). Commensal microorganisms may also drive attenuation of proinflammatory signaling (170). The characteristics of gingival dendritic cell interactions with the complex microbial biofilms in the oral cavity remain undetermined, albeit triggering innate and adaptive immune-response specificity to members of the biofilms must be presumed to play a role in the maintenance of homeostasis, the formation of chronic destructive inflammatory lesions and the adaptive immune response contribution to correcting the dysregulated inflammatory response of disease. How this recognition is modulated by individual oral bacterial species has not been delineated. However, based upon existing models of responses related to regulated homeostasis or dysregulated chronic inflammation, Fig. 2 provides a model for consideration. In this schematic the immature dendritic cells have the capacity to engage more pathogenic bacteria (e.g. P. gingivalis), pioneering commensal bacteria (e.g. Streptococcus gordonii) or bacteria that increase in number with gingival inflammation and probably contribute important features to a climax pathogenic microbial community (e.g. Fusobacterium nucleatum) in the oral cavity. In identifying differences in the outcomes of these interactions, control points that the dendritic cells use to regulate these interactions appropriately include: (i) differences in recognition for binding to the cells, with alterations in effective maturation of the immature dendritic cells; (ii) differences in uptake related to specific receptor engagement and appropriate signaling – in this case, the commensals are not ingested as effectively and processed, leading to a less robust response; (iii) differences in the pattern of cytokines expressed following maturation, resulting in selective engagement of distinct subsets of T-cells, so that pathogens such as P. gingivalis would have a predilection to focus dendritic cell cytokine profiles that would select for T-helper 1 and/or T-helper 17 cell stimulation and chronic inflammation; and (iv) differences in effective antigen presentation, impacted by the characteristics of structures and antigenic epitopes of the microorganism, as well as the processing of the antigens through major histocompatibility complex class I or class II pathways, resulting in selective antigen-specific T-cell activation. As noted in this schematic, a pathogen like P. gingivalis would primarily enhance antigen presentation of the mature dendritic cells towards antigen-specific T-helper 1- and/or T-helper 17-driven expansion and the resulting chronic inflammation.
Fig. 2.
Dendritic cell interactions with various types of oral bacteria, and potential outcomes of dendritic cell functional development that could contribute to discrimination among commensals and pathogens. iDC, immature dendritic cell; Fn, Fusobacterium nucleatum; IFNγ, interferon gamma; IL, interleukin; mDC, mature dendritic cell; Pg, Porphyromoas gingivalis; Sg, Streptococcus gordonii; Th, T helper cell.
Even more complex is how the immature dendritic cells can discriminate, select, and respond to both commensal and pathogenic species within a polymicrobial challenge. The literature provides few studies that address this issue, and none that focus on oral bacteria (70, 103, 145, 287, 321). Scott et al. (290) addressed the effects of intact pathogens on the maturation and effector functions of human dendritic cells following challenge with gram-negative bacteria (E. coli, Salmonella enterica and Salmonella typhimurium), gram-positive cocci (Staphylococcus aureus), atypical bacteria (Mycobacterium tuberculosis and Mycoplasma hominis) and the protozoan, Trichomonas vaginalis. All these pathogens induced similar up-regulation of dendritic cell activation-associated cell-surface markers, but differences in the patterns of interleukin-12, interleukin-10 and interferon were observed, supporting the concept that dendritic cells are plastic in their response to microbial stimuli and that the nature of the pathogen dictates the response of the dendritic cells. Current theories hold that the host immune apparatus must be capable of recognizing potential pathogenic bacteria from among those that are integral to the commensal microbiota and contribute to innate resistance to infection, although data are rather sparse with regard to how dendritic cells manage interactions with complex microbial biofilms and polybacterial challenges that would occur in situ in the oral cavity. Moreover, how this initial immature dendritic cell interaction with a polybacterial infection alters the resultant antigen-presenting capabilities of the mature dendritic cells has not been identified. Consequently, while not exactly the thread of ‘Good Will Hunting’, the dendritic cells have a gift for interacting with bacteria that colonize the host, but clearly benefit from some direction in their microenvironment and are probably regulated by the characteristics of individual bacterial species and complex multispecies challenge.
‘A Bug’s Life’
Periodontal diseases reflect a tissue-destructive process of the hard and soft tissues of the periodontium that is initiated by the accumulation of multispecies bacterial biofilms in the gingival sulcus. We have learned that this accumulation (in both the quantity and the quality of bacteria) results in a chronic immunoinflammatory response of the host to control this noxious challenge, leading to collateral damage of the tissues. As knowledge of the characteristics of the host–bacterial interactions in the oral cavity has expanded, new knowledge has become available on the complexity of the microbial challenge and the repertoire of host responses to this challenge. Recent results from the Human Microbiome Project continue to extend the array of taxa, genera and species of bacteria that inhabit the multiple niches in the oral cavity; however, there is rather sparse information regarding variation in how host cells discriminate commensal from pathogenic species, as well as how the host response is affected by the three-dimensional architecture and interbacterial interactions that occur in the oral biofilms.
As the field of oral microbiology developed through the last few decades, substantial emphasis was placed on more accurately identifying the range of microbial species present in the oral cavity, defining their various niches and better discriminating the microbial components of healthy- and disease-site ecologies (53, 64, 66, 328). In parallel to these microbiologic studies, a range of investigations used cell-culture systems to explore the effect of oral bacteria and their products on the responses of immune and nonimmune cells to microbial challenge. These reports routinely used individual planktonic bacteria or soluble/secreted molecules from the bacteria to stimulate host cells (144, 169). These studies identified a hierarchy of stimulatory capacity of individual species, demonstrating, in broad strokes, that gram-negative bacteria generally induced stronger host cellular responses compared with gram-positive oral bacteria. Additionally, using isolated components from individual bacterial species (e.g. lipopolysaccharide, fimbriae and outer membrane proteins), investigations deduced some information on the relationship of these bacterial biomolecules with individual members of the host repertoire of responses (215). These results also contributed to an understanding of the function of pattern recognition receptors displayed by host cells in the capacity of innate immune responses to maintain homeostasis of oral tissues (153, 323). However, these approaches were limited by a reductionist approach to understanding the complexity of the interactions that probably occur in situ. Consequently, the field must advance by creating data that address the similarities or differences in host outcomes when challenged with a consortium of commensal and pathogenic species and the types of communications that are signaled in the host cells.
Extensive literature over the last three decades has described findings of specific microbial consortia that appear supragingivally and subgingivally in the oral cavity of humans (254, 362). Kolenbrander et al. (151, 180) have laid the groundwork for understanding the characteristics of a sequential acquisition of the oral ecology. These concepts have underpinned recent studies of the structure and organization of oral biofilms. They extended the observations of Costerton et al. (50, 57, 282, 315) that provided a paradigm shift of modeling biofilms as organized and structured three-dimensional assemblies of bacterial species which develop into a multicellular unit in which bacteria form specific scaffolds and passageways in the biofilms, allowing fluid flow for nutrition and waste disposal. The bacteria also have the capacity to detach from the sessile biofilm forms and seed distant sites to create new structures. As importantly, these in-vitro studies have demonstrated an organization of multiple oral species in these biofilms (73), not simply an amorphous conglomeration of the bacteria.
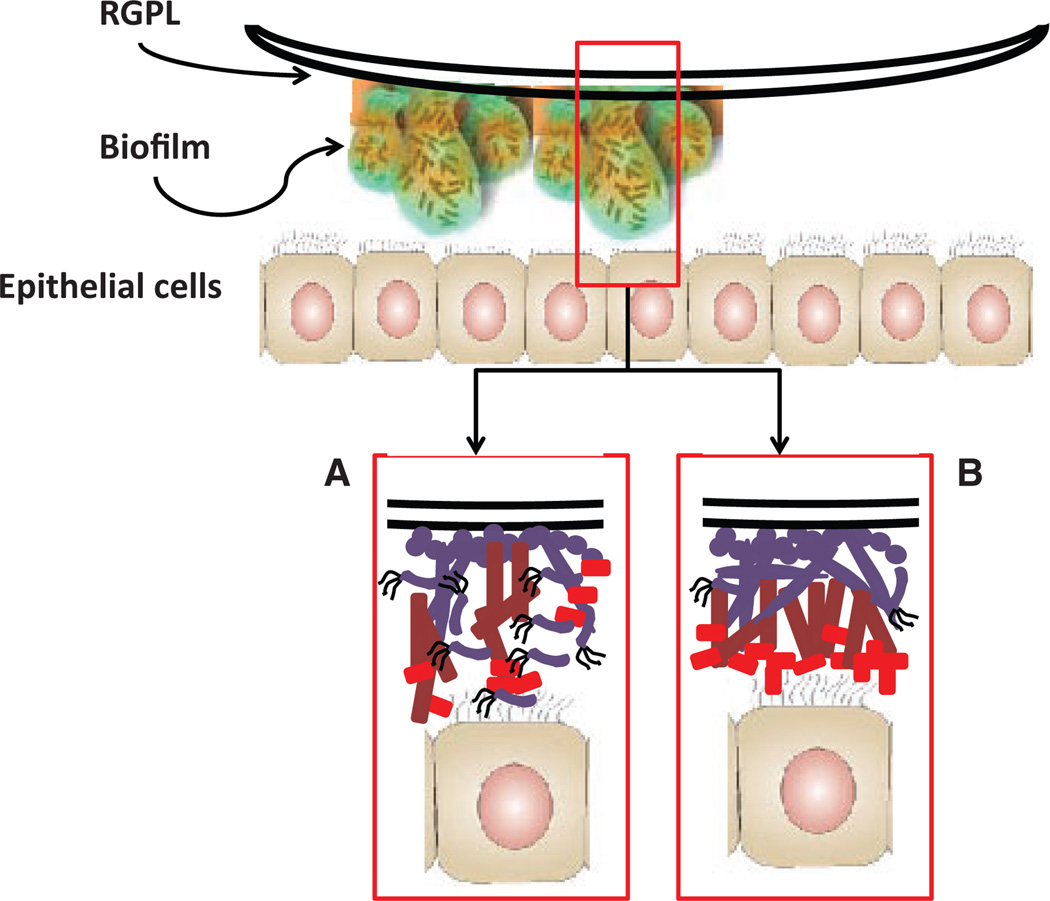
Expanding literature continues to emphasize the importance of biofilms in medical and dental infections, and posits that an important feature of the biofilms is their complex structure and their enhanced resistance to therapeutics and host–response molecules and cells. Various reports have begun to delineate the molecular mechanisms that can occur and which contribute to the unique features of biofilms at the prokaryotic and eukaryotic cell levels (57, 66, 139, 146, 179, 180). However, most of these have focused on proscribed systems of monobacterial infections interacting with host cells. Recently, Guggenheim and colleagues prepared oral multispecies biofilms and used these to challenge epithelial cell cultures (20, 128). While the architecture of these very complex biofilms was described using confocal scanning laser microscopy, the density of the bacteria that interacted with the individual cells remained undetermined, as did the species that may have been primary participants in this process. These reports did document a range of host responses that occurred under aerobic conditions and identified a range of mediators from the epithelial cells, including interleukin-1beta, interleukin-6, interleukin-8 (128) and RANKL/osteoprotegerin (20). We have also developed an in-vitro model in which biofilms are grown on rigid gas-permeable hard contact lenses. Rigid gas-permeable hard contact lenses, which allow substratum-bound epithelial cells (or other host cell types) to interact with the biofilms, can be used to demonstrate the characteristics of single and multi-species biofilms (Fig. 3). We used this novel model of bacterial biofilms to stimulate oral epithelial cells and profiled cytokines and chemokines that would contribute to the local inflammatory environment in the periodontium. Monospecies biofilms were developed with Streptococcus sanguinis, Streptococcus oralis, S. gordonii, Actinomyces naeslundii, F. nucleatum and P. gingivalis on the rigid gas-permeable hard contact lens. We also constructed, using the rigid gas-permeable hard contact lens model, multispecies biofilms to stimulate cytokines/chemokines in the oral epithelial cells. We created three model biofilms: (i) S. gordonii/S. oralis/S. sanguinis, representing the type of biofilm consistent with pioneer microorganisms and biofilms at healthy sites; (ii) S. gordonii/A. naeslundii/F. nucleatum, to represent the type of microbial biofilm that appears to emerge during gingival inflammation and contributes to the ability of more pathogenic species to emerge during the progression of periodontitis; and (iii) S. gordonii/F. nucleatum/P. gingivalis, to represent the type of bacterial interactions that might occur in triggering host cells at diseased sites. The findings of these experiments could be summarized into three primary concepts: (i) select multispecies biofilms stimulated a somewhat distinctive profile of cytokines/chemokines from the oral epithelial cells; (ii) examples of analyte levels were found in which the responses to the multispecies biofilms was significantly greater than that to a simple composite of the individual bacterial biofilms; and (iii) analysis of the analyte responses adjusted for the actual number of bacteria of each species in the multispecies biofilm demonstrated what appeared to be responses to the three-dimensional structure of the biofilms and was significantly greater than simply the number of the species inhabiting the environment.
Fig. 3.
Considerations for multispecies biofilms of oral bacteria interacting with epithelial cells and potential for resulting differential characteristics of subsequent innate immune and/or inflammatory responses. RGPL, rigid gas-permeable hard contact lens.
Consequently, there remain questions regarding the stimulatory capacity of individual bacteria in biofilms, how they compare with planktonic stimuli and how these responses would be altered in the presence of a complex multispecies biofilm. Thus, we have only scratched the surface of ‘A Bug’s Life’ in understanding, at the molecular level, the nuances of host–bacterial interactions that occur between biofilms and resident and inflammatory cells in the oral cavity.
‘The Perfect Storm’
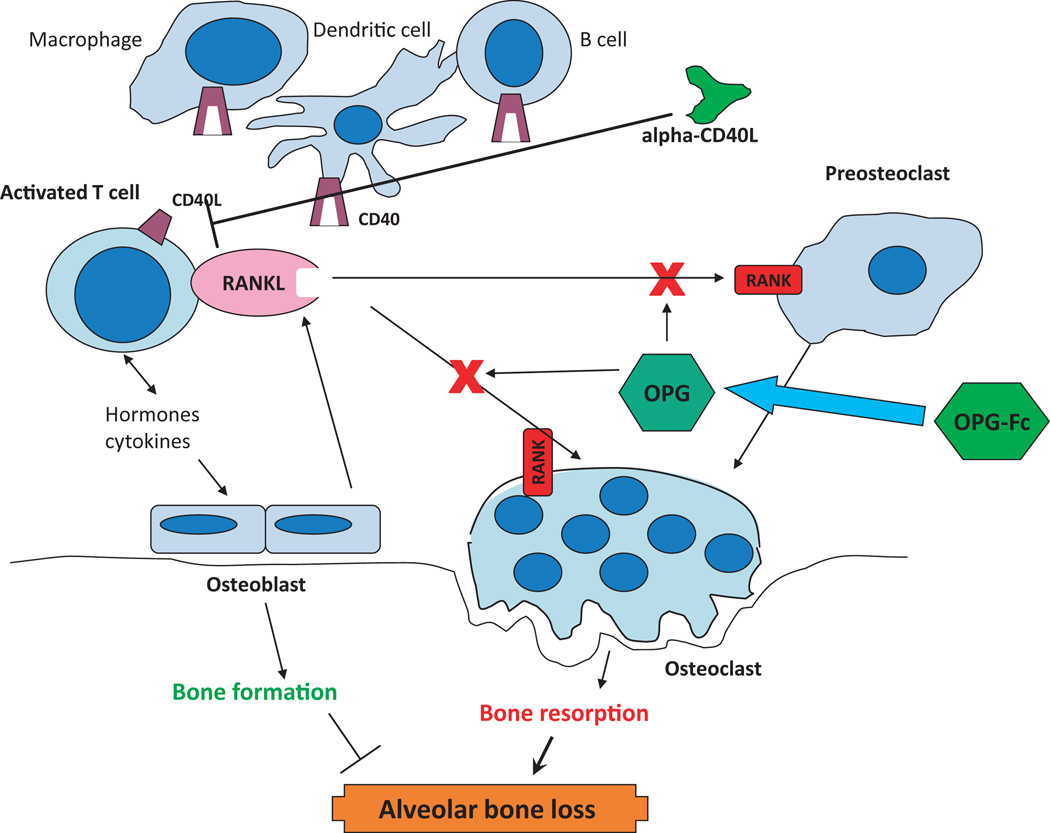
Alveolar bone resorption is the hallmark of destructive periodontitis, resulting from a chronic immunoinflammatory response to the microbial biofilms that inhabit the gingival sulcus. Variations in the rapidity of alveolar bone destruction in humans presumably represent intrinsic genetic differences that regulate the characteristics of the host inflammatory/immune response which links the cells and biomolecules of the immune system to the cellular differentiation and maturation pathways of osteoclastogenesis. Studies using in-vitro and in-vivo rodent models have described the importance of RANK (TNFRSF11A) expression on osteoclast precursors, coupled with expression of RANKL (TNFSF11) on bone stromal cells and on various cells of the innate and adaptive immune systems, in the induction of osteoclastic bone resorption. RANK is present on osteoclast precursors and is capable of initiating osteoclastogenic signal transduction after ligation with RANKL or anti-RANK agonist antibodies (34). Moreover, it is clear that RANK is an osteoclast receptor capable of mediating RANKL function during normal bone homeostasis and in disease, and no other receptors for RANKL have been identified. RANKL is produced by activated T-cells and its expression is up-regulated by many soluble factors affecting bone resorption, including proinflammatory cytokines. RANKL binds receptors on the surface of pre-osteoclasts and stimulates their differentiation into active osteoclasts, thus resulting in bone resorption. This system is regulated by the production of osteoprotegerin (TNFRSF11B). Osteoprotegerin is a soluble decoy receptor for RANKL, neutralizing its ability to bind with RANK and induce a signal. T-cells appear to be a major link between inflammation and bone loss. The osteoprotegerin/RANKL pathway is a key regulator of bone metabolism through its effect on the development and activation of osteoclasts. In addition to its role in osteoclastogenesis, RANKL is also a critical factor in the immune system, with roles in regulating the development of lymph nodes and Peyer’s patches, as an important costimulatory molecule in optimal T-cell activation and in mediating dendritic cell survival (Fig. 4).
