Abstract
As the highly active antiretroviral therapy (HAART) has transitioned human immunodeficiency virus (HIV) infection into a ‘chronic disease’ management strategy, there is growing evidence that infection with non-HIV pathogens in HIV+ patients may have important public health implications in undermining HAART success and acquired immunodeficiency syndrome progression. Several bacterial and host cell products during infections with non-HIV pathogens have shown the capacity to regulate HIV replication in latently infected cells. A high prevalence of oral infections caused by bacteria, viruses and fungi has been described in HIV+ patients, including periodontal disease. The oral cavity appears to be a site of HIV pathogenesis and potential reservoir for the disease as HIV RNA and DNA forms are present in saliva as well as in gingival crevicular fluid, and oral epithelial cells are susceptible to either cell free or cell-associated HIV infection. The clinical and biological bases of potential associations between chronic oral inflammatory disorders, such as periodontal disease, and exacerbation of HIV viraemia have received little attention. This review attempts to evaluate the current understanding of HIV reactivation as a result of co-infection and/or inflammation induced by non-HIV pathogens in HIV-infected patients, and presents a hypothetic model about the potential role of periodontitis as a global oral infection that potentially contributes to HIV recrudescence.
Keywords: infections, HIV-1 reactivation, periodontal disease
Introduction
According to the last report of the Joint United Nations Program on HIV/AIDS (UNAIDS), about 33 million people globally are living with acquired immunodeficiency syndrome (AIDS) (UNIAIDS. 2007 report on the global AIDS epidemic. http://www.unaids.org/en). Acquired immunodeficiency syndrome is a disease caused by infection with the human immunodeficiency virus (HIV) and it is typically characterized by severe immune alterations, particularly targeting lymphocyte subpopulations (e.g. CD4+ T-cells) with consequent immunosuppression and appearance of a variety of potentially fatal opportunistic infections (Chermann et al, 1983). The continually expanding HIV-infected population remains a worldwide issue, with estimates that HIV/AIDS will be the 3rd leading cause of death in the US within the next decade. It has been about 25 years since HIV was identified as the primary aetiologic agent leading to AIDS (Broder and Gallo, 1984). However, while the available treatments cannot completely eliminate AIDS, HIV infections have changed from a progressing fatal condition to a more manageable chronic illness resulting from the continued development of antiretroviral therapeutics (Ho et al, 1995).
The combination of nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitors, or highly active antiretroviral therapy (HAART), not only has significantly decreased the morbidity and mortality of HIV disease, but also is directly related to a significant diminution of opportunistic infections (Smith, 2006). Despite these crucial improvements in controlling the disease progression in patients receiving HAART, a complete eradication of HIV infection is not yet possible, specifically related to the ability of HIV to integrate and produce a latent infection in long-lived host cells, e.g. T cells, macrophages and dendritic cells (Sungkanuparph et al, 2003). The ability of HIV to hide and evade the immune system by integrating into the host genome has become an important target of HIV research, with a goal of elucidating molecular mechanisms that control HIV latency and reactivation, as well as examining a range of new molecules, which target this portion of the HIV life cycle of infection and disease (Stevens et al, 2006).
Chronic immune activation associated with co-infections by non-HIV pathogens (e.g. Pneumocystis carinii, Mycobacterium tuberculosis and Herpesviruses) (Koziel et al, 1999; Goletti et al, 1996; Caselli et al, 2005) has been suggested as a critical factor that influences the severity and progression of AIDS and HAART success/failure, as well as the reduction in survival rate and increase in the risk of HIV transmission in HIV+ patients (Whalen et al, 1995; Blanchard et al, 1997; Ott, 2002). In addition, some pro-inflammatory cytokines/chemokines and mediators produced by immune and non-immune host cells have also been shown to act as inhibitors or stimulators in regulating HIV recrudescence (Collman et al, 2003; Oguariri et al, 2007). Therefore, understanding the role of immune activation associated with non-HIV co-infections in HIV replication may have important clinical significance for the development of novel therapeutic strategies to control AIDS progression during opportunistic infections. There is a growing body of evidence supporting an increased prevalence and severity of a variety of oral diseases associated with immunosuppression coincident with HIV infection that appears early after viral infection. Thus, 40–50% of HIV+ patients exhibit an oral fungal, bacterial or viral infection (Reznik, 2005). Additionally, clinical evidence reveals a positive correlation between HIV viral load and oral infectious diseases, such as periodontal disease, hairy leukoplakia and candidiasis (Alpagot et al, 2004, 2007; Patton, 2000; Ramirez-Amador et al, 2005; Black et al, 2000). However, the potential for oral pathogens to impact HIV cell reservoirs and induce HIV reactivation has not been delineated. The main goal of this review was to describe general concepts about HIV infection, latency and reactivation as well as to consider the evidence of HIV reactivation associated with non-oral opportunistic infections. Finally, based on these considerations, we present periodontal disease as an example of oral chronic infections that may have the ability to induce HIV exacerbation in HIV+ patients. Future studies could explore such a relationship the understanding of which may impact on the management of HIV+ patients and create opportunities to develop new long-term strategies to eradicate HIV.
Biological basis of HIV reactivation
HIV is a ribonucleic acid (RNA) virus that belongs to the retrovirus family and lentivirus sub-group. This group of viruses generally must make a deoxyribonucleic acid (DNA) copy (cDNA) of their RNA to replicate inside the host cell, taking advantage of an enzymatic machinery that includes reverse transcriptase (Freed and Mouland, 2006).