Fig. 4.
Model of the RANK/RANKL/osteoprotegerin pathway for osteoclastic alveolar bone loss in periodontal disease. Also shown are potential sites for blocking these deleterious reactions with various ‘biologicals’ based upon receptor–ligand engagement and intracellular signaling pathways. OPG, osteoprotegerin.
Taubman et al. (131, 169, 327) have identified, using both in vitro cell-based systems and murine models of microbial stimulation of periodontitis, that the osteoprotegerin/RANKL pathway appears critical as a fundamental mechanism of alveolar bone resorption. As noted for other models of chronic inflammation and tissue destruction, this pathway also may provide the primary link between the immune and osteogenic/osteoclastic systems in both physiologic and pathologic bone biology in the oral cavity. The osteoprotegerin/RANKL pathway is a key regulator of bone metabolism through its effect on the development and the activation of osteoclasts. In addition to its role in osteoclastogenesis, RANKL is a critical factor in the immune system, from regulating the development of lymph nodes and Peyer’s patches to serving as an important costimulatory molecule in optimal T-cell activation and mediating dendritic cell survival. Beyond its role in inflammation and bone loss, RANKL also appears to regulate skeletal calcium release and is crucial in the morphogenesis of the lactating mammary gland.
Thus, this framework includes the emigration and local activation of host immune cells expressing and/or releasing RANKL, and the down-regulation of osteoprotegerin and of monocytic osteoclastic precursors expressing RANK. The expression of RANKL on the surface of T-cells, or the release of RANKL into the extracellular milieu at sites of inflammation, is regulated by T-cell activation, leading to the potential for ‘The Perfect Storm’ for destructive bone resorption. Previous studies have indicated that intrinsic levels of osteoprotegerin or extrinsic administration of osteoprotegerin that shift the osteoprotegerin/RANKL ratio in favor of osteoprotegerin, minimize various pathological bone-resorptive processes. The concentrations of osteoprotegerin and RANKL are controlled by many osteotropic hormones and cytokines, leading to changes in the osteoprotegerin/RANKL ratio. These osteotropic hormones and cytokines include: glucocorticoids (osteoprotegerin inhibition/RANKL production by osteoblasts), inflammatory cytokines (interleukin-1beta, interleukin-6, interleukin-11, interleukin-17 and tumor necrosis factor-alpha; induction of RANKL), beta fibroblast growth factor-2 (osteoprotegerin inhibition/RANKL production), parathyroid hormone (inhibits osteoprotegerin/RANKL production) and prostaglandin E2 (34, 135). Estrogen, on the other hand, appears to inhibit the production of RANKL and RANKL-stimulated osteoclastogenesis. The new understanding provided by the RANK/RANKL/osteoprotegerin paradigm for both differentiation and activation of osteoclasts has had tremendous impact on the field of bone biology and has opened new avenues for the development of possible treatments of diseases characterized by excessive bone resorption.
‘Anger Management’ or ‘How to Train Your Dragon’
Genetic control of inflammatory responses
What has evolved in periodontal immunology over the last 10 or so years is a clearer view of molecular events that accompany host responses to the complex microbial ecology in the oral cavity. The field continues to progress related to recent data from the Human Microbiome Project that is redefining the complexity of the autochthonous microbiota throughout the human body, and for the purposes of this review, various sites in the oral cavity. This will require some re-evaluation of the ‘challenge’ side of the host–bacterial equation leading to periodontitis. Additionally, the scientific findings clearly delineate that the tissue damage of periodontitis results not directly from the toxicity of bacterial factors, but from the capacity of the bacterial components to redirect host responses towards those that are chronic and detrimental to the health of tissues and cells, resulting in periodontitis as collateral damage from a dysregulated host response.
Thus, what we are now learning is how to manage the biology of this potentially angry environment, with an eventual goal of ‘training the host–response dragon’. One aspect of this challenge has benefited dramatically as a result of sequencing the human genome. In this post-human genome era, numerous investigations are attempting to identify targeted genetic predisposition to early aggressive and more severe periodontitis. It has been long recognized that within populations there appeared to be measurable differences in individual responses to plaque accumulation, and thus noxious challenge to the periodontal tissues. Thus, certain individuals appeared to produce a rapid and robust inflammatory response to bacterial plaque, while others generated an observably lower clinical inflammatory response. One interpretation was that the differences reflect some genetic contribution to the responses and a greater level of immunoregulation in the high responders, potentially related to resistance to destructive disease. Numerous studies have been provided that support the concept of a genetic contribution in aggressive periodontitis that aggregates within families (284). This finding has resulted in reports of HLA-A9, HLA-B15, HLA-A28 and HLA-DR4 being positively associated with aggressive periodontitis, and HLA-A2 and HLA-A10 being negatively associated (284, 299, 320). Marazita et al. (221) defined an autosomal major locus with dominant transmission for aggressive periodontitis, in both black and non-black subjects. However, how this genetic predisposition may contribute to susceptibility to aggressive periodontitis remains ill-defined. This could relate to neutrophil function (285), the capacity to form protective antibodies (163, 336, 337) or to the presence of receptors providing a more robust transmission and specific adherence capacity for A. actinomycetemcomitans (6, 361), among other options. Subsequent, more classical, genetic studies have identified abnormalities in genes related to molecules, such as cathepsin C (133), associated with periodontitis.
Finally, initial evidence of a linkage disequilibrium in an interleukin-1 genetic single nucleotide polymorphism was identified in aggressive periodontitis (67). These types of approaches have been expanded to interleukin-1 single nucleotide polymorphisms in chronic periodontitis. Numerous studies have followed, describing the types of interleukin-1 single nucleotide polymorphisms in chronic adult periodontitis in multiple populations across the globe (122). These findings, while not absolute, and not always clearly associated with alterations in the product of these genes related to disease, led to a multitude of international studies on single nucleotide polymorphisms for an array of pro- and anti-inflammatory biomolecules, as well as molecules related to adaptive immune responses. Generally, these studies have been conducted on smaller populations of convenience and most frequently do not identify significant differences in single nucleotide polymorphism expression for selected immune and inflammatory molecules. Nevertheless, summarizing these findings would support some likelihood of a genetic impact on control of the ‘dragon’ of the host immune response to periodontal infections that may contribute a somewhat immutable component to an individual’s resistance or disease susceptibility.
Apoptosis in periodontitis
Apoptosis is a mechanism of cell death without inflammation that is crucial for the maintenance of tissue homeostasis at different stages of life, including embryonic development and normal tissue turnover during adulthood (339). The recognition and removal, by phagocytes, of apoptotic cells within tissues is an event that occurs simultaneously with the recognition and removal of microbial pathogens from mucosal surfaces colonized by a high and diverse number of microorganisms, in order to maintain tissue homeostasis. It has been clearly described, for many years, that the main difference of eating apoptotic cells vs. eating microbes by phagocytes is the generation of an inflammatory response exclusively associated with microbial phagocytosis, in contrast to an anti-inflammatory phagocytosis (i.e. production of interleukin-10, transforming growth factor-beta and prostaglandin E2) which is related to the ingestion of apoptotic cells (97, 343). Most recently, it has been demonstrated that the phagocytosis of apoptotic cells, either infected or not with pathogens, dictates whether the dendritic cells that phagocytosed those cells instructed the generation of T-helper 17 or T-regulatory cells (333). In general, these findings support a central role for apoptosis as an essential mechanism that regulates the immunoinflammatory response against pathogens through the generation of anti-inflammatory signals affecting phagocytes at the site of the infection (originally recruited to clear the infection), as well as determining the type of T-helper response (97, 174, 333).
The relationship of pro- and anti-apoptotic events with susceptibility for periodontal disease remains unclear. In general, studies addressing the potential role of apoptosis in periodontitis have shown increased expression of apoptosis of biomolecules, mainly by neutrophils and mononuclear cells (107), as well as the down-regulation of pro-apoptotic molecules in lymphocytic cells associated with chronic adult periodontitis (207). Nevertheless, additional reports have not confirmed a significant role for apoptosis in periodontitis (185). Most of these studies have evaluated apoptotic changes in periodontitis using gingival biopsies from healthy and periodontitis subjects within a broad age range, and for a limited number of molecules related to apoptosis.
An existing paradigm in periodontitis is that it represents a disease of aging, with a higher prevalence and more severe disease occurring in aged individuals (8, 316). Consistent with age being a modifier for the prevalence or severity of periodontal disease, important biological differences in the composition and complexity of the oral microbial ecology, as well as the oral/systemic immunoinflammatory responses during aging, have been described in humans and animal models (84, 243, 286). However, the cellular and molecular changes of the periodontium (including apoptotic events) associated with a higher prevalence of oral diseases (e.g. chronic periodontitis) in aged populations have received little attention.
We have recently tested the hypothesis that the expression of genes associated with apoptotic processes are altered in aged healthy and periodontitis-affected gingival tissue through: (i) an ontological analysis of apoptotic genes expressed in healthy gingival tissue with regards to age; and (ii) investigating age-related variations of apoptotic gene expression in healthy compared with periodontitis-affected gingival tissues using a nonhuman primate model of periodontitis (121). The results showed lower expression of anti-apoptotic genes and higher expression of pro-apoptotic genes in healthy gingival tissue from young (comparative human age 9–12 years) compared with aged (70–85 human years) animals. Few differences in gene expression were observed in healthy gingival tissue between adult and aged animals. Comparison between healthy and periodontitis gingival tissues showed that the up- or down-regulated apoptotic genes in diseased gingival tissue are substantively different in adult animals compared with aged animals. These results suggested that normal apoptotic events in gingival tissues could be reduced with aging, and that unique aspects of apoptotic pathways are potentially involved in the pathophysiology of periodontal disease in adult vs. aged gingival tissues. Based on the anti-inflammatory and immunomodulatory role of apoptosis (driving T-helper 1 or T-helper 17 responses), and evidence showing that the majority of apoptotic events occur in the inflammatory cellular infiltrate during periodontitis, it is tempting to hypothesize that in contrast to young gingival tissues, reduced apoptotic responses in aged gingival tissues could involve failure to control the inflammatory response against the chronic bacterial challenge, resulting in dysregulated clearance of the inflammatory infiltrate. Accordingly, it has been recently shown that the number of mature dendritic cells is higher in gingival biopsies from elderly patients with chronic periodontitis compared with younger periodontitis patients (30). Given the significant role of apoptosis as a regulator of the immuno-inflammatory response, a better understanding of the role of apoptosis in periodontal disease is clearly needed, which will open new possibilities for therapeutic strategies oriented to re-establish the physiological pro-apoptotic mechanisms in diseased gingival tissues, specifically within the inflammatory cell population, as proposed for other chronic inflammatory disorders (209).
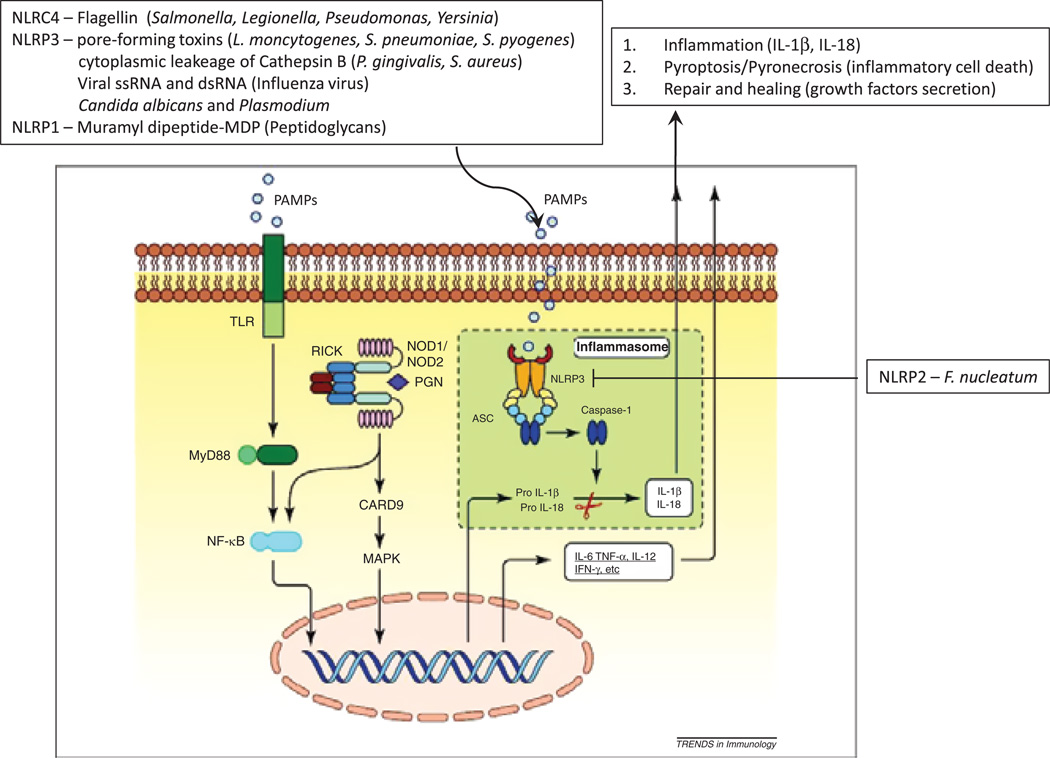
The inflammasome contribution to periodontal disease
It is clear that humans and microbes have co-evolved during millions of years, developing a symbiotic relationship where both parties can interact in a mutually beneficial relationship. For many decades it was thought that a passive physical barrier provided by the epithelial surfaces was the main mechanism of protection against opportunistic pathogens; however, it is now clear that host–microbe interactions are more active and specific at epithelial surfaces (e.g. skin and mucosal surfaces) that involve a growing number of families of cell-innate proteins named pattern recognition receptors. The nucleotide-binding and oligomerization domain-like receptor family of proteins is a group of cytosolic pattern recognition receptors involved in regulating the innate immune responses against pathogen-associated molecular patterns and danger-associated molecular patterns (109). About 22 intracellular nucleotide-binding and oligomerization domain-like receptors have been reported, which are classified into the following subfamilies, based on their N-terminal domain: (i) caspase-recruitment domain-containing nucleotide oligomerization domain with caspase recruitment domain receptors or nucleotide-binding and oligomerization domains (1–5); (ii) pyrin-domain-containing nucleotide oligomerization domain with pyrin domain receptors (1–14); and (iii) baculovirus inhibitor of apoptosis repeat-containing domain (neuronal apoptosis inhibitor proteins) (104, 318). The receptors are activated by bacterial peptidoglycan motifs, diaminopimelic acid from gram-negative bacteria and muramyl dipeptide from both gram-positive and gram-negative bacteria, which signal the activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells and the production of cytokines/chemokines (39, 119, 120). The activation of several members of the nucleotide-binding and oligomerization domain-like receptors gene family (i.e. NLRC4/IPAF (ice-protease activating factor), NLRPs 1, 3, 5, 6 and 7) by a range of pathogen-associated molecular patterns such as muramyl dipeptide, poly(I-C), double-stranded RNA from viruses, bacterial RNA and pore-forming toxins, as well as a range of danger-associated molecular patterns, such as extracellular ATP, uric acid, asbestos, silica, aluminum hydroxide and amyloid-β peptide, contributes to the assembly of a macromolecular protein complex termed the inflammasome (Fig. 5). This process leads to activation of the cysteine protease, caspase-1, which in turn induces inflammation (i.e. secretion of interleukin-1beta, interleukin-18 and interleukin-33), pyroptosis (a specialized type of proinflammatory cell death to control intracellular infectious niches) and repair and healing (fibroblast growth factor-2 secretion and lipid membrane biogenesis) (93, 193, 222, 335). Of note, it has been recently suggested that inflammasome activation influences many metabolic disorders, such as atherosclerosis, type 2 diabetes, gout and obesity (348). Other nucleotide-binding and oligomerization domain-like receptor members (e.g. NLRP2, NLRP10, NLRP12 and NLR family member X1) appear to be negative regulators of inflammation instead of inflammasome activators, decreasing the activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells and reducing type I interferon responses (9, 147, 234, 349), as well as modulators of the adaptive immune response, thus controlling dendritic cell migration from tissues to lymphoid nodes (13). Therefore, this emerging family of cytosolic sensors, with the ability to regulate the host responses to bacteria, inflammation and adaptive immunity, appear to play a crucial role at mucosal surfaces highly exposed to pathogen-associated molecular patterns and danger-associated molecular patterns, to maintain tissue homeostasis. Most recently, it has been suggested that nucleotide-binding and oligomerization domain-like receptors could be critical innate sensors for discriminating and controlling pathogenic species (i.e. bacteria expressing specialized secretion systems, pore-forming toxins and increased invasiveness ability) within the context of complex bacterial communities constantly colonizing the mucosal surfaces during health, mainly through a regulated activation of the inflammasomes (28).
Fig. 5.
Schematic of inflammasome with identification of various microbial products that have been shown to interact with various nucleotide-binding and oligomerization domains and resulting types of downstream responses. Adapted from Zaki et al., 2011 (360). ASC, apoptosis-associated speck-like protein containing a CARD; CARD, caspase-recruitment domain; IFN-γ, interferon gamma; IL, interleukin; NF-κB, nuclear factor of kappa light polypeptide gene enhancer in B-cells; NLRC, nucleotide oligomerization domain with caspase recruitment domain receptors; NLRP, nucleotide oligomerization domain with pyrin domain receptors; NOD, nucleotide-binding and oligomerization domain; PAMP, pathogen-associated molecular pattern; PGN, peptidoglycan; RICK, receptor interacting serine threonine kinase 2; TGFβ, transforming growth factor-beta; TLR, toll-like receptor; TNFα, tumor necrosis factor-alpha.
Evidence for the role of the inflammasome in periodontal disease is still very poor; however, the critical regulatory role that this macromolecular protein complex plays in inflammation triggered by pathogen-associated molecular patterns and danger-associated molecular patterns, and the solid evidence indicating that cytokines belonging to the interleukin-1 family, such as interleukin-1beta and interleukin-18, play a critical role in periodontitis (124, 251), makes it an interesting and important target to study in periodontal disease. Accordingly, recent in vivo and in vitro studies have shown variation in the expression of some inflammasome genes, in response to oral biofilms and planktonic bacteria, as well as the ability of some oral periodontopathogenic species (e.g. P. gingivalis and A. actinomycetemcomitans) to activate the inflammasome (10, 19, 31, 32, 358). In addition, the anti-inflammatory effect of NLRP2 activation, along with the ability of F. nucleatum to activate NLRP2 inducing the production of human beta defensins (154), suggest that this nucleotide-binding and oligomerization domain-like receptor could be a critical regulator of infection and inflammation of the oral mucosa.
Similarly, based on the premise that the aged population is at higher risk for infections and inflammatory disorders, we analyzed gingival changes in the expression of nucleotide-binding and oligomerization domain-like receptors and inflammasome-related genes that occur with aging during health and periodontitis. We were able to identify a subset of nucleotide-binding and oligomerization domain-like receptors and immunity-related GTPases that appears to change with aging in healthy gingival tissue, and most importantly, aging-related periodontitis was associated with decreased expression of specific nucleotide-binding and oligomerization domain-like receptors and immunity-related GTPases (O. A. Gonzalez, J. L. Ebersole, unpublished data). These observations suggest that the appropriate expression of these intracellular innate sensors during aging could be compromised, which would increase the risk for oral chronic inflammation determined by the partial or inefficient control of oral pathogens. The mechanisms by which these variations could be related to a higher risk and/or severity of periodontitis with aging will require further research.
‘The Departed’
Immunonutrition
Various reports describe an array of effects of altered nutrition on resistance to infections and host responses. Recently ‘immunonutrition’ has gained increasing importance in general health strategies. Impaired antioxidant defenses may contribute to disease progression with HIV, and treatment with antioxidants may slow the progression of AIDS. Protein-energy malnutrition is associated with greater malaria morbidity and mortality, and controlled trials of supplementation with either vitamin A or zinc have shown a substantial reduction in clinical malaria attacks. Treatment of established infections with vitamin A is effective in measles-associated complications. Short periods of zinc supplementation substantially improve immune defense in individuals with sickle cell anemia, renal disease, chronic gastrointestinal disorders, AIDS and diarrhea, as well as having a significant benefit on clinical outcomes. Many well-recognized disorders are associated with excessive intake of dietary fat, including obesity, insulin resistance, coronary heart disease and some forms of cancer; in particular, saturated, trans and arachidonic fatty acids have been linked to the development of chronic disease. However, research shows that n-3 polyunsaturated fatty acids, specifically fish oils (e.g. cold-water fish such as salmon, cod, tuna or mackerel) or plant oils (e.g. flax and linseed), can contribute to the prevention and treatment of disease. The effect of dietary lipid intake on resistance to infection suggests that fatty acids are involved in the modulation of immune responses through complex pathways.
In addition to nutritional impacts on infections, various studies have identified the capacity of dietary modulation to affect a myriad of host responses. In animal studies, inflammatory stimuli are influenced by dietary intake of copper, zinc, selenium, N-acetylcysteine, vitamin E and a cocktail of antioxidant nutrients that lead to a reduction in inflammatory symptoms. Several human and rodent studies have demonstrated that dietary fish oil ameliorates autoimmune diseases such as rheumatoid arthritis, Sjögren’s syndrome and systemic lupus erythematosus, as well as malignancy and cardiovascular diseases (46, 95, 192, 198, 212, 301). It has been shown that vitamin E, vitamin A, fatty acids and dietary caloric restriction can also positively impact various immune- cell functions, antibody levels, levels of oxidative stress and aging processes (224, 276). Dietary supplementation with n-3 polyunsaturated fatty acids may inhibit the synthesis of lipid mediators of inflammation (platelet-activating factor, prostaglandins and leukotrienes) that have potent anti-inflammatory effects. In addition, the biologic mechanisms of n-3 polyunsaturated fatty acid function include modulating lymphocyte proliferation, cytokine production, signal transduction, gene expression, oxygen radical generation and antioxidant levels (such as superoxide dismutase and catalase) (192, 314).
Nutrition and periodontitis
The relationship of nutrition to periodontal disease remains somewhat speculative. Various epidemiological studies have related nutrition to periodontitis by identifying increased disease in obese patients. These observations may well be unlinked to specific nutritional components, but reflect a general relationship between dietary intake and susceptibility to periodontitis. Recent studies have linked calcium intake to periodontal disease, presumably reflecting the wealth of knowledge relating this nutritional factor to bone density and osteoporosis. In response to periodontal pathogens, polymorphonuclear leukocytes release destructive oxidants, proteinases and other factors that can damage host tissues. Free radical-mediated oxidative stress has been implicated in adverse tissue changes in a number of diseases. Oxidants (hydrogen peroxide, free radicals and hypochlorous acid) also enhance the production of interleukin-1, interleukin-8 and tumor necrosis factor in response to inflammatory stimuli, and antioxidant defenses protect the host against the damaging influences of this type of cytokine/oxidant response. A number of reports have also described the potential contribution of antioxidants to oral inflammation and disease. These studies have generally been oriented toward providing exogenous antioxidants as dietary supplements, with a few of the studies actually demonstrating a moderating effect.
The main polyunsaturated fatty acids in most human diets are linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3). Fish oils are rich, dietary sources of the n-3 polyunsaturated fatty acids, eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3). Fish oil has effects on diverse physiological processes via its distribution to virtually every cell in the body, thus impacting cell functions, cell communication, production of various biomolecules and antioxidant activities (7, 40, 101). This range of effects has been proposed to account for the potent anti-inflammatory properties of n-3 fatty acids in human and rodent disease models (40, 101). Much of the attention over the last three decades has focused on the beneficial effects of dietary fish oils, particularly the n-3 fatty acid components, modulating a variety of chronic inflammatory diseases such as cardiovascular disease, rheumatoid arthritis, asthma, Alzheimer’s disease and Crohn’s disease.
Campan et al. (36) reported that human experimental gingivitis was modified by n-3 polyunsaturated fatty acids with findings of a lower level of inflammation, although the study was underpowered to provide any definitive conclusions. A recent report evaluated the ability of a topical application of n-6 fatty acids to modify experimental gingivitis in humans. The topical application of n-3 or n-6 fatty acids failed to inhibit the development of experimental gingivitis; however, rinsing with n-6 fatty acids could reduce the level of gingival crevicular fluid in established experimental gingivitis, although the biology of this effect remains enigmatic (75). It has been reported that the n-6 polyunsaturated fatty acid levels in the serum are higher in periodontitis patients, suggesting that an imbalance between n-6 and n-3 polyunsaturated fatty acids may contribute to susceptibility to oral bone loss. A pilot study of dietary fatty acid supplementation (borage oil) in the treatment of adult periodontitis found an improvement in gingival inflammation, but the study reported that further clinical studies using a larger number of subjects over longer periods of time were needed (275).
Resolution of inflammation
Acute inflammation in response to infection or tissue damage is usually characterized by heat, redness, swelling and pain at a visual level, and by edema and accumulation of leukocytes, and then by infiltration of mononuclear cells at a cellular level. Various lipid inflammatory mediators derived from arachidonic acid are crucial in the early stages of the inflammatory process. During inflammation, neutrophils are produced that have generally beneficial effects in countering disease, but in the longer term (or if malfunctioning) they may eventually cause trauma and tissue damage through infiltration into tissues. Consequently, a well-integrated inflammatory response and an intrinsic process for resolution are essential to re-establish homeostasis. Thus, over the last decade intriguing results, with direct relevance to periodontitis, have been produced in understanding the molecular events that govern termination of the acute inflammatory response.
An additional hallmark of inflammation is that cells die via apoptosis and need to be cleared from the system for effective tissue healing. In this regard, Serhan and colleagues (289, 296) discovered that lipoxins, which were described as a series of short-lived anti-inflammatory lipid mediators, endogenously produced eicosanoids. These authors have identified that the appearance of lipoxins (including lipoxin A4 and lipoxin B4) at inflammatory sites signals resolution of the inflammation. Both lipoxin A4 and lipoxin B4 signal through the lipoxin A4 receptor and effectively inhibit the activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells, chemotaxis and thus migration of inflammatory cells out of the vasculature, and the generation of oxygen radicals. Consequently, as part of the resolution of inflammation, the lipoxins signal macrophages to ingest the apoptotic cells. Additionally, during acute inflammation, proinflammatory cytokines, including interleukin-1beta and interferon gamma, can induce the production of lipoxins and anti-inflammatory cytokines (e.g. interleukin-4) that link the progression and the resolution of the inflammatory response.
Recent studies by Serhan and colleagues (106, 294, 295, 310) have reported intrinsic biochemical pathways that occur in resolving inflammatory exudates. The pathways were shown to generate a new family of locally acting mediators from the n-3 polyunsaturated fatty acids, eicosapentaenoic acid and docosahexaenoic acid (293). The authors termed these molecules resolvins and protectins as a result of their capacity to control the duration and the magnitude of inflammation. They are produced by enzymatic actions of the cyclooxygenase-2 pathway, particularly related to chemical reactions in the presence of aspirin (310). These anti-inflammatory lipids reduce the cellular component of inflammation by inhibiting the production and transportation of inflammatory cells and chemicals to the sites of inflammation. They have also shown that resolvins and protectins can remove chemokines that are generated in the inflamed tissues from apoptotic neutrophils and T-cells, as part of the molecular mechanisms of resolution. Subsequently in the body’s commitment to return tissues to health, the lipoxins, resolvins and protectins, and maresins, promote active resolution of the inflammation through removal of the leukocytes and other cellular debris, making them part of the molecular mechanisms that contribute to the restoration of tissue integrity once the need for the inflammatory response subsides. This orchestrated ‘healing’ also appears to include clearing remnants of the host defenses, the infecting microorganisms or other molecules that perpetuate the inflammation. These findings changed the paradigm of resolution from the concept of a passive process of the host to an active, regulated process controlled by specific molecular entities that resolve inflammation. Thus, this family of molecules appears to have an important role in regulating and inhibiting the harmful effects of continued inflammation, and represent biomolecules derived from the arachidonate pathway that oppose the effects of some of the proinflammatory eicosanoids with a likely impact on periodontal disease (338). In the movie ‘The Departed’ two men from opposite sides of the law, acting undercover to break an organized crime ring, were depicted, with the end result being questionable loyalties and blurring identities. Similarly, the proinflammatory and resolving molecules from the arachidonic acid substrates resulting from similar enzymatic pathways, requires complete understanding and close attention to their detailed roles in inflammation, to clarify the ‘identities and loyalties’.
Consequently, it appears obvious that these compounds and their metabolism have substantial potential for the development of biological therapeutic intervention in acute inflammation or chronic inflammatory disease(s), such as periodontitis. The corollary to this approach is with their proven anti-inflammatory, pro-resolving and protective properties, potential defective resolution mechanism(s) may underlie a range of inflammatory disease phenotype(s). Thus, continued understanding of these potent agonists of resolution, mapping of their resolution circuits and delineating their signaling pathways should continue to help clarify the molecular basis of inflammatory diseases.
‘Back to the Future’
Human microbiome and periodontal immunobiology
Successful colonization of the oral cavity depends on the presence of bacterial attachment sites on the conditioning layer, derived from saliva and gingival crevicular fluid, coating the oral hard- and soft-tissue surfaces (180) and microbial accumulation by autogenic and allogenic succession. Initial bacterial colonization by pioneering microorganisms alters the environment and enhances subsequent colonization by species more suited for the new environment (autogenic succession). Allogenic succession also occurs with environmental changes driven by a factor(s) other than those derived from the pioneer microorganisms, including host-controlled factors (181, 260). The resulting microbial communities or biofilms are complex ecosystems of bacteria that develop over time and are somewhat unique to various ecological niches (260, 363).
The ecology in an individual evolves over time at the level of the quantity and quality of phyla, genera and species (48, 257, 258), as well as the genomic profile of the individual species (72, 259, 339, 359). However, this evolution generally leads to an equilibrium between the microbiota and the environment, as a climax community. Climax biofilm communities are thought to be unique to each individual and ecologic niche in the oral cavity (181, 260). Microorganisms that colonize the oral soft and hard tissues and maintain a symbiotic relationship with the host are referred to as commensal bacteria (90). Pathogenic bacteria are those that are harmful to the host. Microbial biofilm communities on the subgingival tooth surfaces subjacent to the gingival tissues are composed of ca. 700 species (190, 258). The microbial ecology of the subgingival environments of periodontally healthy and diseased sites is distinct (190, 257, 258). Accumulation of tooth-associated bacterial biofilm (plaque) elicits gingival inflammation as a result of bacterial virulence factors and vascular dilation. In sites colonized by pathogendominated biofilms, the inflammatory response results in the destruction of connective tissue and alveolar bone, the classic features of periodontitis. The tissue destruction is actually a result of the host response elicited by the pathogens, rather than by direct toxic/noxious actions of the bacterial virulence factors (175, 325). Continuing research is identifying the effects of various environmental factors that affect the microbial composition in the oral cavity, as well as the host response. While smoking is a well-recognized risk factor for periodontal attachment loss, smokers often exhibit less gingival bleeding than would be predicted (68). This is probably a result of the effects of the toxic cigarette chemicals on the local vascular functions (68, 69). Minimal data are available to understand how the complex oral biofilms interact with host cells under various environmental conditions, and the resulting differences in outcomes of the host-cell responses trying to control the microbial challenge.
Host–bacterial interactions and immune adaptation
The immune system comprises both the innate and the adaptive immune responses that are used to manage bacterial infections. The adaptive immune response results from a cognate interaction of receptors on immune cells engaging antigens as ligands, resulting in the initiation of cell-mediated and/or humoral immune responses. Antigenic triggering of immunoglobulin receptors on B-cells leads to the maturation and differentiation of these cells into plasmacytes that produce antibodies, which are the effector molecules of humoral immunity (175, 302). Important to the objectives of this project, the host oral cavity is routinely colonized by a range of commensal bacteria, as well as by a varied number of potentially pathogenic species. While these bacteria all represent ‘non-self’, it remains unclear how the immune system differentiates commensals that are important in maintaining health from those bacteria with greater virulence capabilities (258). It has been suggested that the immune system has the ability to recognize commensal bacteria differently from pathogens, thus leading to a different type of immune response (90, 302, 340). However, the details of these characteristics, specifically with regards to the oral cavity, remain to be determined.
We have recently generated data to address this concept by: (i) determining the level of IgG to periodontal pathogens and oral commensal bacteria in smokers; and (ii) determining how antibody responses are affected by the extent of smoking and degree of periodontal disease (134). It was hypothesized that the level of IgG to periodontal pathogens would be greater than that observed to oral commensal bacteria. The extent of smoking and/or periodontal disease was expected to modify this relationship (i.e. higher levels of antibody to pathogens and lower levels of antibody to commensals) and contribute to a greater risk of progressing periodontitis. Thus, serum IgG reactive with three pathogenic and five commensal species of oral bacteria in 301 current smokers (21–66 years of age) was examined and related to clinical features of periodontitis. Antibody to the pathogens and salivary cotinine levels were positively related to disease severity; however, the antibody levels were best described by the level of clinical disease and were unrelated to the amount of smoking. The data showed a greater immune response to pathogens than to commensals; this was specifically related to disease extent and most notable in black male subjects. Significant correlations in individual patient responses to the pathogens and commensals were lost with an increasing extent of periodontitis and serum antibody to the pathogens. Antibody to P. gingivalis was particularly distinct with respect to the discriminatory nature of the immune responses in recognizing the pathogens.
A second area of interest in studies of adaptive immune responses is the conundrum of chronic colonization and multiple episodes of periodontal disease exacerbations over an extended time frame in patients with substantial levels of serum antibody to the pathogens. As noted in an earlier section of this review, human systemic and local antibody responses to a wide array of oral microorganisms have: (i) characterized a range of antigens from multiple pathogenic species; and (ii) determined variations in the levels of antigen-specific serum antibody to these microorganisms in longitudinal, prospective designs that often correlated with infection and disease progression. The extension of these observations is that putative oral pathogens represent a chronic infection of susceptible hosts and appear to emerge as opportunistic pathogens. Implied in this concept is that characteristics of the pathogen and/or the susceptible host must be favorable for the maintenance of this infection. Thus, a logical question that needs to be addressed is ‘Is the variability in host antibody responses to these oral pathogens that colonize periodontitis patients critical for the lifelong colonization of the oral cavity, and does this phenomenon reflect the ability of these microorganisms to demonstrate antigenic diversity as a strategy to effectively help circumvent host elimination from the ecology?’