Infection is initiated by the encounter of a HIV virion with a cell expressing the CD4, cluster determinant receptor. The interaction between the CD4 molecule and the viral surface glycoprotein, gp120, induces a conformational change of gp120, allowing it to interact with chemokine receptors, CCR5 or CXCR4. This binding leads to fusion of the virus and the cell membrane, which further enables the virus to enter into the host cell. The viral glycoprotein, gp41, also appears critical for the viral fusion process. In the cytoplasm of the host cell, the HIV reverse transcriptase converts viral RNA into DNA, and this new DNA associated with viral and host proteins (pre-integration complex) then translocates into the nucleus, where it is spliced into the host’s DNA via the activity of another viral enzyme integrase. The viral DNA that becomes integrated into the host genome is known as a provirus. At this point of the viral cycle, HIV can remain latently infecting some host cells. Although CD4+ T cells appear to be the primary targets of HIV, other immune and non-immune cells with or without CD4 molecules on their surfaces can also be infected. Thus, macrophages, dendritic cells, CD8+ T cells, endothelial cells, epithelial cells and fibroblasts also can harbour HIV proviruses in their genome (Freed and Mouland, 2006).
Viral latency
Viral latency is defined as a reversible state in which the viral genome is present in the host cell without production of infectious virus particles, a scenario that has accompanied the success of HAART therapy. However, the molecular, cellular and immunological features that are involved in latency are diverse and depend on the specific biology of specific viruses, but in all cases, latency consists of an infection cycle that can be separated in three phases: (I) establishment, (II) maintenance and (III) reactivation (Redpath et al, 2001).
At the cellular level, during the establishment of latency, there is an inhibition of viral gene expression at an early stage following entry into the host cell, with most productive viral cycle genes being quiescent. At the establishment phase, host cell factors seem to be the determinant for suppressing viral gene expression and replication. During the maintenance phase, while virus expression is not required, some viral transcripts may play an important role, such that viral gene transcription is not necessarily completely silenced in latency. The reactivation phase transits from a state with no replicating virus to the production of new virions (Redpath et al, 2001).
Two major forms of HIV latency have been described, pre- and post-integration latency (Lassen et al, 2004). HIV post-integrated latency has been shown in numerous cell types, including CD4+ T cells, macrophages, dendritic cells, NK cells, B cells, endothelial cells, glial cells and epithelial cells (Blankson et al, 2002). Apparently, the most stable reservoirs in the post-integration state of latency are CD4+ T cells, with a predicted halflife of 43 months (Finzi et al, 1999), although a low level of transcription has been identified. Thus, down-regulation of viral transcriptional activity after integration into the host genome seems to be related to several mechanisms, including inaccessibility of the integrated provirus to the cellular transcriptional machinery, the absence in resting cells of transcription factors necessary for HIV reactivation, the presence of transcriptional repressors and the premature termination of HIV transcription elongation due to the absence of the viral Tat protein and its cofactors (He et al, 2002).
The population of latently infected cells in HIV+ patients seems to be refractory to HAART, and even in patients receiving antiretroviral therapy, HIV replication has been shown, which could account for the persistence of infection and resulting AIDS (Blankson et al, 2002). Importantly, emerging evidence shows that the high mutation rate of HIV in the presence of HAART is associated with drug resistance and diminished efficacy, which tend to increase in both treatment-naïve and treated patients (Little et al, 2002; Kuritzkes, 2007). Therefore, the study of mechanisms that modulate HIV replication during the latent stage is important to understand the pathogenesis of this infection as a chronic disease and to develop new therapies for eradicating latently infected cells or preventing the activation of HIV replication from these cell reservoirs.
Mechanisms involved in HIV reactivation
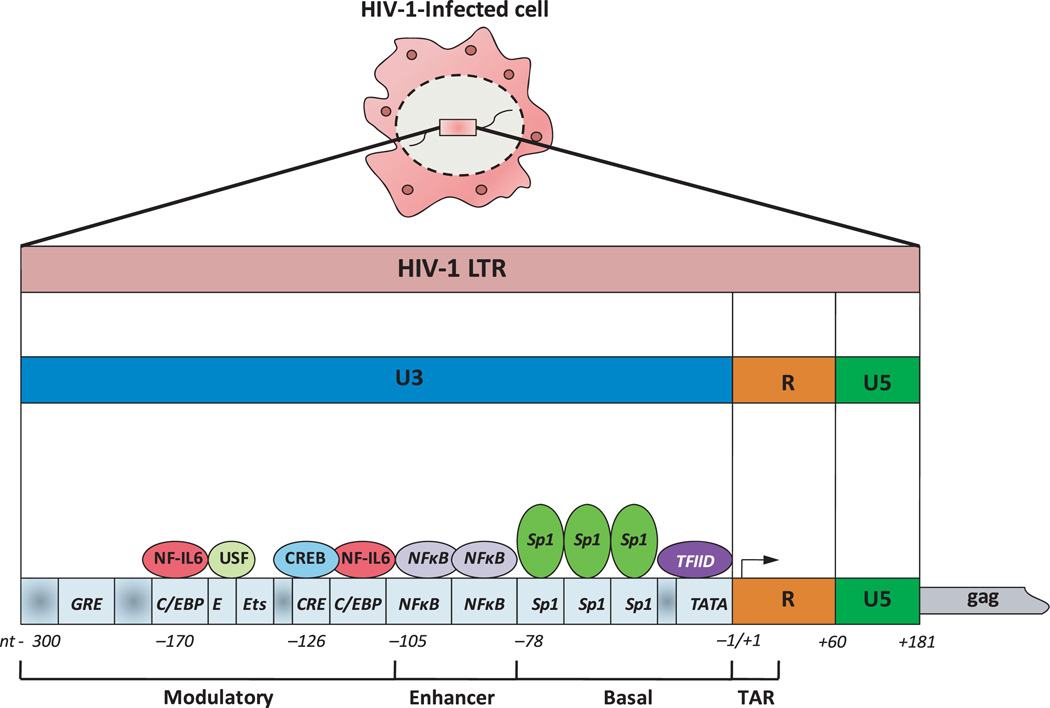
As a critical part of HIV life cycle, reactivation of the provirus results in the transcription of new viral RNA (genomic and mRNA) and synthesis of new viral proteins, which further leads to assembly and production of new virions during which the viral proteases play an important role. These particular events are regulated by both viral (e.g. Tat) and cellular transcription factors (e.g. NFκB, C/EBP, Sp1, CREB and AP-1) with binding sites present in the HIV promoter, as shown in Figure 1 (Garcia and Gaynor, 1994; Pereira et al, 2000). Two different phases could be regulating HIV transcription and controlling reactivation: (I) the early phase or initiation of transcription that is mainly activated by cellular transcription factors and only few transcripts elongate throughout the viral genome, resulting in transcription of the viral transactivator Tat. (II) The late phase of transcription occurs when enough Tat protein has accumulated, which then binds to the transactivation response element or TAR (an RNA hairpin loop formed at the 5′ end of all nascent HIV transcripts) and recruits the cellular protein kinase complex termed TAK/P-TEFb complex (Tat-associated kinase/positive transcriptional elongation factor b). Further, this complex causes the hyperphosphorylation of the RNA polymerase II and dramatically increases its ability to elongate (Freed, 2004). This set of molecular events clearly shows that HIV is able to adapt transcription of its integrated proviral genome by exploiting the specific cellular environment. In fact, the molecular mechanisms of HIV reactivation seem to differ depending upon the cell type, degree of cell activation and micro environmental factors, including cytokines and other soluble factors (Rohr et al, 2003). Interestingly, it also has been suggested that HIV-1 reactivation from latently infected cells can be a Tat-independent mechanism, where only activated cellular transcription factors such as NFκB and interferon regulatory factor-1 (IRF-1) will promote proviral transcription irrespective of the presence of Tat (Sgarbanti et al, 2008).
Figure 1.
Structure of human immunodeficiency virus 1-long terminal repeat (HIV-1 LTR) promoter. Transcription of HIV-1 provirus is regulated by both viral and cellular transcription factors with binding sites in the HIV-1 LTR that contains a number of cis-regulatory regions: (i) transactivation response element (TAR), encodes an mRNA that binds the viral transactivator Tat; (ii) core or basal region, which contains the initiator, the TATA box and three Sp1-binding sites; (iii) enhancer region which binds NFκB and (iv) modulatory region that has several target sequences for a variety of cellular transcription factors, such as NF-interleukin 6 (NF-IL6), cyclic AMP response element protein (CREB), etc. In the absence of the viral Tat protein, transcription of HIV-1 LTR is initiated but elongation is not successful, with production of few non-functional transcripts. Tat protein stimulates transcriptional elongation by interacting with mRNA TAR and promoting the formation of a transcriptional complex composed of Tat, cyclin T1 and CDK9, which then recruit RNA polymerase II to the initiation site
Among all HIV latently infected cells, macrophages have received particular attention because unlike CD4+ T cells, which are depleted by apoptosis during AIDS, these antigen presenting cells appear to be resistant to virus-mediated apoptotic death, promoting a long-term persistence of the HIV infection and potential contribution to developing of immunodeficiency. In addition to being a substantial viral reservoir, infected macrophages may also transfer HIV to uninfected T cells (Wahl et al, 2003). Unlike T cells, the mechanisms for HIV reactivation in macrophages involve the loss of the C/EBP family of transcriptional repressors which bind three different sites of the HIVLTR and may be mediated by T cell-macrophage interactions (Tesmer et al, 1993). However, the activity of NFκB transcription factor is critical to HIV reactivation in both T cells and macrophages (Henderson et al, 1995; Zhang et al, 1995).
Co-infections and HIV reactivation
Previous studies analysing the impact of vaccination for influenza and tetanus in HIV-infected patients found a temporal increase in the RNA viral load that returned to pre-vaccination levels in 3–4 weeks (Staprans et al, 1995; Stanley et al, 1996; O’Brien et al, 1995). These unexpected observations led to the hypothesis that co-infections in HIV+ patients may contribute to disease progression; a concept supported by a relationship between microbial infections and the clinical evolution of AIDS. Most of these studies have examined mycobacterial infections, which are associated with the production of large amounts of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, each of which has also been identified to elicit HIV reactivation in monocytes/macrophages (Poli et al, 1994). Thus, immunosupression in HIV-1+ patients enables opportunistic infections to lead to significant increase in the abundance of HIV virions. These findings suggested that HIV-associated immunodeficiency increases susceptibility to opportunistic infections, and opportunistic infections promote HIV reactivation that may enhance AIDS progression (Wahl et al, 2003). Mycobacterium tuberculosis is the most common opportunistic infection resulting in AIDS-related fatalities, and seems to be specifically associated with an increased viral mutation as well as accelerated course of HIV infection (Condos et al, 2000). Coincidently, in patients with successful tuberculosis therapy, a reduction in the viral load has been demonstrated (Whalen et al, 1995; Pape et al, 1993).