The basis for this concept is that phenotypic changes in bacteria grown in vitro are paralleled by the dramatic ability of certain pathogens to alter the surface characteristics that they present to their environment(s). Variation in the antigenic composition of pathogens generally comes in two forms: antigenic ‘variation’ (shift); and antigenic ‘diversity’ (drift). Antigenic variation (shift) is generally limited to defining intrastrain variability. Antigenic variation is a specific strategy, which requires the ability to abruptly replace one type of surface antigen with another. It generally requires multiple nonallelic genes for surface proteins and usually some form of gene rearrangement (3). The characteristic of antigenic variation has been best described for Borrelia spp., Neisseria spp. and protozoa. The evolutionary relationship of antigenic variation to that observed between strains of the same species is not always evident. The antigenic differences within species is termed antigenic diversity (drift) and is often related to surface structures that are exposed to the defense mechanisms of the host, such as the H, K and O antigens of E. coli (1).
Antigenic diversity occurs by the gradual accumulation of mutations in genes that code for the targets of the immune system, which in gram-negative bacteria are frequently the outer membrane components. Antigenic diversity (i.e. heterogeneity) associated with outer membrane protein variations has been indicated for Bordetella spp., Campylobacter spp., Haemophilus influenzae, Pseudomonas aeruginosa, Bacteroides spp. and other pathogens (11). It appears to occur in all pathogens, but develops in some more than in others and may be more greatly affected in chronic infections than in acute infections. These mutations result in considerable population heterogeneity and in a drift toward selection of an antigenic type that is stable in the particular host–parasite interaction. This process represents a nonspecific strategy for evasion of host immunity.
Most of our knowledge concerning host antibody responses to extracellular bacterial pathogens has been derived from studies examining bacteria that result in acute infections and often life-threatening diseases (74). Knowledge of the fundamental aspects of the immunology of chronic infection still lags behind that of responses to injection of antigens or acute infections (233). Thus, in oral pathogenic infections in which the microorganisms continually colonize the subgingival ecology in the presence of an active host immune response with accompanying active or remitted disease, little is known concerning the evolution and interplay of the host responses that regulate these pathogens. Thus, while the existence of robust host immune responses to oral pathogens, such as A. actinomycetemcomitans and P. gingivalis, has been known for decades, limited data describing in-situ antigenic diversity (i.e. heterogeneity) are available in individual subjects; this reflects a modified antigenic repertoire, which contributes to the chronicity of the host–parasite interaction that is observed in periodontal infections. Accompanying this dearth of information are minimal data that describe the genetic heterogeneity of the pathogens within individual subjects over time, which are related to the characteristics of the host adaptive immune response and to disease emergence/progression. It would appear that the oral cavity represents a unique model for examining these dynamic parameters of host–bacterial interactions to members of the autochthonous microbiota, both pathogens and commensals.
Oral–systemic health linkages
The ‘Focal Infection Theory’ expresses the concept that a local infection affecting a small area of the body can lead to subsequent infections or symptoms in other parts of the body as a result either of the translocation of the infection or by the production and release of toxins into the vasculature. The modern expression of this theory arose in the late 19th century. At that time, W. D. Miller proposed that oral microorganisms derived from dental caries could play a role in lung and gastric infections, and even in brain abscesses, among other medical conditions (25). In 1900, William Hunter claimed that poor oral health could be etiologic for systemic diseases (137, 331). By 1912, Frank Billings had formally introduced the focal infection theory to American physicians, emphasizing that most infections were preventable, including those potentially derived from oral infections (reviewed in 117). While these pronouncements extrapolated the focal infection theory toward illogical conclusions and untoward clinical responses of practitioners, it did lay the groundwork a century ago that ‘the mouth is attached to the body’. Over succeeding decades in the century, this concept appeared to be lost by dichotomizing biomedical researchers into a cohort of investigators focusing on dental and oral health sciences and a cohort of physicians detailing diseases in the rest of the body.
In the 1980s, epidemiologic publications were surfacing regarding the association between periodontitis and some systemic conditions, especially coronary heart disease, stroke, diabetes and preterm birth/low birthweight. These findings were not lost on the dental profession, and appeared to be a ‘Back to the Future’ consideration of the validity of the theory of focal infection. The potential value of these reports was reinforced by comments that events in the oral cavity can influence systemic disease, which was highlighted by the US Surgeon General’s report in 2000 (41, 96, 249). This area has continued to expand, although the ability to transition associational data to a causal relationship has met with substantial challenges based upon the available evidence (205, 231, 246, 247). However, continually expanding evidence on the role of chronic systemic inflammation in many human conditions (e.g. cardiovascular disease, preterm birth/low birthweight, obesity and diabetes) has underscored some underlying commonality for susceptibility and contribution of chronic oral infections to this process. This relationship has been supported by studies of the ‘acute phase response’. Following injury, the body responds by increasing the hepatic synthesis of a number of plasma proteins, which increases their concentration in plasma and at the site of injury. Fever is one of the first acute-phase responses that was described and may occur following many types of inflammatory stimuli. The cytokine, interleukin-1, is now believed to be identical to the endogenous pyrogens that contribute to fever. Several components of the complement system are acute-phase reactants because they increase during infection. When bound to bacteria, C-reactive protein promotes the binding of complement, which facilitates the uptake of these bacteria by phagocytes. Acute-phase proteins also include protease inhibitors in human plasma that are involved in the inflammatory response by blocking proteases which degrade tissue surrounding an inflammatory process and cause damage leading to chronic inflammation. Various other acute-phase reactants bind iron and copper, and contribute to wound healing. A range of biomedical sciences have been examining the levels of these systemic analytes, many produced by hepatocytes, as biomarkers of chronic inflammation and risk for systemic disease (80). Of interest are the findings that patients with chronic periodontitis exhibit elevated levels of multiple acute-phase proteins that often decrease with therapy, including systemic administration of antiinflammatory drugs (80). We have recently shown that periodontitis patients undergoing elective oral surgery for removal of all teeth, exhibited elevated levels of various acute-phase proteins (e.g. C-reactive protein) that significantly decreased over a 1-year interval, but still remained above normal levels.
An intersection exists between this area of periodontal sciences and results being developed from the Human Genome Project and expansion of functional genomics and systems biology. As such, acute inflammation plays an important and necessary role in the protective immune response. Chronic inflammation, however, can invoke an unfettered production of cytokines, growth factors and reactive oxygen/nitrogen species that eventually foster some ‘loss of function’ as a result of destruction, fibrosis and/or necrosis of tissues and organs. Chronic inflammation has been associated with numerous conditions throughout the body, including: periodontal disease; autoimmune disorders (rheumatoid arthritis); respiratory disorders; digestive disorders; diseases of the vasculature (atherosclerosis); metabolic diseases (diabetes); and aging disorders (Alzheimer’s disease and dementia). Perhaps even more remarkable than the diversity of tissues affected by chronic inflammation is the observation that patients exhibiting one form of chronic inflammation appear to be more prone to develop other chronic inflammatory diseases at secondary sites within their body (51, 52, 59, 63, 102, 230, 242, 248, 265, 266, 312). This is exemplified by studies of periodontitis, which have demonstrated a strong association of periodontal disease with the development or presence of other chronic inflammatory diseases and/or infection(s) outside the oral cavity. For example, rheumatoid arthritis patients are eight times more likely to have periodontal disease compared with people without signs of autoimmune disease (265). Patients with severe periodontal disease are more likely to suffer from coronary artery disease and stroke than are individuals with healthy gums (51, 52). Chronic periodontal infections and inflammation have been associated with the impairment of memory and other cognitive functions, and periodontal disease increases the risk of dementia later in life (242, 266, 312, 313). Finally, it has been demonstrated that adults with tooth loss owing to poor dental health are more likely to develop rheumatoid arthritis, chronic kidney disease and dementia than are individuals who still have their teeth (59, 102, 312, 313). The common thread in each of these diseases is the presence of chronic inflammation. These findings suggest that: (i) a chronic inflammatory response in one area of the body may create a systemic environment primed for tissue damage and disease in other areas of the body; (ii) some individuals are more prone to chronic inflammation; and/or (iii) a combination of systemic environmental and genetic factors influences the risk of chronic inflammatory-based disease development and maintenance.
It is also interesting to note that the general process of aging appears to be associated with low-grade chronic inflammation. Studies have demonstrated that centenarians, individuals living to be 100 years of age or older, have a robust anti-inflammatory genetic profile (202, 203). If such a genetic profile is associated with a longer life and postponing or even preventing cancer, tissue damage/destruction and/or disease, then one could assume that an alternate proinflammatory genetic profile, potentially linked to chronic inflammation, could likewise be defined and may be the common denominator of multiple chronic inflammatory disease states. Evidence for a genetic predisposition to a strong proinflammatory response has already been demonstrated in cases of periodontal disease, rheumatoid arthritis and myocardial infarction among young adults, and premature aging (45, 123, 148, 183, 202, 227). Thus, a genetic predisposition to a robust proinflammatory response could be the common underlying precursor to many chronic inflammatory diseases, and as such may represent a systemic-wide ‘first hit’ in a multiple-hit model of disease formation. Accordingly, additional systemic/environmental factors and/or genetic variations could supply ‘a second and/or third hit’ to a specific tissue site, ultimately leading to disease development within that specific tissue (i.e. periodontal disease in the oral cavity, rheumatoid arthritis in the joints, Crohn’s in the digestive tract, etc.…). Taken together, these observations suggest that (i) an unhealthy or ‘out-of-balance’ systemic environment, primed for tissue damage, exists in some individuals, which facilitates new chronic inflammatory disease development at auxiliary tissue sites; (ii) some individuals are inherently more prone to the development of chronic inflammatory diseases; and (iii) genetic factors/variations may influence the generation or maintenance of the systemic environment imbalance. To date, there are few, if any, studies that have identified a specific set of genetic markers that could predict an individual’s predisposition to chronic inflammation with an effective detection range spanning a multiplicity of chronic inflammatory diseases.
With the introduction of genome wide association studies, and the enormous monetary and time investments going into genome wide association study-based disease studies, it is imperative that research scientists take advantage of the knowledge gained from the studies when designing disease-based studies – instead of trying to reinvent the wheel. Many early genome wide association studies were small in size, focused on a single disease and had the best resolution for identifying only the genetic markers with strong disease associations (for example: rs1537415-G in the GLT6D1 gene for periodontal disease, rs6910071-G in the HLA-DRB1 gene for rheumatoid arthritis and rs2076756-G in the caspase-recruitment domain 15/nucleotide-binding and oligomerization domain 2 associated with Crohn’s disease) (105, 281, 311). The studies that followed increased their power by enlarging subject enrollment and utilizing more total markers for better chromosomal coverage, and they have begun to make multiple disease comparisons (91, 92, 204, 268, 330, 334). Importantly, as genome wide association studies have expanded, it has become increasingly apparent that genetic markers can effectively be utilized as risk predictors of complex diseases (157). Recent studies with multiple disease comparisons are beginning to uncover genetic markers/variations with moderate disease associations that span multiple disease states (91, 92, 204, 268, 334). A landmark genome wide association study was designed to examine 2,000 subjects in each of seven different disease states and 3,000 shared controls (330). In addition to the standard genome wide association studies analysis, scientists with the Wellcome Trust Case Control Consortium have examined the influence of a cumulative number of genetic variations within different biological pathways to gain a better understanding of genetic determinants (unique and overlapping) influencing human chronic inflammatory diseases such as rheumatoid arthritis, Type 1 diabetes mellitus and Crohn’s disease compared with four noninflammatory diseases (92).
Genetic variations come in many forms, including single point mutations, additions/deletions and duplications. The most common form of variation is the single nucleotide polymorphism. There are an estimated 1.4 million single nucleotide polymorphisms identified in the human genome alone. These types of genetic variations can act to enhance, diminish or have no influence on processes such as gene transcription, protein translation and/or protein function based on where they reside in the DNA. Individuals inheriting a number of single nucleotide polymorphisms, which favor/enhance a proinflammatory response (and/or diminish an anti-inflammatory response) would be predisposed to a proinflammatory profile that could contribute to a systemic environment favoring chronic inflammatory disease development. During the innate immune response, a delicate balancing act is started early on to keep the newly initiated proinflammatory response from proceeding unchecked and leading to unwanted tissue damage or destruction. Surveillance cells of the immune system (natural killer cells, monocytes, tissue macrophage and/or dendritic cells) set off a proinflammatory response with the release of numerous cytokines, including interferon gamma, interleukin-1beta and tumor necrosis factor-alpha. These cytokines jump-start the clonal expansion of effector cells (T-cells and potentially B-cells), stimulate T-cell and/or macrophage production of additional cytokines and chemokines (such as interleukin-2, interleukin-6 and interleukin-8) and facilitate immune-cell activation. This type of immune response is maintained until clearance of the immune threat is achieved and anti-inflammatory mechanisms return the system to baseline (i.e. release of interleukin-10 and/or transforming growth factor-beta; mechanisms of apoptosis, etc.…).
Thus, the final verdict has still not been reached regarding causal linkages of chronic oral infections and inflammation with systemic diseases, but investigations at the molecular, genetic, epidemiologic and animal model levels continue to generate intriguing data, stimulating continued discussion related to risk assessment, diagnosis and more personalized monitoring/therapy.
Salivary biodiagnostics
Our understanding of the mucosal immune system has made substantial progress over almost the last 50 years in providing an understanding of the unique features of this immune system, as well as the crucial role that it plays at mucosal surfaces throughout the body. Early on, numerous studies of the oral cavity focused on the characteristics of these mucosal responses, particularly as they related to immune components and the potential capacity to interfere with the oral infection of dental caries. Abundant literature was produced regarding the specificity of adaptive immune responses in saliva, as well as clearly demonstrating the antigenic specificity to vaccines that elicited oral antibody to Streptococcus mutans or selected virulence components (306, 308). The role of this immune system in periodontal disease has been much less concrete. Nevertheless, following publication of the results from the salivary proteome project, the breadth of biomolecules that exist in salivary secretions sparked a renewed interest in the likely functions of these secretions in maintaining homeostasis in the oral cavity (277). In addition, these results increased the emphasis of the potential for use of these biomolecules as biomarkers for both oral and systemic diseases (217, 309).
Thus, salivary diagnostics is an emerging area that has progressed through several important developments in the past decade, including the infusion of federal funds to integrate nanotechnologies and microfluidic engineering concepts into developing compact point-of-care devices for rapid analysis of this secretion, as a diagnostic tool (232). Scientists have recognized that early detection of periodontal disease would benefit from accurate, reliable, sensitive and specific biomarkers for biological fluids. Thus, during the past 30+ years, scientists have investigated oral fluids as an alternative diagnostic approach (142). Initially, the focus was on oral fluids emanating from individual teeth. This gingival crevicular fluid is an inflammatory exudate that is typically in low abundance during health, but increases in quantity and in the complexity of inflammatory molecules at diseased sites. It was considered to have several diagnostic advantages, because inflammatory mediators and tissue-destructive molecules associated with periodontitis appear, and can be detected, in gingival crevicular fluid. However, gingival crevicular fluid analyses are time consuming, requiring multiple sampling of individual teeth onto filter paper strips (i.e. up to 32 teeth or via clinical decision preselecting the teeth to sample). The procedure is labor intensive and somewhat technically demanding, requiring equipment for calibrating and measuring fluid volumes. Finally, the assessment of analytes is expensive because each sample must be evaluated individually and the required assays are laboratory-based and generally cannot be performed chairside.
Given some of the problems inherent in sampling gingival crevicular fluid, the analysis of salivary biomarkers offers some advantages. Acquisition of saliva is easy, noninvasive, rapid and requires less manpower and fewer materials than does the analysis of gingival crevicular fluid. However, in contrast to gingival crevicular fluid, whole saliva clearly provides different diagnostic information. Saliva, for example, represents a pooled sample from all periodontal sites, thereby giving an overall assessment of a particular disease or risk status at the subject-level (as opposed to site-level or tooth-level). Knowledge of the levels of specific salivary biomarkers can, in turn, give patients and health-care providers the ability to determine whether disease is present, entry into treatment is needed, specific treatment should be provided or the treatment has been successful.
A goal in this area would be to advance the use of saliva as a diagnostic tool for oral and systemic disease assessment. The current status of the field demonstrates that biomarkers of the periodontium: (i) reflect the biology of the disease; (ii) are present, measurable and significantly elevated in the saliva of patients with periodontal inflammation and tissue destruction; (iii) appear to reflect the severity/extent of inflammation and tissue destruction; (iv) change in response to therapy; and (v) are not yet considered as routine contributors to the standard of care because large-scale studies that validate these findings are lacking (116, 200, 232, 297, 329).