Basically, two general cellular responses have been described in monocytes/macrophages obtained from HIV+ patients with tuberculosis when exposed to M. tuberculosis: (i) increased transcriptional activity of HIV genes and virus production (Zhang et al, 1995; Goletti et al, 1996); and (ii) increased susceptibility of additional cells to infection with HIV (Toossi et al, 1993). Similar findings have been reported for infection with Streptococcus pneumoniae (Bush et al, 1996), Neisseria gonorrhea and Chlamydia trachomatis (Rotchford et al, 2000). These results have contributed to the inclusion of prophylactic antibiotics as part of AIDS treatment, with noted benefit for these immunocompromised patients in industrialized countries (Blanchard et al, 1997).
The HIV reactivation induced by other pathogens commonly found in HIV+ patients has also been studied. Thus, herpes simplex virus-1 (HSV-1) appears to use the same NFκB pathway to up-regulate transcriptional activity of HIVLTR in latently infected cells (Amici et al, 2004). Measles virus inhibits the in vitro production of HIV protein, p24, in peripheral blood mononuclear cells (PBMCs) latently infected cells; a finding that was also observed in HIV+ children with measles. The reduction of HIV RNA levels was correlated with a reduction in T cell proliferation during measles infection, potentially related to the CD4+ cells that where crucial for HIV replication (Garcia et al, 2005). Whether the measles virus is involved in inhibition of HIVLTR promoter activation remains unknown. Additionally, controversial results have been found using antigens of the parasite Plasmodium falciparum. In vitro studies suggest increased HIV replication in T CD4+ cells, whereas epidemiological studies have not detected a more rapid clinical progression of HIV disease. Recently, it was found that P. falciparum antigens can reduce the HIV infectivity in CD4+ T cells apparently through production of IFNγ and down-regulation of the CCR5 receptor, which could explain the clinical outcomes (Moriuchi et al, 2002). Using rhesus monkeys, it was also shown that the parasite Schistosoma mansoni exhibited the ability to reactivate HIV replication (Ayash-Rashkovsky et al, 2007). Finally, Trichomonas vaginalis, one of the most common non-viral sexually transmitted pathogens worldwide, exhibited the ability to not only disrupt epithelial monolayer but also enhance HIV replication in newly infected peripheral blood mononuclear cells (Guenthner et al, 2005).
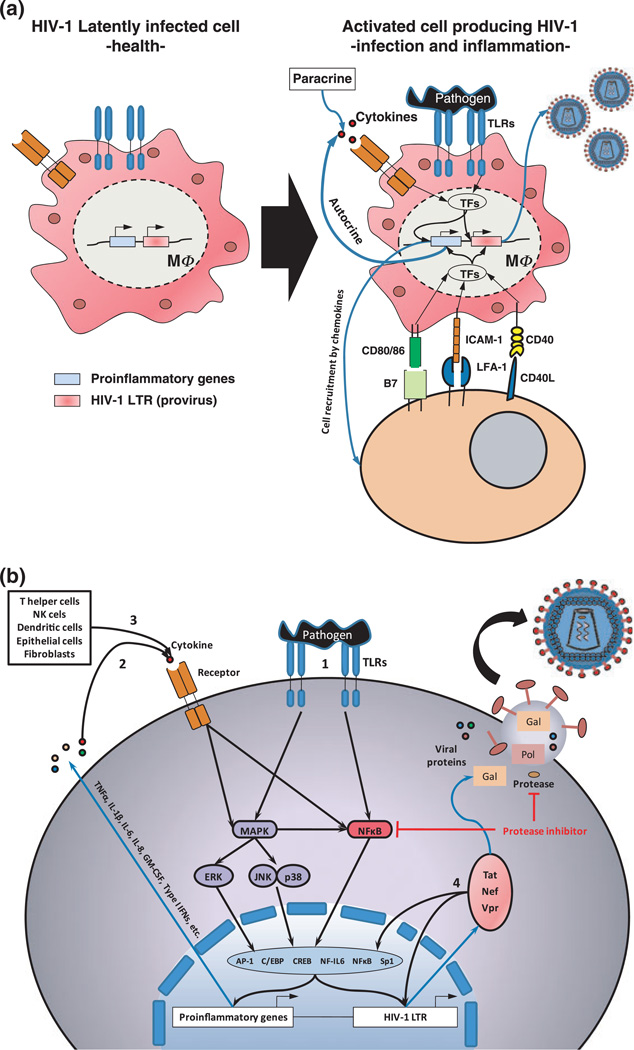
Although the cellular and molecular mechanisms by which opportunistic pathogens could be stimulating HIV reactivation in latently infected cells remain somewhat unclear, a growing body of evidence suggests an important role for Toll-like receptors (TLRs), and signalling molecules involved in TLR-induced cell activation (Figure 2). To date, HIVLTR transactivation and HIV replication associated with TLR2, TLR4 and TLR9 has been shown (Equils et al, 2003, 2004). Treatment of HIV-infected cells with enterobacterial lipopolyssacharide (LPS) exhibited substantial NFκB activation and HIVLTR transactivation, which appears to involve the IL-1R-associated signalling molecules MyD88, IRAK, TRAF-6, as well as p38 MAPK and PI3 kinase in macrophages (Equils et al, 2001, 2004). However, in a recent study, Nordone et al (2007) found that TLR4 stimulation did not induce HIV production in different cell lines, by using an ultra-purified LPS from Escherichia coli, and suggested that up-regulation of NFκB alone is not sufficient to activate HIV promoter. These results suggest a potential failure or impairment of TLR-4 signalling pathways during HIV infection. It is important to consider whether HIV reactivation is being evaluated in a model of acute or chronic infection, which may elicit different results associated with the same TLR (Nordone et al, 2007). It has been shown that re-stimulation of monocytes/macrophages previously exposed to various TLR ligands, LPS (TLR4 ligand), CpG (TLR9 ligand) and lipoteichoic acid (TLR2 ligand), fails to elicit pro-inflammatory cytokines such as TNFα, IL-1, IL-6, IL-12, as part of the tolerance mechanisms of the immune system to control inflammatory responses, particularly at mucosal surfaces highly colonized by commensals microorganisms (Dobrovolskaia and Vogel, 2002). Surprisingly, this ‘tolerogenic response’ does not affect the HIV gene and protein expression after re-stimulation in vitro despite a dramatic reduction in pro-inflammatory cytokine production. These results suggest that HIV could be escaping normal mechanisms of host infection control, such as microbe-induced tolerance (Bafica et al, 2004). Most recently, it has been elegantly shown by Brenchley et al that microbial translocation mainly of Gram-negative bacteria from the gut to systemic circulation is a cause of systemic activation in chronic HIV infection and AIDS progression in vivo. Importantly, effective antiretroviral therapy appeared to reduce microbial translocation only partially, which suggests that persistence of abnormalities of gastrointestinal mucosal surface with a limited reconstitution of gastrointestinal CD4+ T cells as well as impaired B-cell function could be accompanying HAART (Brenchley et al, 2006).
Figure 2.
Model of human immunodeficiency virus 1 (HIV-1) reactivation during co-infections (a) HIV-1 latently infected cells in the absence of infection and inflammation remain undistinguishable from non-HIV-1 infected cells without production of new virions. During co-infections, activation of the HIV-1-infected cell by different membrane receptors [Toll-like receptors (TLRs), cytokine receptors, integrins, co-stimulatory molecules, etc.] induces activation of human immunodeficiency virus 1-long terminal repeat (HIV-1 LTR) provirus with the consequent production of new virions. (b) Activation of cell membrane receptors in HIV-1-infected cells turns on downstream signalling pathways that lead to activation of transcription factors with the ability for regulating not only proinflammatory genes but also HIV-1 LTR transcription and production of new virions. TLR activation leads to the production of pro-inflammatory cytokines as well as production of new HIV-1 virions (1). Pro-inflammatory cytokines produced by the HIV-1-infected cell itself in response to bacterial challenge (2), as well as cytokines from other cellular sources (e.g. T helper cells, natural killer (NK) cells, dendritic cells (DCs), epithelial cells, fibroblasts, etc.) (3), induce a synergistic HIV-1 viral production. Some viral proteins as Tat, Nef and Vpr also have the ability to induce HIV-1 LTR reactivation and cytokine production (4). A potential for some protease inhibitors used in highly active antiretroviral therapy to inhibit TLR-4 signalling through blocking NFκB has been suggested
Although most studies evaluating HIV reactivation induced directly by microorganisms have been focused on monocytes/macrophages, some findings have revealed that bacterial stimulation also has the capacity to enhance HIV replication in CD4+ T cells (Moriuchi et al, 2000). Most recently the demonstration that T cells also express TLRs (Gelman et al, 2004, 2006) suggests the possibility that co-infecting non-HIV pathogens can directly reactivate HIV proviral forms in CD4+ T cells, the most important viral reservoir. In addition to TLR-associated viral reactivation, it has also been recently demonstrated that different TLR agonists induce activation in CD8+ T cells and death in CD4+ T cells, events that positively correlate with the immunological changes typically observed during AIDS progression (Funderburg et al, 2008).
Cytokines/chemokines and soluble mediators associated with HIV reactivation
Immune activation during the host response to HIV infection itself or the presence of exogenous stimuli has an important effect on the HIV life cycle. Thus, the constant immune activation during chronic infections characterized by the constant release of pro-inflammatory cytokines can result in increased HIV replication and also lead to phenotypical and genotypical changes in the virus (Figure 2) (Devadas et al, 2004). A group of cytokines/chemokines and soluble mediators have been found to have the capacity to either stimulate or inhibit HIV replication, or in certain cases do both (Table 1). Thus, pro-inflammatory cytokines, such as TNFα, TNF-β, IL-1β and IL-6, are associated with an increased HIV replication in T cells and monocytes-derived macrophages (MDM) (Poli et al, 1990; Devadas et al, 2004), IL-2 and IL-7 up-regulate HIV activation in T cells (Wang et al, 2005) and macrophage-colony stimulating factor stimulates HIV in MDM. On the other hand, IL-10, TGFβ, IL-13, IL-16 and IL-27 inhibit HIV in MDM (Wang and Rice, 2006; Montaner et al, 1997; Truong et al, 1999; Imamichi et al, 2008), and IFNγ, IL-4 and granulocyte/macrophage-colony stimulating factor appear to have a pivotal role being activators or inhibitors under selected conditions for HIV reactivation (Biswas et al, 1992; Naif et al, 1994; Kedzierska et al, 2000; Osiecki et al, 2005). Although IL-10 plays a critical role down-regulating the cellular immune response to HIV-1, it appears to be involved in TNFα-mediated activation of HIV in monocytes/macrophages (Rabbi et al, 1998). IL-18 is a pro-inflammatory cytokine that acts on Th1 cells to produce IFNγ in the presence of IL-12, whereas in the absence of IL-12, it promotes the differentiation of Th2 cells (Nakanishi et al, 2001). It has been shown that IL-18 stimulates the replication of HIV in chronically infected promonocytic cells (Pugliese et al, 2002). Most recently, a significant increase in IL-17 production by CD4+ and CD4− T cells has been associated with HIV infection; however, its role in HIV reactivation remains unknown (Maek et al, 2007).