Many analytes associated with periodontitis have been detected in saliva. Cytokines, chemokines, enzymes and immunoglobulins are host-derived factors that can provide potentially important information regarding periodontal status. We have focused on markers that hold potential diagnostic significance relevant to three important biological phases of periodontal disease (i.e. the inflammatory phase, the connective tissue-degradation phase and the bone turnover phase). Figure 6 provides an overview of these biomarkers. As a result of several case–control studies, a few promising analytes have been identified. However, before salivary diagnostics becomes established in clinical practice, greater development and understanding of normal day-to-day variations in biomarker levels are required (332), as well as identification of the measures that are indicative of significant disease activity. In the near future, biomarker panels will be employed to gain the specificity needed for the utility of saliva as a diagnostic fluid, but these will only be achieved once the proper combination of markers are validated in longitudinal studies that confirm their reliability with respect to day-to-day variations and potential confounders. Our recent findings suggest that several biomarkers are associated with distinct biological stages of these diseases and show promise as practical biomarkers in identifying and managing periodontal disease. The majority of these studies have progressed through biomarker discovery, with the identified molecules requiring more robust clinical studies to enable substantive validation for the diseases. It is predicted that, with continued advances in the field, the use of a combination of biomarkers in multiplex panels will yield improved rapid screening opportunities for health-care professionals. Future developments in the field of salivary diagnostics are likely to lead to point-of-care devices that can revolutionize our approaches to screening, risk assessment and therapeutic management of a range of health conditions. In turn, these developments will allow individualized treatments to be provided before significant tissue destruction occurs.
Fig. 6.
Schematic of considerations of potential biomarker targets for salivary diagnostic panels for periodontal disease. GM-CSF, granulocyte–macrophage colony-stimulating factor; IL, interleukin; MCP-1, monocyte chemotactic protein 1; Mip-1α, macrophage inflammatory protein-1alpha; MMP, matrix metalloproteinase; PGE2, prostaglandin E2; TIMP, tissue inhibitor of metalloproteinase.
Models of periodontal infections, disease and host responses
A number of animal model systems for periodontitis have been utilized in the study of the host–bacteria inter-relationships: (i) rodent models of periodontal disease have been useful in developing an understanding of the characteristics of adaptive immune response mechanisms in periodontitis; (ii) the canine model has been used to focus on gingival inflammation and the effects of topical antimicrobials, as well as to study the effects of nonsteroidal anti-inflammatory drugs on alveolar bone biology; (iii) studies in sheep have related to the etiology and treatment of an economically devastating periodontitis-like disease; and (iv) rabbit models of periodontal infections examined host mediators involved in resolving inflammation. All of these animal models have varied oral anatomy, dentition and structure of periodontal tissues. In addition, the oral pathophysiology, oral bacterial ecology and inflammatory responses also demonstrate some differences across species. Thus, these models each have strengths and weaknesses with regards to the facility to study various aspects of the complex disease of periodontitis, as well as limitations in homology to human disease (126, 127, 214, 288, 345). However, importantly, rodent models have generally been limited to studies of oral infection with individual microorganisms that represent exogenous pathogens in these rodents. For this reason, these studies require extensive manipulation of the oral microbiota, through the use of broad-spectrum antibiotics, such that the biofilm composition is quite distinct from those of humans and nonhuman primates. Nevertheless, the ability to parse the various aspects of immune responses and other critical genes using knockout or transgenic mice has been an invaluable tool in providing fundamental understanding of the potential host–bacteria interactions that could take place in periodontal pathology.
However, the nonhuman primate model, which has the advantage of being phylogenetically similar to humans, has provided the essential bridge for understanding the interaction of selected members of the subgingival microbiota with the host relevant to the longitudinal alterations that occur in the clinical and microbiological progression of ligature-induced periodontitis (288). A considerable body of research has demonstrated the homology of oral structures between humans and the nonhuman primate. Histological manifestations of spontaneous gingivitis and periodontitis in the nonhuman primate suggest a pattern similar to the human periodontal experience (354). This model has also permitted assessment of the role of the emerging microbiota and the immune response to selected members of this microbiota to either protect against disease progression, or to exacerbate the inflammatory process during the longitudinal progression of the inflammatory disease (79, 125, 263, 264). Biological changes can be measured in the local periodontal environment, as well as systemically. In this model the local microbiota can also be manipulated to select for specific microorganisms that can be inoculated subgingivally and result in a rapid and significant ‘burst’ of bone loss consistent with progressing periodontitis and directly attributable to the emergence of pathogen in the ecology (140). Nonhuman primates demonstrate significant bone loss from ligature-induced disease that enables the examination of microbiological, immunological and clinical features of periodontal disease and its prevention and treatment. In this regard, Holt et al. (140) have demonstrated that P. gingivalis inoculated subgingivally resulted in a rapid and significant ‘burst’ of bone loss, consistent with progressing periodontitis and directly attributable to the emergence of P. gingivalis in the ecology. Soluble receptors to interleukin-1 and tumor necrosis factor significantly inhibited recruitment of inflammatory cells in close proximity to bone, reduced osteoclast formation and reduced bone loss in ligature-induced periodontitis in a nonhuman primate animal model (14, 60). Thus, there appears to be a relationship between the microbiological and immunological studies of gingivitis and periodontitis in humans and those described for ligature-induced progression of periodontitis in nonhuman primates. It is clear that the primate model has provided the essential bridge for understanding the interaction of selected members of the subgingival microbiota with the host, particularly as reflected by the longitudinal alterations that occur in the clinical and microbiological progression of ligature-induced periodontitis. Additionally, the importance of periodontal disease as a model of host–bacteria interactions and inflammatory disease lies in our ability to isolate and characterize bacterial and host factors from the oral cavity in a noninvasive manner and correlate these changes with pathologic changes occurring in host tissues. The accessibility of oral tissues and the development of chronic inflammation in the oral cavity in response to microbial biofilms will provide tools for examining the ontogeny of inflammatory/immune processes, as related to disease expression, using this animal model.
The nonhuman primate model has also been used successfully to examine clinical and biological changes associated with oral–systemic relationships, including the link between periodontal disease and preterm birth/low birthweight and HIV/AIDS transmission. The model has also been instrumental in measuring the effectiveness of pharmaceutical agents in the management of periodontal disease. Consequently, the ‘lessons learned’ included a continuing need to not only identify or create new models to study the parameters of this disease, but also clearly to recognize the value and limitations of existing models so that they will be most effectively utilized to continue progress in the science of periodontics.
‘A Fish Called Wanda’
In conclusion, there has been substantial progress in our understanding of the molecular mechanisms of host–bacteria interactions that result in the clinical presentation and outcomes of destructive periodontitis. The science has embarked from observations of variations in responses related to disease expression with a focus for utilization of the responses in diagnosis and therapeutic outcomes, to current investigations using cutting-edge fundamental biological processes to attempt to model the initiation and progression of soft- and hard-tissue destruction of the periodontium. The four eclectic people in the movie ‘A Fish Called Wanda’ developed a team to effectively commit a crime, but then spent the rest of the time attempting to double-cross each other for the spoils of their efforts. We have learned how the armamentarium of the innate immune, inflammatory and adaptive immune responses ‘team’ in unique ways, depending upon host, microbial and environmental controls, to maintain or re-establish homeostasis. However, these inherent ‘good guys’ can double-cross the control points for a regulated response, resulting in dysregulated chronic inflammation and collateral damage of the periodontium. As importantly, the next era in the immunobiology of periodontal disease will need to engage more sophisticated experimental designs for clinical studies to enable robust translation of basic biologic processes that are in action early in the transition from health to disease, those that stimulate microenvironmental changes that select for a more pathogenic microbial ecology and those that represent a rebalancing of the complex host responses and a resolution of inflammatory tissue destruction.
References
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver RP. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba S, Tagami H. Dendritic cell activation induced by various stimuli, e.g. exposure to microorganisms, their products, cytokines, and simple chemicals as well as adhesion to extracellular matrix. J Dermatol Sci. 1998;20:1–13. doi: 10.1016/s0923-1811(99)00005-5. [DOI] [PubMed] [Google Scholar]
- 3.Aiba T, Akeno N, Kawane T, Okamoto H, Horiuchi N. Matrix metalloproteinases-1 and -8 and TIMP-1 mRNA levels in normal and diseased human gingivae. Eur J Oral Sci. 1996;104:562–569. doi: 10.1111/j.1600-0722.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 5.Al-Shangiti AM, Nair SP, Chain BM. The interaction between staphylococcal superantigen-like proteins and human dendritic cells. Clin Exp Immunol. 2005;140:461–469. doi: 10.1111/j.1365-2249.2005.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alaluusua S, Asikainen S, Lai CH. Intrafamilial transmission of Actinobacillus actinomycetemcomitans. J Periodontol. 1991;62:207–210. doi: 10.1902/jop.1991.62.3.207. [DOI] [PubMed] [Google Scholar]
- 7.Alam SQ, Bergens BM, Alam BS. Arachidonic acid, prostaglandin E2 and leukotriene C4 levels in gingiva and submandibular salivary glands of rats fed diets containing n-3 fatty acids. Lipids. 1991;26:895–900. doi: 10.1007/BF02535974. [DOI] [PubMed] [Google Scholar]
- 8.Albandar JM, Tinoco EM. Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000. 2002;29:153–176. doi: 10.1034/j.1600-0757.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, Ranjan P, Monroe KM, Pickles RJ, Sambhara S, Ting JP. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Association of Public Health Dentistry: recommendations for teaching about the prescription of dietary fluoride supplements. J Public Health Dent. 1989;49:237–241. doi: 10.1111/j.1752-7325.1989.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 11.Amsterdam D, Papsian CJ. Bacteria. In: Wicher K, editor. Microbial antigenodiagnosis. Boca Raton, FL: CRC Press, Inc; 1987. pp. 4–32. [Google Scholar]
- 12.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–494. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 13.Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, Schneider M, Roney KE, O’Connor BP, Moore CB, Morrison A, Sutterwala FS, Bertin J, Koller BH, Liu Z, Ting JP. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2010;185:4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 15.Aukhil I, Lopatin DE, Syed SA, Morrison EC, Kowalski CJ. The effects of periodontal therapy on serum antibody (IgG) levels to plaque microorganisms. J Clin Periodontol. 1988;15:544–550. doi: 10.1111/j.1600-051x.1988.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 16.Bainbridge BW, Darveau RP. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol Scand. 2001;59:131–138. doi: 10.1080/000163501750266710. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 18.Baranowska HI, Palmer RM, Wilson RF. A comparison of antibody levels to Bacteroides gingivalis in serum and crevicular fluid from patients with untreated periodontitis. Oral Microbiol Immunol. 1989;4:173–175. doi: 10.1111/j.1399-302x.1989.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 19.Belibasakis GN, Johansson A. Aggregatibacter actinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine. 2012;59:124–130. doi: 10.1016/j.cyto.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Belibasakis GN, Meier A, Guggenheim B, Bostanci N. Oral biofilm challenge regulates the RANKL-OPG system in periodontal ligament and dental pulp cells. Microb Pathog. 2011;50:6–11. doi: 10.1016/j.micpath.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Benko S, Magyarics Z, Szabo A, Rajnavolgyi E. Dendritic cell subtypes as primary targets of vaccines: the emerging role and cross-talk of pattern recognition receptors. Biol Chem. 2008;389:469–485. doi: 10.1515/bc.2008.054. [DOI] [PubMed] [Google Scholar]
- 22.Berglundh T, Liljenberg B, Tarkowski A, Lindhe J. Local and systemic TCR V gene expression in advanced periodontal disease. J Clin Periodontol. 1998;25:125–133. doi: 10.1111/j.1600-051x.1998.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 23.Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol Suppl. 1999;57:16–21. [PubMed] [Google Scholar]
- 24.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64:456–460. [PubMed] [Google Scholar]
- 25.Billings F. Focal infection as the cause of general disease. Bull N Y Acad Med. 1930;6:759–773. [PMC free article] [PubMed] [Google Scholar]
- 26.Bimstein E, Ebersole JL. Serum antibody levels to oral microorganisms in children and young adults with relation to the severity of gingival disease. Pediatr Dent. 1991;13:267–272. [PubMed] [Google Scholar]
- 27.Blach-Olszewska Z. Innate immunity: cells, receptors, and signaling pathways. Arch Immunol Ther Exp (Warsz) 2005;53:245–253. [PubMed] [Google Scholar]
- 28.Blander JM, Sander LE. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat Rev Immunol. 2012;12:215–225. doi: 10.1038/nri3167. [DOI] [PubMed] [Google Scholar]
- 29.Bode KA, Schmitz F, Vargas L, Heeg K, Dalpke AH. Kinetic of RelA activation controls magnitude of TLR-mediated IL-12p40 induction. J Immunol. 2009;182:2176–2184. doi: 10.4049/jimmunol.0802560. [DOI] [PubMed] [Google Scholar]
- 30.Bodineau A, Coulomb B, Tedesco AC, Seguier S. Increase of gingival matured dendritic cells number in elderly patients with chronic periodontitis. Arch Oral Biol. 2009;54:12–16. doi: 10.1016/j.archoralbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA, Belibasakis GN. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 2009;157:415–422. doi: 10.1111/j.1365-2249.2009.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bostanci N, Meier A, Guggenheim B, Belibasakis GN. Regulation of NLRP3 and AIM2 inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell Immunol. 2011;270:88–93. doi: 10.1016/j.cellimm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Boucher BJ. Vitamin D insufficiency and diabetes risks. Curr Drug Targets. 2011;12:61–87. doi: 10.2174/138945011793591653. [DOI] [PubMed] [Google Scholar]
- 34.Braun T, Schett G. Pathways for bone loss in inflammatory disease. Curr Osteoporos Rep. 2012;10:101–108. doi: 10.1007/s11914-012-0104-5. [DOI] [PubMed] [Google Scholar]
- 35.Califano JV, Schenkein HA, Tew JG. Immunodominant antigens of Actinobacillus actinomycetemcomitans serotype b in early-onset periodontitis patients. Oral Microbiol Immunol. 1992;7:65–70. doi: 10.1111/j.1399-302x.1992.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 36.Campan P, Planchand PO, Duran D. Pilot study on n-3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 1997;24:907–913. doi: 10.1111/j.1600-051x.1997.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 37.Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. Sci World J. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 39.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 40.Chandrasekar B, Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994;200:893–898. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- 41.Chen I. The Surgeon General’s report on oral health: implications for research and education. N Y State Dent J. 2000;66:38–42. [PubMed] [Google Scholar]
- 42.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 43.Chinen J, Shearer WT. Basic and clinical immunology. J Allergy Clin Immunol. 2005;116:411–418. doi: 10.1016/j.jaci.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 45.Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, Yu BP. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 46.Cleland LG, Caughey GE, James MJ, Proudman SM. Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. J Rheumatol. 2006;33:1973–1979. [PubMed] [Google Scholar]
- 47.Cohen N, Morisset J, Emilie D. Induction of tolerance by Porphyromonas gingivalis on APCS: a mechanism implicated in periodontal infection. J Dent Res. 2004;83:429–433. doi: 10.1177/154405910408300515. [DOI] [PubMed] [Google Scholar]
- 48.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 50.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 51.Cotti E, Dessi C, Piras A, Mercuro G. Can a chronic dental infection be considered a cause of cardiovascular disease? A review of the literature. Int J Cardiol. 2011;148:4–10. doi: 10.1016/j.ijcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Cronin A. Periodontal disease is a risk marker for coronary heart disease? Evid Based Dent. 2009;10:22. doi: 10.1038/sj.ebd.6400634. [DOI] [PubMed] [Google Scholar]
- 53.Curtis MA, Zenobia C, Darveau RP. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe. 2011;10:302–306. doi: 10.1016/j.chom.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cutler CW, Jotwani R. Antigen-presentation and the role of dendritic cells in periodontitis. Periodontol 2000. 2004;35:135–157. doi: 10.1111/j.0906-6713.2004.003560.x. [DOI] [PubMed] [Google Scholar]