Table 1.
Soluble factors produced by oral host cells with potential to modulate HIV reactivation
| Epithelial cells | Gingival fibroblasts | |||
|---|---|---|---|---|
| Protein | mRNA | Protein | mRNA | |
| Stimulators | ||||
| TNF-alpha | Yes | Yes | - | Yes |
| TNF-beta | - | - | - | - |
| IL-1 beta | Yes | Yes | - | Yes |
| IL-6 | Yes | Yes | Yes | Yes |
| IL-7 | Yes | Yes | - | - |
| IL-2 | - | - | - | - |
| IL-18 | Yes | Yes | - | - |
| M-CSF | Yes | Yes | - | - |
| Monocyte chemotactic protein-1 (MCP-1) | Yes | Yes | Yes | Yes |
| Prostaglandin E2 | Yes | Yes | Yes | Yes |
| Inhibitors | ||||
| Alpha and beta defensins 1,2,3 | Yes | Yes | No | No |
| Alpha-1 antitrypsin | - | - | - | - |
| CD8-positive cell product anti-thrombin III | - | - | - | - |
| Interferon alpha and beta | - | - | - | - |
| IL-10 | Yes | Yes | - | - |
| IL-13 | - | - | - | - |
| IL-16 | - | - | - | - |
| IL-27 | - | - | - | - |
| TGF-beta | - | Yes | Yes | Yes |
| RANTES (in the presence of TNF-alpha) | Yes | Yes | Yes | Yes |
| Macrophage inflammatory protein-1 α (MIP-1 alpha) | Yes | Yes | Yes | Yes |
| Macrophage inflammatory protein beta (MIP-1 beta) | Yes | Yes | - | - |
| Monocyte chemotactic protein-2 (MCP-2) | - | - | - | - |
| Stromal-derived factor 1 (SDF-1) | Yes | Yes | Yes | Yes |
| Leukaemia inhibitory factor (LIF) (Placenta component) | - | - | - | - |
| Macrophage-derived chemokine (MDC) | - | - | - | - |
| Rnase | - | - | - | - |
| Secretory leucocyte protease inhibitor (SLPI) | - | - | - | - |
| Prostaglandin A | - | - | - | - |
| Pivotal role | ||||
| IFN-gamma | Yes | Yes | Yes | Yes |
| IL-4 | - | - | - | - |
| GM-CSF | Yes | Yes | Yes | Yes |
| IL-8 | Yes | Yes | Yes | Yes |
HIV, human immunodeficiency virus; TNF, tumour necrosis factor; IL, interleukin; M-CSF, macrophage-colony stimulating factor; TGF, transforming growth factor; RANTES, regulated on activation, normal T-cell expressed and secreted; IFN, interferon; GM-CSF, granulocyte/macrophage-colony stimulating factor.
-, Neither mRNA nor protein has been detected or evaluated.
As was mentioned earlier in this review, some chemokine receptors are important co-receptors for fusion between HIV envelope and target cell membrane during the first events of viral infection. These receptors belong to the superfamily of seven transmembrane G-protein coupled receptors, and have become a tempting target for the development of new anti retroviral drugs (viral fusion inhibitors). For instance, the C-C chemokines, macrophage inflammatory protein (MIP)-1alpha, MIP-1beta and regulated on activation, normal T-cell expressed and secreted are important inhibitors of macrophage-tropic strains of HIV, whereas the alpha chemokine stromal-derived factor-1 down-regulates infection of T-tropic strains of HIV (Demonte et al, 2004; Kedzierska and Crowe, 2001). In contrast, Monocyte chemotactic protein-1 has been shown to be capable of stimulating HIV replication (Borghi et al, 2000). On the other hand, the role of IL-8 (CXC chemokine) in HIV reactivation is still controversial; however, little evidence shows that it could have a pivotal role depending upon the state of infection. Thus, HIV acutely infected cells exposed to IL-8 seem to increase viral replication (Lane et al, 2001), and in contrast chronically infected cells treated with IL-8 fail to exhibit HIV exacerbation (Tiemessen et al, 2000). Apparently, cell signalling triggered by IL-8 receptor CXCR1 but not CXCR2 could be inducing internalization of CCR5, which would reduce the extent of HIV infection (Richardson et al, 2003).