- 55.Cutler CW, Jotwani R, Palucka KA, Davoust J, Bell D, Banchereau J. Evidence and a novel hypothesis for the role of dendritic cells and Porphyromonas gingivalis in adult periodontitis. J Periodontal Res. 1999;34:406–412. doi: 10.1111/j.1600-0765.1999.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 56.D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 57.Davey ME, Costerton JW. Molecular genetics analyses of biofilm formation in oral isolates. Periodontol 2000. 2006;42:13–26. doi: 10.1111/j.1600-0757.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 58.de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, Salamero J, Cazenave JP, Hanau D, Mori L, Puzo G, De Libero G. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 59.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–76. [PubMed] [Google Scholar]
- 60.Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, Graves DT. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontol. 2001;28:233–240. doi: 10.1034/j.1600-051x.2001.028003233.x. [DOI] [PubMed] [Google Scholar]
- 61.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dereka XE, Tosios KI, Chrysomali E, Angelopoulou E. Factor XIIIa+ dendritic cells and S-100 protein+ Langerhans’ cells in adult periodontitis. J Periodontal Res. 2004;39:447–452. doi: 10.1111/j.1600-0765.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- 63.Detert J, Pischon N, Burmester G, Buttgereit F. The association between rheumatoid arthritis and periodontal disease. Arthritis Res Ther. 2010;12:218. doi: 10.1186/ar3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz PI. Microbial diversity and interactions in subgingival biofilm communities. Front Oral Biol. 2012;15:17–40. doi: 10.1159/000329669. [DOI] [PubMed] [Google Scholar]
- 67.Diehl SR, Wang Y, Brooks CN, Burmeister JA, Califano JV, Wang S, Schenkein HA. Linkage disequilibrium of interleukin-1 genetic polymorphisms with early-onset periodontitis. J Periodontol. 1999;70:418–430. doi: 10.1902/jop.1999.70.4.418. [DOI] [PubMed] [Google Scholar]
- 68.Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J Periodontol. 2004;75:16–22. doi: 10.1902/jop.2004.75.1.16. [DOI] [PubMed] [Google Scholar]
- 69.Dietrich T, Hoffmann K. A comprehensive index for the modeling of smoking history in periodontal research. J Dent Res. 2004;83:859–863. doi: 10.1177/154405910408301107. [DOI] [PubMed] [Google Scholar]
- 70.Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–144. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dixon GL, Newton PJ, Chain BM, Katz D, Andersen SR, Wong S, van der Ley P, Klein N, Callard RE. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect Immun. 2001;69:4351–4357. doi: 10.1128/IAI.69.7.4351-4357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dogan B, Kipalev AS, Okte E, Sultan N, Asikainen SE. Consistent intrafamilial transmission of Actinobacillus actinomycetemcomitans despite clonal diversity. J Periodontol. 2008;79:307–315. doi: 10.1902/jop.2008.070270. [DOI] [PubMed] [Google Scholar]
- 73.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douglas SD. Host responses to bacterial, mycotic, and parasitic diseases. In: Rose NR, Friedman H, Fahey JL, editors. Manual of clinical laboratory immunology. Washington, DC: American Society for Microbiology; 1986. pp. 332–487. [Google Scholar]
- 75.Eberhard J, Heilmann F, Acil Y, Albers HK, Jepsen S. Local application of n-3 or n-6 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 2002;29:364–369. doi: 10.1034/j.1600-051x.2002.290413.x. [DOI] [PubMed] [Google Scholar]
- 76.Ebersole JL. Systemic humoral immune responses in periodontal disease. Crit Rev Oral Biol Med. 1990;1:283–331. doi: 10.1177/10454411900010040601. [DOI] [PubMed] [Google Scholar]
- 77.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–166. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 78.Ebersole JL. Immune responses in periodontal diseases. In: Wilson TG Jr, Kornman KS, editors. Fundamentals of periodontics. 2nd edn. Chicago, IL: Quintessence Publishing Co., Inc.; 2003. pp. 111–143. [Google Scholar]
- 79.Ebersole JL, Brunsvold M, Steffensen B, Wood R, Holt SC. Effects of immunization with Porphyromonas gingivalis and Prevotella intermedia on progression of ligature-induced periodontitis in the nonhuman primate Macaca fascicularis. Infect Immun. 1991;59:3351–3359. doi: 10.1128/iai.59.10.3351-3359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol 2000. 2000;23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 81.Ebersole JL, Cappelli D, Steffen MJ. Antigenic specificity of gingival crevicular fluid antibody to Actinobacillus actinomycetemcomitans. J Dent Res. 2000;79:1362–1370. doi: 10.1177/00220345000790060301. [DOI] [PubMed] [Google Scholar]
- 82.Ebersole JL, Sandoval MN, Steffen MJ, Cappelli D. Serum antibody in Actinobacillus actinomycetemcomitans-infected patients with periodontal disease. Infect Immun. 1991;59:1795–1802. doi: 10.1128/iai.59.5.1795-1802.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ebersole JL, Steffen MJ. Human antibody responses to outer envelope antigens of Porphyromonas gingivalis serotypes. J Periodontal Res. 1995;30:1–14. doi: 10.1111/j.1600-0765.1995.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 84.Ebersole JL, Steffen MJ, Gonzalez-Martinez J, Novak MJ. Effects of age and oral disease on systemic inflammatory and immune parameters in nonhuman primates. Clin Vaccine Immunol. 2008;15:1067–1075. doi: 10.1128/CVI.00258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–141. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 86.Ebersole JL, Taubman MA, Smith DJ. Gingival crevicular fluid antibody to oral microorganisms. II. Distribution and specificity of local antibody responses. J Periodontal Res. 1985;20:349–356. doi: 10.1111/j.1600-0765.1985.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 87.Ebersole JL, Taubman MA, Smith DJ, Frey DE, Haffajee AD, Socransky SS. Human serum antibody responses to oral microorganisms. IV. Correlation with homologous infection. Oral Microbiol Immunol. 1987;2:53–59. doi: 10.1111/j.1399-302x.1987.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 88.Ebersole JL, Taubman MA, Smith DJ, Haffajee AD. Effect of subgingival scaling on systemic antibody responses to oral microorganisms. Infect Immun. 1985;48:534–539. doi: 10.1128/iai.48.2.534-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eckersten C, Pylvanen L, Schroder U, Twetman S, Wennhall I, Matsson L. Prevalence of dental fluorosis in children taking part in an oral health programme including fluoride tablet supplements from the age of 2 years. Int J Paediatr Dent. 2010;20:347–352. doi: 10.1111/j.1365-263X.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 90.Edelman SM, Kasper DL. Symbiotic commensal bacteria direct maturation of the host immune system. Curr Opin Gastroenterol. 2008;24:720–724. doi: 10.1097/MOG.0b013e32830c4355. [DOI] [PubMed] [Google Scholar]
- 91.Eleftherohorinou H, Hoggart CJ, Wright VJ, Levin M, Coin LJ. Pathway-driven gene stability selection of two rheumatoid arthritis GWAS identifies and validates new susceptibility genes in receptor mediated signalling pathways. Hum Mol Genet. 2011;20:3494–3506. doi: 10.1093/hmg/ddr248. [DOI] [PubMed] [Google Scholar]
- 92.Eleftherohorinou H, Wright V, Hoggart C, Hartikainen AL, Jarvelin MR, Balding D, Coin L, Levin M. Pathway analysis of GWAS provides new insights into genetic susceptibility to 3 inflammatory diseases. PLoS ONE. 2009;4:e8068. doi: 10.1371/journal.pone.0008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 94.Emingil G, Tervahartiala T, Mantyla P, Maatta M, Sorsa T, Atilla G. Gingival crevicular fluid matrix metalloproteinase (MMP)-7, extracellular MMP inducer, and tissue inhibitor of MMP-1 levels in periodontal disease. J Periodontol. 2006;77:2040–2050. doi: 10.1902/jop.2006.060144. [DOI] [PubMed] [Google Scholar]
- 95.Ergas D, Eilat E, Mendlovic S, Sthoeger ZM. n-3 fatty acids and the immune system in autoimmunity. Isr Med Assoc J. 2002;4:34–38. [PubMed] [Google Scholar]
- 96.Evans CA, Kleinman DV. The Surgeon General’s report on America’s oral health: opportunities for the dental profession. J Am Dent Assoc. 2000;131:1721–1728. doi: 10.14219/jada.archive.2000.0118. [DOI] [PubMed] [Google Scholar]
- 97.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farida R, Wilson M, Ivanyi L. Serum IgG antibodies to lipopolysaccharides in various forms of periodontal disease in man. Arch Oral Biol. 1986;31:711–715. doi: 10.1016/0003-9969(86)90001-4. [DOI] [PubMed] [Google Scholar]
- 100.Ferlazzo G, Morandi B, D’Agostino A, Meazza R, Melioli G, Moretta A, Moretta L. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 101.Fernandes G, Venkatraman JT. Role of omega-3 fatty acids in health and disease. Nutr Res. 1993;13(Suppl 1):S19–S45. [Google Scholar]
- 102.Fisher MA, Taylor GW, Shelton BJ, Jamerson KA, Rahman M, Ojo AO, Sehgal AR. Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis. 2008;51:45–52. doi: 10.1053/j.ajkd.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 103.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol. 2006;79:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 104.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 105.Franke A, McGovern DPB, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel J-F, Cottone M, Stronati L, Denson T, De Vos M, D’Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot J-P, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panes J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PCF, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D’Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 2011;437:185–197. doi: 10.1042/BJ20110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gamonal J, Bascones A, Acevedo A, Blanco E, Silva A. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J Periodontol. 2001;72:517–525. doi: 10.1902/jop.2001.72.4.517. [DOI] [PubMed] [Google Scholar]
- 108.Gary-Gouy H, Lebon P, Dalloul AH. Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J Interferon Cytokine Res. 2002;22:653–659. doi: 10.1089/10799900260100132. [DOI] [PubMed] [Google Scholar]
- 109.Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discovery. 2009;8:465–479. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 110.Gemmell E, Carter CL, Seymour GJ. Chemokines in human periodontal disease tissues. Clin Exp Immunol. 2001;125:134–141. doi: 10.1046/j.1365-2249.2001.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gemmell E, McHugh GB, Grieco DA, Seymour GJ. Costimulatory molecules in human periodontal disease tissues. J Periodontal Res. 2001;36:92–100. doi: 10.1034/j.1600-0765.2001.360205.x. [DOI] [PubMed] [Google Scholar]
- 112.Genco RJ, Zambon JJ, Christersson LA. The origin of periodontal infections. Adv Dent Res. 1988;2:245–259. doi: 10.1177/08959374880020020901. [DOI] [PubMed] [Google Scholar]
- 113.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 114.Gervassi A, Alderson MR, Suchland R, Maisonneuve JF, Grabstein KH, Probst P. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect Immun. 2004;72:7231–7239. doi: 10.1128/IAI.72.12.7231-7239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gibbons RV. Germs, Dr. Billings, and the theory of focal infection. Clin Infect Dis. 1998;27:627–633. doi: 10.1086/514705. [DOI] [PubMed] [Google Scholar]
- 118.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 119.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 120.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 121.Gonzalez OA, Stromberg AJ, Huggins PM, Gonzalez-Martinez J, Novak MJ, Ebersole JL. Apoptotic genes are differentially expressed in aged gingival tissue. J Dent Res. 2011;90:880–886. doi: 10.1177/0022034511403744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gore EA, Sanders JJ, Pandey JP, Palesch Y, Galbraith GM. Interleukin-1beta+3953 allele 2: association with disease status in adult periodontitis. J Clin Periodontol. 1998;25:781–785. doi: 10.1111/j.1600-051x.1998.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 123.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 125.Graves DT, Delima AJ, Assuma R, Amar S, Oates T, Cochran D. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol. 1998;69:1419–1425. doi: 10.1902/jop.1998.69.12.1419. [DOI] [PubMed] [Google Scholar]
- 126.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol. 2012;15:117–132. doi: 10.1159/000329675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guggenheim B, Gmur R, Galicia JC, Stathopoulou PG, Benakanakere MR, Meier A, Thurnheer T, Kinane DF. In vitro modeling of host-parasite interactions: the ‘subgingival’ biofilm challenge of primary human epithelial cells. BMC Microbiol. 2009;9:280. doi: 10.1186/1471-2180-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guiney DG, Hasegawa P, Cole SP. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect Immun. 2003;71:4163–4166. doi: 10.1128/IAI.71.7.4163-4166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gunsolley JC, Tew JG, Gooss C, Marshall DR, Burmeister JA, Schenkein HA. Serum antibodies to periodontal bacteria. J Periodontol. 1990;61:412–419. doi: 10.1902/jop.1990.61.7.412. [DOI] [PubMed] [Google Scholar]
- 131.Han X, Lin X, Seliger AR, Eastcott J, Kawai T, Taubman MA. Expression of receptor activator of nuclear factor-kappaB ligand by B cells in response to oral bacteria. Oral Microbiol Immunol. 2009;24:190–196. doi: 10.1111/j.1399-302X.2008.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 133.Hart TC, Hart PS, Michalec MD, Zhang Y, Marazita ML, Cooper M, Yassin OM, Nusier M, Walker S. Localisation of a gene for prepubertal periodontitis to chromosome 11q14 and identification of a cathepsin C gene mutation. J Med Genet. 2000;37:95–101. doi: 10.1136/jmg.37.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayman L, Steffen MJ, Stevens J, Badger E, Tempro P, Fuller B, McGuire A, Al-Sabbagh M, Thomas MV, Ebersole JL. Smoking and periodontal disease: discrimination of antibody responses to pathogenic and commensal oral bacteria. Clin Exp Immunol. 2011;164:118–126. doi: 10.1111/j.1365-2249.2010.04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haynes DR. Bone lysis and inflammation. Inflamm Res. 2004;53:596–600. doi: 10.1007/s00011-004-1303-z. [DOI] [PubMed] [Google Scholar]
- 136.Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 137.Herschfeld JJ. William Hunter and the role of “oral sepsis” in American dentistry. Bull Hist Dent. 1985;33:35–45. [PubMed] [Google Scholar]
- 138.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444–2450. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 139.Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–990. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- 140.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–557. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 141.Hope JC, Thom ML, McCormick PA, Howard CJ. Interaction of antigen presenting cells with mycobacteria. Vet Immunol Immunopathol. 2004;100:187–195. doi: 10.1016/j.vetimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 142.Horton JE, Zimmermann ER, Collings CK. Use of salivary reductase tests in periodontal disease detection. Periodontics. 1968;6:212–213. [PubMed] [Google Scholar]
- 143.Hu L, Bray MD, Osorio M, Kopecko DJ. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect Immun. 2006;74:2697–2705. doi: 10.1128/IAI.74.5.2697-2705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang C, Altimova Y, Strange S, Ebersole J. Polybacterial challenge effects on cytokine/chemokine production by macrophages and dendritic cells. Inflamm Res. 2011;60:119–125. doi: 10.1007/s00011-010-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, Lander ES, Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 146.Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2:435–444. doi: 10.4161/viru.2.5.16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imamura R, Wang Y, Kinoshita T, Suzuki M, Noda T, Sagara J, Taniguchi S, Okamoto H, Suda T. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J Immunol. 2010;184:5874–5884. doi: 10.4049/jimmunol.0900779. [DOI] [PubMed] [Google Scholar]
- 148.Incalcaterra E, Caruso M, Balistreri CR, Candore G, Lo Presti R, Hoffmann E, Caimi G. Role of genetic polymorphisms in myocardial infarction at young age. Clin Hemorheol Microcirc. 2010;46:291–298. doi: 10.3233/CH-2010-1353. [DOI] [PubMed] [Google Scholar]
- 149.Itoh H, Ohsawa Y, Yoshie H, Yamazaki K. Oligoclonal accumulations of T-cell clones in gingivitis and periodontitis lesions. Oral Microbiol Immunol. 2002;17:324–329. doi: 10.1034/j.1399-302x.2002.170511.x. [DOI] [PubMed] [Google Scholar]
- 150.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jakubovics NS, Kolenbrander PE. The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 2010;16:729–739. doi: 10.1111/j.1601-0825.2010.01701.x. [DOI] [PubMed] [Google Scholar]
- 152.Janeway CA, Jr, Travers P. Immunobiology: the immune system in health and disease. 2nd edn. New York, NY: Garland Publishing, Inc.; 1996. [Google Scholar]
- 153.Jeon DI, Park SR, Ahn MY, Ahn SG, Park JH, Yoon JH. NOD1 and NOD2 stimulation triggers innate immune responses of human periodontal ligament cells. Int J Mol Med. 2012;29:699–703. doi: 10.3892/ijmm.2012.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ji S, Shin JE, Kim YS, Oh JE, Min BM, Choi Y. Toll-like receptor 2 and NALP2 mediate induction of human beta-defensins by Fusobacterium nucleatum in gingival epithelial cells. Infect Immun. 2009;77:1044–1052. doi: 10.1128/IAI.00449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jin Y, Fuller L, Ciancio G, Burke GW, 3rd, Tzakis AG, Ricordi C, Miller J, Esquenzai V. Antigen presentation and immune regulatory capacity of immature and mature-enriched antigen presenting (dendritic) cells derived from human bone marrow. Hum Immunol. 2004;65:93–103. doi: 10.1016/j.humimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 156.Johnson V, Johnson BD, Sims TJ, Whitney CW, Moncla BJ, Engel LD, Page RC. Effects of treatment on antibody titer to Porphyromonas gingivalis in gingival crevicular fluid of patients with rapidly progressive periodontitis. J Periodontol. 1993;64:559–565. doi: 10.1902/jop.1993.64.6.559. [DOI] [PubMed] [Google Scholar]
- 157.Jostins L, Barrett JC. Genetic risk prediction in complex disease. Hum Mol Genet. 2011;20:R182–R188. doi: 10.1093/hmg/ddr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jotwani R, Cutler CW. Fimbriated Porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect Immun. 2004;72:1725–1732. doi: 10.1128/IAI.72.3.1725-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jotwani R, Palucka AK, Al-Quotub M, Nouri-Shirazi M, Kim J, Bell D, Banchereau J, Cutler CW. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol. 2001;167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003;33:2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 161.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 163.Kalmar JR. Antimicrobial dysfunction in localized juvenile periodontitis neutrophils. In: Genco RJ, editor. Molecular pathogenesis of periodontal disease. Washington, DC: American Society for Microbiology; 1994. pp. 337–349. [Google Scholar]