Among a growing group of soluble factors with the ability to modulate HIV replication, inflammatory mediators associated to the innate immune response, such as antimicrobial peptides, α-defensins and β-defensins 1, 2 and 3 produced by epithelial cells, RNases, prostaglandin A (PGA) and the secretory leucocyte protease inhibitor (SLPI), also can inhibit HIV reactivation (Quinones-Mateu et al, 2003; Dumais et al, 1998; Ankel et al, 1991; McNeely et al, 1997). In contrast, the inflammatory mediator prostaglandin E2 (PGE2) seems to be able to increase viral replication in T-cells (Dumais et al, 1998). Evidence suggests that some cytokines such as IL-1 and TNFα are able to activate the transcription factor NFκB, which in turn can further induce viral gene expression through interaction with the long terminal repeat (LTR) of HIV provirus (Folks et al, 1989); however, not all cytokines involve the same pathway, and thus IFNγ, IL-6 and TNFα also up-regulate IFN regulatory factor-1 (IRF-1) which leads to cell activation and drives HIV-1 replication (Fauci, 1996; Fujita et al, 1989). Interestingly, IRF-1 can bind the HIV transactivator protein Tat and NFκB, which allows further induction of HIV promoter activation and virus replication in an amplified transcriptional activity. In addition to HIV gene reactivation, other IRF members such as IRF-8 can negatively regulate this process, a potential molecular mechanism that could be associated with latency stages (Sgarbanti et al, 2008, 2002). Type-1 IFNs have also been proposed as part of the negative transcriptional regulatory mechanisms that alveolar macrophages use during resting stages without an identified infection (Khan et al, 1989). Thus, IFNs appear to alter the expression of C/EBPβ, producing a dominant negative transcription factor and repressing the HIV promoter. In fact, resting alveolar macrophages obtained from non-inflamed lung behave like IFNβ-treated macrophages, expressing the inhibitory 16-kDa C/EBPβ and inhibiting HIV replication, likely contributing to viral latency in a non-inflamed lung (Honda et al, 1998). In addition to the IFN-response as a negative regulator of HIVLTR transcription, the antiinflammatory cytokine IL-10 also induces the C/EBPβ repressor in macrophages but not in monocytes through a pathway mediated by STAT3 (Tanaka et al, 2005). It has been hypothesized that the inclusion of C/EBP sites in the HIVLTR may be another example of HIV usurping normal immune regulation for its own advantage to develop viral latency. Thus, pathways that inhibit cytokine production may also promote viral latency, protecting provirally infected macrophages from immune surveillance (Weiden et al, 2000).
More recently, it was shown that interactions between activated CD4+ and CD8+ lymphocytes and macrophages are critical for HIVLTR transcription and viral replication, which resemble the clinical features observed during tuberculosis in HIV+ patients (Figure 2). Such cell interactions lead to the loss of inhibitory C/EBPβ and an activation of NF-κB, with a consequent increase in HIV-1 replication in a process that seems to be developed in two steps: (i) The contact leads to a loss of inhibitory C/EBPβ that de-represses the HIVLTR; and (ii) soluble lymphocyte factors (e.g. IL-1β, IL-6 and TNFβ) elicit NF-κB activation that enhances HIVLTR transcription (Hoshino et al, 2002). The same type of responses following cell–cell contact has been reported for neutrophils. Thus, the interaction of macrophages and polymorphonuclear neutrophils (PMNs) abolished the inhibitory C/EBPβ which led to de-repression of the HIVLTR in macrophages. The contact-mediated activation was dependent on crosslinking of 3 co-stimulatory receptors (CD40, CD80/86 and ICAM-1) by their respective ligands (CD40L, CD28 and LFA-1), all of which are expressed in lipid raft fractions of PMNs (Hoshino et al, 2007). The presence of repressors and de-repressors seems to influence a large group of inflammatory genes containing promoters with C/EBP sites. Therefore, as proposed by Tanaka et al, the interplay between repressors and de-repressors is pivotal in determining if a given stimulus leads to up or down-regulation of inflammation and HIV replication (Tanaka et al, 2005).
Like many pro-inflammatory cytokine genes, the long tandem repeats (LTR) promoter sequence of the HIV provirus that is integrated in the genome of latently infected host cells contains binding sites not only for NFκB but also for other transcription factors such as Sp1, CRE and CCAAT/ Enhancer Binding Protein (C/EBPβ) (Figure 1). As mentioned earlier, alveolar macrophages strongly express C/EBPβ that correlates with very low levels of HIV levels in un-inflamed lung (Nakata et al, 1995). In contrast, pro-inflammatory cytokines induced during tuberculosis directly increased the HIVLTR activity and HIV replication. One outcome was that TNFα increased HIV production in mononuclear phagocytes through transcriptional activation of the LTR promoter by NFκB (Bernstein et al, 1991; Goletti et al, 1996). It has been hypothesized that tuberculosis switches the pulmonary microenvironment from one that suppresses HIV replication to one that stimulates it (Zhang et al, 1995). Interestingly, when monocytes are differentiated into macrophages in vitro, several stimuli, such as LPS, M. tuberculosis or other pro-inflammatory stimuli, suppress HIV replication, an effect mediated by type-1 IFNs (Weiden et al, 2000).
Different cellular transcription factors with the ability to bind HIV promoter are normally activated through cytokine and chemokine receptors as well as TLRs. This suggests that HIV replication induced by cytokines/chemokines could be a net result of complex interactions between several transcription factors during inflammation (Figures 1 and 2). To understand the molecular mechanisms of HIV reactivation or inhibition associated to TLR agonists is currently a focus of intense research, as potential modulation of particular signalling pathways associated to these factors could be helpful for the development of new anti-HIV therapeutic strategies based on targeting HIV transcription.
Interestingly enough, most of the described cellular and molecular factors of the innate immune system associated with HIV reactivation in monocytes/macrophages have also been broadly described as critical players in oral infectious diseases, such as periodontal disease (e.g. macrophages, neutrophils, CD4+ and CD8+ T cells, etc.). Therefore, particular microbial and immune events associated with the oral environment, such as polymicrobial infections and tolerance, may be relevant for understanding a potential HIV reactivation during oral co-infections.