- 164.Kanaya S, Nemoto E, Ogawa T, Shimauchi H. Porphyromonas gingivalis lipopolysaccharides induce maturation of dendritic cells with CD14+ CD16+ phenotype. Eur J Immunol. 2004;34:1451–1460. doi: 10.1002/eji.200324549. [DOI] [PubMed] [Google Scholar]
- 165.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun. 2004;72:2671–2678. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kawai T, Akira S. Pathogen recognition with toll-like receptors. Curr Opin Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 167.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 168.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kebschull M, Papapanou PN. Periodontal microbial complexes associated with specific cell and tissue responses. J Clin Periodontol. 2011;38(Suppl 11):17–27. doi: 10.1111/j.1600-051X.2010.01668.x. [DOI] [PubMed] [Google Scholar]
- 170.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclearcytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 171.Kido J, Nakamura T, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. Calprotectin in gingival crevicular fluid correlates with clinical and biochemical markers of periodontal disease. J Clin Periodontol. 1999;26:653–657. doi: 10.1034/j.1600-051x.1999.261004.x. [DOI] [PubMed] [Google Scholar]
- 172.Kikuchi T, Hahn CL, Tanaka S, Barbour SE, Schenkein HA, Tew JG. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect Immun. 2004;72:5089–5096. doi: 10.1128/IAI.72.9.5089-5096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kikuchi T, Willis DL, Liu M, Purkall DB, Sukumar S, Barbour SE, Schenkein HA, Tew JG. Dendritic-NK cell interactions in P. gingivalis-specific responses. J Dent Res. 2005;84:858–862. doi: 10.1177/154405910508400915. [DOI] [PubMed] [Google Scholar]
- 174.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 175.Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontol 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 176.Kinane DF, Johnston FA, Evans CW. Depressed helper-to-suppressor T-cell ratios in early-onset forms of periodontal disease. J Periodontal Res. 1989;24:161–164. doi: 10.1111/j.1600-0765.1989.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 177.Kinane DF, Mooney J, MacFarlane TW, McDonald M. Local and systemic antibody response to putative periodontopathogens in patients with chronic periodontitis: correlation with clinical indices. Oral Microbiol Immunol. 1993;8:65–68. doi: 10.1111/j.1399-302x.1993.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 178.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 179.Kolenbrander PE. Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source. Int J Oral Sci. 2011;3:49–54. doi: 10.4248/IJOS11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 181.Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 182.Kopitar AN, Ihan Hren N, Ihan A. Commensal oral bacteria antigens prime human dendritic cells to induce Th1, Th2 or Treg differentiation. Oral Microbiol Immunol. 2006;21:1–5. doi: 10.1111/j.1399-302X.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 183.Kornman KS, di Giovine FS. Genetic variations in cytokine expression: a risk factor for severity of adult periodontitis. Ann Periodontol. 1998;3:327–338. doi: 10.1902/annals.1998.3.1.327. [DOI] [PubMed] [Google Scholar]
- 184.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 185.Koulouri O, Lappin DF, Radvar M, Kinane DF. Cell division, synthetic capacity and apoptosis in periodontal lesions analysed by in situ hybridisation and immunohistochemistry. J Clin Periodontol. 1999;26:552–559. doi: 10.1034/j.1600-051x.1999.260810.x. [DOI] [PubMed] [Google Scholar]
- 186.Kranzer K, Eckhardt A, Aigner M, Knoll G, Deml L, Speth C, Lehn N, Rehli M, Schneider-Brachert W. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect Immun. 2004;72:4416–4423. doi: 10.1128/IAI.72.8.4416-4423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kranzer K, Sollner L, Aigner M, Lehn N, Deml L, Rehli M, Schneider-Brachert W. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infect Immun. 2005;73:4180–4189. doi: 10.1128/IAI.73.7.4180-4189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kudela P, Paukner S, Mayr UB, Cholujova D, Schwarczova Z, Sedlak J, Bizik J, Lubitz W. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J Immunother. 2005;28:136–143. doi: 10.1097/01.cji.0000154246.89630.6f. [DOI] [PubMed] [Google Scholar]
- 189.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 190.Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kunimatsu K, Yamamoto K, Ichimaru E, Kato Y, Kato I, Cathepsins B. H and L activities in gingival crevicular fluid from chronic adult periodontitis patients and experimental gingivitis subjects. J Periodontal Res. 1990;25:69–73. doi: 10.1111/j.1600-0765.1990.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 192.La Guardia M, Giammanco S, Di Majo D, Tabacchi G, Tripoli E, Giammanco M. Omega 3 fatty acids: biological activity and effects on human health. Panminerva Med. 2005;47:245–257. [PubMed] [Google Scholar]
- 193.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 194.Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–229. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 195.Lamster IB, Celenti R, Ebersole JL. The relationship of serum IgG antibody titers to periodontal pathogens to indicators of the host response in crevicular fluid. J Clin Periodontol. 1990;17:419–425. doi: 10.1111/j.1600-051x.1990.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 196.Lamster IB, Oshrain RL, Celenti RS, Fine JB, Grbic JT. Indicators of the acute inflammatory and humoral immune responses in gingival crevicular fluid: relationship to active periodontal disease. J Periodontal Res. 1991;26:261–263. doi: 10.1111/j.1600-0765.1991.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 197.Larsson M, Majeed M, Ernst JD, Magnusson KE, Stendahl O, Forsum U. Role of annexins in endocytosis of antigens in immature human dendritic cells. Immunology. 1997;92:501–511. doi: 10.1046/j.1365-2567.1997.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lau CS. Collateral benefits of fish oil therapy for rheumatoid arthritis. J Rheumatol. 2006;33:1931–1933. [PubMed] [Google Scholar]
- 199.Lauvau G, Glaichenhaus N. Mini-review: presentation of pathogen-derived antigens in vivo. Eur J Immunol. 2004;34:913–920. doi: 10.1002/eji.200424944. [DOI] [PubMed] [Google Scholar]
- 200.Lee A, Ghaname CB, Braun TM, Sugai JV, Teles RP, Loesche WJ, Kornman KS, Giannobile WV, Kinney JS. Bacterial and salivary biomarkers predict the gingival inflammatory profile. J Periodontol. 2012;83:79–89. doi: 10.1902/jop.2011.110060. [DOI] [PubMed] [Google Scholar]
- 201.Leon F, Smythies LE, Smith PD, Kelsall BL. Involvement of dendritic cells in the pathogenesis of inflammatory bowel disease. Adv Exp Med Biol. 2006;579:117–132. doi: 10.1007/0-387-33778-4_8. [DOI] [PubMed] [Google Scholar]
- 202.Lio D, Scola L, Crivello A, Bonafe M, Franceschi C, Olivieri F, Colonna-Romano G, Candore G, Caruso C. Allele frequencies of +874T−>a single nucleotide polymorphism at the first intron of interferon-gamma gene in a group of Italian centenarians. Exp Gerontol. 2002;37:315–319. doi: 10.1016/s0531-5565(01)00198-x. [DOI] [PubMed] [Google Scholar]
- 203.Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafe M, Cavallone L, Marchegiani F, Olivieri F, Franceschi C, Caruso C. Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10 −1082 promoter SNP and its interaction with TNF-alpha −308 promoter SNP. J Med Genet. 2003;40:296–299. doi: 10.1136/jmg.40.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu Y, Xu H, Chen S, Chen X, Zhang Z, Zhu Z, Qin X, Hu L, Zhu J, Zhao GP, Kong X. Genome-wide interaction-based association analysis identified multiple new susceptibility Loci for common diseases. PLoS Genet. 2011;7:e1001338. doi: 10.1371/journal.pgen.1001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC, Jr, Baddour LM. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 206.Lu H, Califano JV, Schenkein HA, Tew JG. Immunoglobulin class and subclass distribution of antibodies reactive with the immunodominant antigen of Actinobacillus actinomycetemcomitans serotype b. Infect Immun. 1993;61:2400–2407. doi: 10.1128/iai.61.6.2400-2407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lucas H, Bartold PM, Dharmapatni AA, Holding CA, Haynes DR. Inhibition of apoptosis in periodontitis. J Dent Res. 2009;89:29–33. doi: 10.1177/0022034509350708. [DOI] [PubMed] [Google Scholar]
- 208.Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA, Maliszewski C, Shortman K, Cebon J, Maraskovsky E. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 209.Lugering A, Lebiedz P, Koch S, Kucharzik T. Apoptosis as a therapeutic tool in IBD? AnnNY Acad Sci. 2006;1072:62–77. doi: 10.1196/annals.1326.013. [DOI] [PubMed] [Google Scholar]
- 210.Lundqvist C, Hammarstrom ML. T-cell receptor gamma delta-expressing intraepithelial lymphocytes are present in normal and chronically inflamed human gingiva. Immunology. 1993;79:38–45. [PMC free article] [PubMed] [Google Scholar]
- 211.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Macdonald A. Omega-3 fatty acids as adjunctive therapy in Crohns disease. Gastroenterol Nurs. 2006;29:295–301. doi: 10.1097/00001610-200607000-00005. quiz 302–203. [DOI] [PubMed] [Google Scholar]
- 213.Mackler BF, Schur P, Waldrop T, Coker E, Rossen R. IgG subclasses in human periodontal disease: III. Serum concentrations of IgG subclass immunoglobulins and circulating immune complexes. J Dent Res. 1979;58:1701–1704. doi: 10.1177/00220345790580070901. [DOI] [PubMed] [Google Scholar]
- 214.Madden TE, Caton JG. Animal models for periodontal disease. Methods Enzymol. 1994;235:106–119. doi: 10.1016/0076-6879(94)35135-x. [DOI] [PubMed] [Google Scholar]
- 215.Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005;32(Suppl 6):57–71. doi: 10.1111/j.1600-051X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 216.Mahanonda R, Sa-Ard-Iam N, Yongvanitchit K, Wisetchang M, Ishikawa I, Nagasawa T, Walsh DS, Pichyangkul S. Upregulation of co-stimulatory molecule expression and dendritic cell marker (CD83) on B cells in periodontal disease. J Periodontal Res. 2002;37:177–183. doi: 10.1034/j.1600-0765.2002.00664.x. [DOI] [PubMed] [Google Scholar]
- 217.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am. 2011;55:159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Maliszewski CR, Moser M. Role of CD8alpha+ and CD8alpha- dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 219.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–282. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 220.Mantel PY, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol. 2011;677:303–338. doi: 10.1007/978-1-60761-869-0_21. [DOI] [PubMed] [Google Scholar]
- 221.Marazita ML, Burmeister JA, Gunsolley JC, Koertge TE, Lake K, Schenkein HA. Evidence for autosomal dominant inheritance and race-specific heterogeneity in early-onset periodontitis. J Periodontol. 1994;65:623–630. doi: 10.1902/jop.1994.65.6.623. [DOI] [PubMed] [Google Scholar]
- 222.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 223.Martin SA, Falkler WA, Jr, Suzuki JB, Hawley CE, Mackler BF. Local and systemic immunoglobulins reactive to Bacteroides gingivalis in rapidly progressive and adult periodontitis. J Periodontal Res. 1986;21:351–364. doi: 10.1111/j.1600-0765.1986.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 224.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 225.Matyszak MK, Young JL, Gaston JS. Uptake and processing of Chlamydia trachomatis by human dendritic cells. Eur J Immunol. 2002;32:742–751. doi: 10.1002/1521-4141(200203)32:3<742::AID-IMMU742>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 226.McArthur WP, Clark WB. Specific antibodies and their potential role in periodontal diseases. J Periodontol. 1993;64:807–818. doi: 10.1902/jop.1993.64.8s.807. [DOI] [PubMed] [Google Scholar]
- 227.McClane SJ, Rombeau JL. Cytokines and inflammatory bowel disease: a review. JPEN J Parenter Enteral Nutr. 1999;23:S20–S24. doi: 10.1177/014860719902300506. [DOI] [PubMed] [Google Scholar]
- 228.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 229.Mege JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis. 2011;24:230–234. doi: 10.1097/QCO.0b013e328344b73e. [DOI] [PubMed] [Google Scholar]
- 230.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–787. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 231.Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matseoane S, Tschida PA. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 232.Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, Redding SW, Ebersole JL, McDevitt JT. Current developments in salivary diagnostics. Biomark Med. 2010;4:171–189. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mitchel GF. Co-evolution of parasites and adaptive immune responses. Immunol Today. 1991;22:A2–A5. doi: 10.1016/S0167-5699(05)80002-7. [DOI] [PubMed] [Google Scholar]
- 234.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JP. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 235.Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–697. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- 236.Mouton C, Desclauriers M, Allard H, Bouchard M. Serum antibodies to Bacteroides gingivalis in periodontitis: a longitudinal study. J Periodontal Res. 1987;22:426–430. doi: 10.1111/j.1600-0765.1987.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 237.Mowat AM. Dendritic cells and immune responses to orally administered antigens. Vaccine. 2005;23:1797–1799. doi: 10.1016/j.vaccine.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 238.Muller-Berghaus J, Olson WC, Moulton RA, Knapp WT, Schadendorf D, Storkus WJ. IL-12 production by human monocyte-derived dendritic cells: looking at the single cell. J Immunother. 2005;28:306–313. doi: 10.1097/01.cji.0000163594.74533.10. [DOI] [PubMed] [Google Scholar]
- 239.Murray PA, Burstein DA, Winkler JR. Antibodies to Bacteroides gingivalis in patients with treated and untreated periodontal disease. J Periodontol. 1989;60:96–103. doi: 10.1902/jop.1989.60.2.96. [DOI] [PubMed] [Google Scholar]
- 240.Niedergang F, Kweon MN. New trends in antigen uptake in the gut mucosa. Trends Microbiol. 2005;13:485–490. doi: 10.1016/j.tim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 241.Niemiec BA. Periodontal therapy. Top Companion Anim Med. 2008;23:81–90. doi: 10.1053/j.tcam.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 242.Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80:1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nonnenmacher C, Mutters R, de Jacoby LF. Microbiological characteristics of subgingival microbiota in adult periodontitis, localized juvenile periodontitis and rapidly progressive periodontitis subjects. Clin Microbiol Infect. 2001;7:213–217. doi: 10.1046/j.1469-0691.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 244.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, Man YG, Borges V, Schedin P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176:1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.O’Mahony L, O’Callaghan L, McCarthy J, Shilling D, Scully P, Sibartie S, Kavanagh E, Kirwan WO, Redmond HP, Collins JK, Shanahan F. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am J Physiol Gastrointest Liver Physiol. 2006;290:G839–G845. doi: 10.1152/ajpgi.00112.2005. [DOI] [PubMed] [Google Scholar]
- 246.Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, Stewart DD, Murtha AP, Cochran DL, Dudley DJ, Reddy MS, Geurs NC, Hauth JC. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstet Gynecol. 2009;114:551–559. doi: 10.1097/AOG.0b013e3181b1341f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA, Couper DJ, Stewart DD, Falkner KL, Graham SP, Grossi S, Gunsolley JC, Madden T, Maupome G, Trevisan M, Van Dyke TE, Genco RJ. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80:190–201. doi: 10.1902/jop.2009.080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ogrendik M. Rheumatoid arthritis is linked to oral bacteria: etiological association. Mod Rheumatol. 2009;19:453–456. doi: 10.1007/s10165-009-0194-9. [DOI] [PubMed] [Google Scholar]
- 249.Oral health in America: a report of the Surgeon General. J Calif Dent Assoc. 2000;28:685–695. [PubMed] [Google Scholar]
- 250.Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006;21:256–260. doi: 10.1111/j.1399-302X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 251.Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin 18 and periodontal disease. J Dent Res. 2007;86:586–593. doi: 10.1177/154405910708600702. [DOI] [PubMed] [Google Scholar]
- 252.Page RC. Host response tests for diagnosing periodontal diseases. J Periodontol. 1992;63:356–366. doi: 10.1902/jop.1992.63.4s.356. [DOI] [PubMed] [Google Scholar]
- 253.Page RC, Sims TJ, Engel LD, Moncla BJ, Bainbridge B, Stray J, Darveau RP. The immunodominant outer membrane antigen of Actinobacillus actinomycetemcomitans is located in the serotype-specific high-molecular-mass carbohydrate moiety of lipopolysaccharide. Infect Immun. 1991;59:3451–3462. doi: 10.1128/iai.59.10.3451-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Palmer RJ., Jr Supragingival and subgingival plaque: paradigm of biofilms. Compend Contin Educ Dent. 2010;31:104–106. 108. 110 passim; quiz 124, 138. [PubMed] [Google Scholar]
- 255.Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlén G. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol. 2000;71:885–897. doi: 10.1902/jop.2000.71.6.885. [DOI] [PubMed] [Google Scholar]
- 256.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 257.Paster BJ, Dewhirst FE. Molecular microbial diagnosis. Periodontol 2000. 2009;51:38–44. doi: 10.1111/j.1600-0757.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 259.Perez-Chaparro PJ, Gracieux P, Lafaurie GI, Donnio PY, Bonnaure-Mallet M. Genotypic characterization of Porphyromonas gingivalis isolated from subgingival plaque and blood sample in positive bacteremia subjects with periodontitis. J Clin Periodontol. 2008;35:748–753. doi: 10.1111/j.1600-051X.2008.01296.x. [DOI] [PubMed] [Google Scholar]
- 260.Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009;191:6804–6811. doi: 10.1128/JB.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Persson GR. Immune responses and vaccination against periodontal infections. J Clin Periodontol. 2005;32(Suppl 6):39–53. doi: 10.1111/j.1600-051X.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 262.Persson GR, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, Page RC. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect Immun. 1994;62:1026–1031. doi: 10.1128/iai.62.3.1026-1031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Persson GR, Engel LD, Moncla BJ, Page RC. Macaca nemestrina: a non-human primate model for studies of periodontal disease. J Periodontal Res. 1993;28:294–300. doi: 10.1111/j.1600-0765.1993.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 264.Persson GR, Engel LD, Whitney CW, Weinberg A, Moncla BJ, Darveau RP, Houston L, Braham P, Page RC. Macaca fascicularis as a model in which to assess the safety and efficacy of a vaccine for periodontitis. Oral Microbiol Immunol. 1994;9:104–111. doi: 10.1111/j.1399-302x.1994.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 265.Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, Landau H, Brinkmann PG, Schlattmann P, Zernicke J, Buttgereit F, Detert J. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–986. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 266.Rai B, Kaur J, Anand SC. Possible relationship between periodontitis and dementia in a North Indian old age population: a pilot study. Gerodontology. 2010;29:e200–e205. doi: 10.1111/j.1741-2358.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- 267.Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004;12:509–517. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 268.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, Chung SA, Graham RR, Zidovetzki R, Kelly JA, Kaufman KM, Jacob CO, Vyse TJ, Tsao BP, Kimberly RP, Gaffney PM, Alarcón-Riquelme ME, Harley JB, Langefeld CD for The International Consortium on the Genetics of Systemic E. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rams TE, Listgarten MA, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species-specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J Periodontal Res. 2006;41:228–234. doi: 10.1111/j.1600-0765.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 270.Ray JD, Jr, Eubanks DL. Dental homecare: teaching your clients to care for their pet’s teeth. J Vet Dent. 2009;26:57–60. doi: 10.1177/089875640902600115. [DOI] [PubMed] [Google Scholar]
- 271.Reinhardt RA, McDonald TL, Bolton RW, DuBois LM, Kaldahl WB. IgG subclasses in gingival crevicular fluid from active versus stable periodontal sites. J Periodontol. 1989;60:44–50. doi: 10.1902/jop.1989.60.1.44. [DOI] [PubMed] [Google Scholar]
- 272.Ren L, Leung WK, Darveau RP, Jin L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and toll-like receptors 2 and 4 in chronic periodontitis. J Periodontol. 2005;76:1950–1959. doi: 10.1902/jop.2005.76.11.1950. [DOI] [PubMed] [Google Scholar]
- 273.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–2826. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 275.Rosenstein ED, Kushner LJ, Kramer N, Kazandjian G. Pilot study of dietary fatty acid supplementation in the treatment of adult periodontitis. Prostaglandins Leukot Essent Fatty Acids. 2003;68:213–218. doi: 10.1016/s0952-3278(02)00272-7. [DOI] [PubMed] [Google Scholar]
- 276.Roth GS, Lane MA, Ingram DK. Caloric restriction mimetics: the next phase. Ann N Y Acad Sci. 2005;1057:365–371. doi: 10.1196/annals.1356.027. [DOI] [PubMed] [Google Scholar]
- 277.Ruhl S. The scientific exploration of saliva in the postproteomic era: from database back to basic function. Expert Rev Proteomics. 2012;9:85–96. doi: 10.1586/epr.11.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol. 1998;3:40–50. doi: 10.1902/annals.1998.3.1.40. [DOI] [PubMed] [Google Scholar]
- 279.Satthaporn S, Eremin O. Dendritic cells (I): biological functions. J R Coll Surg Edinb. 2001;46:9–19. [PubMed] [Google Scholar]
- 280.Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Camargo LE, Line SR. Interleukin 10 gene promoter polymorphisms are associated with chronic periodontitis. J Clin Periodontol. 2004;31:443–448. doi: 10.1111/j.1600-051X.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 281.Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, Arlt A, Rosenstiel P, Noack B, Groessner-Schreiber B, Jepsen S, Loos BG, Schreiber S. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. 2010;19:553–562. doi: 10.1093/hmg/ddp508. [DOI] [PubMed] [Google Scholar]
- 282.Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: an archetypical biofilm disease. J Am Dent Assoc. 2009;140:978–986. doi: 10.14219/jada.archive.2009.0307. [DOI] [PubMed] [Google Scholar]
- 283.Schenck K, Helgeland K, Tollefsen T. Antibodies against lipopolysaccharide from Bacteroides gingivalis before and after periodontal treatment. Scand J Dent Res. 1987;95:112–118. doi: 10.1111/j.1600-0722.1987.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 284.Schenkein HA. Genetics of early-onset periodontal diseases. Washington, DC: American Society for Microbiology; 1994. [Google Scholar]
- 285.Schenkein HA, Van Dyke TE. Early-onset periodontitis: systemic aspects of etiology and pathogenesis. Periodontol 2000. 1994;6:7–25. doi: 10.1111/j.1600-0757.1994.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 286.Schlegel-Bregenzer B, Persson RE, Lukehart S, Braham P, Oswald T, Persson GR. Clinical and microbiological findings in elderly subjects with gingivitis or periodontitis. J Clin Periodontol. 1998;25:897–907. doi: 10.1111/j.1600-051x.1998.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 287.Schoppet M, Huppertz HI, Simm A, Bubert A. Infection of dendritic cells by enterobacteriaceae. Med Microbiol Immunol. 2000;188:191–196. doi: 10.1007/s004300000038. [DOI] [PubMed] [Google Scholar]
- 288.Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993;64:497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- 289.Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 290.Scott K, Manunta M, Germain C, Smith P, Jones M, Mitchell P, Dessi D, Branigan Bamford K, Lechler RI, Fiori PL, Foster GR, Lombardi G. Qualitatively distinct patterns of cytokines are released by human dendritic cells in response to different pathogens. Immunol. 2005;116:245–254. doi: 10.1111/j.1365-2567.2005.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Seledtsov VI, Seledtsova GV. A balance between tissue-destructive and tissue-protective immunities: a role of toll-like receptors in regulation of adaptive immunity. Immunobiology. 2012;217:430–435. doi: 10.1016/j.imbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 292.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 293.Serhan CN. Novel omega-3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 294.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 297.Sexton WM, Lin Y, Kryscio RJ, Dawson DR, 3rd, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. 2011;38:434–441. doi: 10.1111/j.1600-051X.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodontal Res. 1993;28:478–486. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 299.Shapira L, Eizenberg S, Sela MN, Soskolne A, Brautbar H. HLA A9 and B15 are associated with the generalized form, but not the localized form, of early-onset periodontal diseases. J Periodontol. 1994;65:219–223. doi: 10.1902/jop.1994.65.3.219. [DOI] [PubMed] [Google Scholar]
- 300.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 302.Singer RE, Moss K, Beck JD, Offenbacher S. Association of systemic oxidative stress with suppressed serum IgG to commensal oral biofilm and modulation by periodontal infection. Antioxid Redox Signal. 2009;11:2973–2983. doi: 10.1089/ars.2009.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Singh A, Mehdi AA, Srivastava RN, Verma NS. Immunoregulation of bone remodelling. Int J Crit Illn Inj Sci. 2012;2:75–81. doi: 10.4103/2229-5151.97271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sirard JC, Bayardo M, Didierlaurent A. Pathogen-specific TLR signaling in mucosa: mutual contribution of microbial TLR agonists and virulence factors. Eur J Immunol. 2006;36:260–263. doi: 10.1002/eji.200535777. [DOI] [PubMed] [Google Scholar]
- 305.Slots J, Rams TE. Microbiology of periodontal disease. In: Slots J, Taubman MA, editors. Contemporary oral microbiology and immunology. St. Louis: Mosby-Year Book, Inc; 1992. pp. 425–445. [Google Scholar]
- 306.Smith DJ. Dental caries vaccines: prospects and concerns. Crit Rev Oral Biol Med. 2002;13:335–349. doi: 10.1177/154411130201300404. [DOI] [PubMed] [Google Scholar]
- 307.Smith DJ, Gadalla LM, Ebersole JL, Taubman MA. Gingival crevicular fluid antibody to oral microorganisms. III. Association of gingival homogenate and gingival crevicular fluid antibody levels. J Periodontal Res. 1985;20:357–367. doi: 10.1111/j.1600-0765.1985.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 308.Smith DJ, Mattos-Graner RO. Secretory immunity following mutans streptococcal infection or immunization. Curr Top Microbiol Immunol. 2008;319:131–156. doi: 10.1007/978-3-540-73900-5_6. [DOI] [PubMed] [Google Scholar]
- 309.Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, Li Y, Kurreeman FA, Zhernakova A, Hinks A, Guiducci C, Chen R, Alfredsson L, Amos CI, Ardlie KG, Barton A, Bowes J, Brouwer E, Burtt NP, Catanese JJ, Coblyn J, Coenen MJ, Costenbader KH, Criswell LA, Crusius JB, Cui J, de Bakker PI, De Jager PL, Ding B, Emery P, Flynn E, Harrison P, Hocking LJ, Huizinga TW, Kastner DL, Ke X, Lee AT, Liu X, Martin P, Morgan AW, Padyukov L, Posthumus MD, Radstake TR, Reid DM, Seielstad M, Seldin MF, Shadick NA, Steer S, Tak PP, Thomson W, van der Helm-van Mil AH, van der Horst-Bruinsma IE, van der Schoot CE, van Riel PL, Weinblatt ME, Wilson AG, Wolbink GJ, Wordsworth BP, Wijmenga C, Karlson EW, Toes RE, de Vries N, Begovich AB, Worthington J, Siminovitch KA, Gregersen PK, Klareskog L, Plenge RM. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun Study. J Am Dent Assoc. 2007;138:1314–1322. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 313.Stein PS, Kryscio RJ, Desrosiers M, Donegan SJ, Gibbs MB. Tooth loss, apolipoprotein E, and decline in delayed word recall. J Dent Res. 2010;89:473–477. doi: 10.1177/0022034509357881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Stephensen CB. Fish oil and inflammatory disease: is asthma the next target for n-3 fatty acid supplements? Nutr Rev. 2004;62:486–489. doi: 10.1111/j.1753-4887.2004.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 315.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 316.Streckfus CF, Parsell DE, Streckfus JE, Pennington W, Johnson RB. Relationship between oral alveolar bone loss and aging among African-American and Caucasian individuals. Gerontology. 1999;45:110–114. doi: 10.1159/000022072. [DOI] [PubMed] [Google Scholar]
- 317.Sundquist M, Rydstrom A, Wick MJ. Immunity to Salmonella from a dendritic point of view. Cell Microbiol. 2004;6:1–11. doi: 10.1046/j.1462-5822.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 318.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82:259–264. doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 319.Suzuki JB, Martin SA, Vincent JW, Falkler WA., Jr Local and systemic production of immunoglobulins to periodonto-pathogens in periodontal disease. J Periodontal Res. 1984;19:599–603. doi: 10.1111/j.1600-0765.1984.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 320.Takashiba S, Noji S, Nishimura F, Ohyama H, Kurihara H, Nomura Y, Taniguchi S, Murayama Y. Unique intronic variations of HLA-DQ beta gene in early-onset periodontitis. J Periodontol. 1994;65:379–386. doi: 10.1902/jop.1994.65.5.379. [DOI] [PubMed] [Google Scholar]
- 321.Talaat KR, Bonawitz RE, Domenech P, Nutman TB. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J Infect Dis. 2006;193:196–204. doi: 10.1086/498912. [DOI] [PubMed] [Google Scholar]
- 322.Tanaka S, Fakher M, Barbour SE, Schenkein HA, Tew JG. Influence of proinflammatory cytokines on Actinobacillus actinomycetemcomitans specific IgG responses. J Periodontal Res. 2006;41:1–9. doi: 10.1111/j.1600-0765.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 323.Tang L, Zhou XD, Wang Q, Zhang L, Wang Y, Li XY, Huang DM. Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch Oral Biol. 2011;56:1064–1072. doi: 10.1016/j.archoralbio.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 324.Tanner ACR. Microbial succession in the development of periodontal disease. In: Hamada S, Holt SC, McGhee JR, editors. Periodontal disease: pathogens & host immune responses. Tokyo: Quintessence Publishing Co., Ltd; 1991. pp. 13–26. [Google Scholar]
- 325.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. v. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 326.Taubman MA, Eastcott JW, Simauchi H, Takeichi O, Smith DJ. Modulatory role of T lymphocytes in periodontal inflammation. In: Genco RJ, editor. Molecular pathogenesis of periodontal disease. Washington, DC: American Society for Microbiology; 1994. pp. 147–157. [Google Scholar]
- 327.Taubman MA, Kawai T, Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J Clin Periodontol. 2007;34:367–369. doi: 10.1111/j.1600-051X.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 328.Teles FR, Teles RP, Uzel NG, Song XQ, Torresyap G, Socransky SS, Haffajee AD. Early microbial succession in redeveloping dental biofilms in periodontal health and disease. J Periodontal Res. 2012;47:95–104. doi: 10.1111/j.1600-0765.2011.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44:411–417. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Thoden van Velzen SK, Abraham-Inpijn L, Moorer WR. Plaque and systemic disease: a reappraisal of the focal infection concept. J Clin Periodontol. 1984;11:209–220. doi: 10.1111/j.1600-051x.1984.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 332.Thomas MV, Branscum A, Miller CS, Ebersole J, Al-Sabbagh M, Schuster JL. Within-subject variability in repeated measures of salivary analytes in healthy adults. J Periodontol. 2009;80:1146–1153. doi: 10.1902/jop.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Torchinsky MB, Garaude J, Blander JM. Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol. 2010;22:55–62. doi: 10.1016/j.coi.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Torkamani A, Topol EJ, Schork NJ. Pathway analysis of seven common diseases assessed by genome-wide association. Genomics. 2008;92:265–272. doi: 10.1016/j.ygeno.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 336.Van Dyke TE. Role of neutrophils in host defense to periodontal infections. In: Hamada S, Holt SC, McGhee JR, editors. Periodontal disease: pathogens & host immune responses. Tokyo: Quintessence Publishing Co; 1991. pp. 251–261. [Google Scholar]
- 337.Van Dyke TE, Duncan RL, Cutler CW, Kalmar JR, Arnold RR. Mechanisms and consequences of neutrophil interaction with subgingival microbiota. In: Guggenheim B, editor. Periodontology Today. Basel: Karger; 1988. pp. 290–217. [Google Scholar]
- 338.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 339.van Winkelhoff AJ, Rijnsburger MC, van der Velden U. Clonal stability of Porphyromonas gingivalis in untreated periodontitis. J Clin Periodontol. 2008;35:674–679. doi: 10.1111/j.1600-051X.2008.01285.x. [DOI] [PubMed] [Google Scholar]
- 340.Vaughan AT, Gorringe A, Davenport V, Williams NA, Heyderman RS. Absence of mucosal immunity in the human upper respiratory tract to the commensal bacteria Neisseria lactamica but not pathogenic Neisseria meningitidis during the peak age of nasopharyngeal carriage. J Immunol. 2009;182:2231–2240. doi: 10.4049/jimmunol.0802531. [DOI] [PubMed] [Google Scholar]
- 341.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 342.Veckman V, Miettinen M, Pirhonen J, Siren J, Matikainen S, Julkunen I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J Leukoc Biol. 2004;75:764–771. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 343.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 344.Wahl SM, Costa GL, Mizel DE, Allen JB, Skaleric U, Mangan DF. Role of transforming growth factor beta in the pathophysiology of chronic inflammation. J Periodontol. 1993;64:450–455. [PubMed] [Google Scholar]
- 345.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 346.Watanabe H, Marsh PD, Ivanyi L. Antigens of Actinobacillus actinomycetemcomitans identified by immunoblotting with sera from patients with localized human juvenile periodontitis and generalized severe periodontitis. Arch Oral Biol. 1989;34:649–656. doi: 10.1016/0003-9969(89)90020-4. [DOI] [PubMed] [Google Scholar]
- 347.Weinberg MA, Bral M. Laboratory animal models in periodontology. J Clin Periodontol. 1999;26:335–340. doi: 10.1034/j.1600-051x.1999.260601.x. [DOI] [PubMed] [Google Scholar]
- 348.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases – did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis- induced pro-inflammatory signals. J Biol Chem. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Williams RC, Beck JD, Offenbacher SN. The impact of new technologies to diagnose and treat periodontal disease. A look to the future. J Clin Periodontol. 1996;23:299–305. doi: 10.1111/j.1600-051x.1996.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 351.Wilson ME. IgG antibody response of localized juvenile periodontitis patients to the 29 kilodalton outer membrane protein of Actinobacillus actinomycetemcomitans. J Periodontol. 1991;62:211–218. doi: 10.1902/jop.1991.62.3.211. [DOI] [PubMed] [Google Scholar]
- 352.Wilson ME, Schifferle RE. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect Immun. 1991;59:1544–1551. doi: 10.1128/iai.59.4.1544-1551.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wilton JM, Bampton JL, Hurst TJ, Caves J, Powell JR. Interleukin-1 beta and IgG subclass concentrations in gingival crevicular fluid from patients with adult periodontitis. Arch Oral Biol. 1993;38:55–60. doi: 10.1016/0003-9969(93)90155-f. [DOI] [PubMed] [Google Scholar]
- 354.Wirthlin MR. Natural and experimental periodontal diseases in non-human primates. J West Soc Periodontol Periodontal Abstr. 1991;39:89–108. [PubMed] [Google Scholar]
- 355.Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Gunther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol. 2002;118:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 356.Xu T, Lundqvist A, Ahmed HJ, Eriksson K, Yang Y, Lagergard T. Interactions of Haemophilus ducreyi and purified cytolethal distending toxin with human monocyte-derived dendritic cells, macrophages and CD4+ T cells. Microbes Infect. 2004;6:1171–1181. doi: 10.1016/j.micinf.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 357.Yamazaki K, Nakajima T, Ohsawa Y, Tabeta K, Yoshie H, Sakurai K, Seymour GJ. Selective expansion of T cells in gingival lesions of patients with chronic inflammatory periodontal disease. Clin Exp Immunol. 2000;120:154–161. doi: 10.1046/j.1365-2249.2000.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yoshino T, Laine ML, van Winkelhoff AJ, Dahlen G. Genotype variation and capsular serotypes of Porphyromonas gingivalis from chronic periodontitis and periodontal abscesses. FEMS Microbiol Lett. 2007;270:75–81. doi: 10.1111/j.1574-6968.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 360.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contribution to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zambon JJ, Christersson LA, Slots J. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. J Periodontol. 1983;54:707–711. doi: 10.1902/jop.1983.54.12.707. [DOI] [PubMed] [Google Scholar]
- 362.Zijnge V, Ammann T, Thurnheer T, Gmur R. Subgingival biofilm structure. Front Oral Biol. 2012;15:1–16. doi: 10.1159/000329667. [DOI] [PubMed] [Google Scholar]
- 363.Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmur R, Harmsen HJ. Oral biofilm architecture on natural teeth. PLoS ONE. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]