Hypothesis: oral co-infections as a risk factor for HIV recrudescence
Historically, a high prevalence of oral infectious diseases has been strongly associated with HIV infection, including pseudo-membranous candidiasis (OC), oral hairy leukoplakia (OHL), HIV gingivitis and periodontitis and Kaposi’s sarcoma (Coogan et al, 2005). The advent of HAART appears to reduce the frequency of HIV-associated oral pathology (i.e. OHL and OC), particularly in industrialized countries; however, the available evidence only supports a significant reduction for OC in patients under antiretroviral therapy (Patton et al, 2000; Greenspan et al, 2004). In addition, new oral clinical manifestations associated with HAART therapy have been described, such as an increased prevalence of oral warts associated with human papiloma virus (HPV), which appears to represent a form of immune reconstitution syndrome (King et al, 2002). Whether oral infectious diseases in HIV+ patients have an impact in HIV exacerbation remains unclear. However, results obtained from cross-sectional studies have shown a positive correlation between OC or OHL and HIV-1 viral load (Patton, 2000; Chattopadhyay et al, 2005; Greenspan et al, 2000; Black et al, 2000). Interestingly, Alpagot et al in a prospective study have recently shown a positive correlation between HIV viral load and periodontal disease as well as increased presence of PGE2, metalloproteinase-9 and tissue inhibitor of metalloproteinase- 1 in gingival crevicular fluid (Alpagot et al, 2007, 2006). In addition, a significant increase in the levels of Fusobacterium nucleatum, Prevotella intermedia, as well as IFNγ and TGFβ in gingival crevicular fluid has also been shown, which may be risk factors for rapid progression of periodontitis in HIV+ patients (Alpagot et al, 2003, 2004, 2007, 2008). A biological explanation for this association remains undetermined.
On the other hand, it has been shown that normal oral mucosa tissues display infiltrating immune cells, such as Langerhans dendritic cells, macrophages and CD4+ T cells, as part of the immune response elicited against oral bacteria (Juhl et al, 1988; Jotwani and Cutler, 2003; Schroeder and Listgarten, 1997). These cells are an important target for HIV infection and replication, as well as for providing a long-lived host cell population to act as a reservoir for latent HIV (Chou et al, 2000). The oral cavity seems to be a site of HIV pathogenesis and potential reservoir for the disease in HIV+ patients, as both HIV RNA and DNA proviral forms are present in saliva as well as in gingival crevicular fluid (Maticic et al, 2000; Shugars et al, 2001). Nevertheless, substances that inactivate HIV (e.g. SLPI), lower viral loads and specific anti-HIV antibodies that mediate antibody-dependent cell-mediated cytotoxicity appear to reduce the infectivity of virions in saliva (McNeely et al, 1995; Shugars et al, 1999; Kim et al, 2006).
Interestingly enough, the possibility for HIV to infect oral epithelial cells has been demonstrated in vitro. Thus, oral epithelial cells are susceptible to either cell free or cell-associated HIV-1 infection through the glycosphingolipid galactosylceramide (GalCer) receptor and CXCR4 co-receptor (Liu et al, 2003; Moore et al, 2003). In addition, CCR5-tropic virus infecting oral epithelial cells can be transferred to circulating CD4+ T lymphocytes and macrophages (Liu et al, 2003). These results are consistent with a positive expression of all HIV receptors and co-receptors found in healthy gingiva, i.e. Langerin, DC-SIGN, macrophage mannose receptor (MMR), GalCer, CCR5 and CXCR4, which suggests that these tissues are susceptible to infection by HIV. Among these receptors, DC-SIGN, MMR, CXCR4 and CCR5 show increased expression in periodontitis, where CCR5+ cells were mostly T cells, macrophages and dermal dendritic cells (Jotwani et al, 2004). Most recently, the ability of HIV to infect and replicate in oral mucosa epithelial cells in vivo has also been demonstrated (Rodriguez-Inigo et al, 2005). Thus, immune and oral epithelial cells latently infected with HIV in gingival tissues may be an important source for HIV reactivation during chronic inflammatory events triggered by oral pathogens. There remains an incomplete understanding concerning the origin of latently infected immune cells in the oral mucosa. These cells could potentially involve a direct HIV infection of oral mucosal immune cells by viruses crossing the oral epithelial barrier, or may also be recruited from the systemic circulation of HIV-infected patients. The individual or combined contribution to the local infected cells in the gingival tissue has not been demonstrated; thus, additional investigations are required as these regional latently infected cells probably may have a significant impact on the potential for HIV replication, transmission, as well as AIDS progression.
The oral cavity is colonized by an enormous variety and number of microorganisms that interact with each other in complex biofilms, including commensal, opportunistic and pathogenic species. Some of these microorganisms, particularly Gram-negative bacteria have been proposed as periodontopathogens, due to their greater capacity to elicit an inflammatory response with a consequent destruction of supportive tissues of the teeth (Feng and Weinberg, 2006). Most of the bacteriological studies in HIV-1+ patients (Table 2) have demonstrated that the prevalence of periodontal pathogens appears to be similar between HIV-infected and HIV-uninfected subjects (Goncalves Lde et al, 2004; Gornitsky et al, 1991; Patel et al, 2003; Tsang and Samaranayake, 2001; Rams et al, 1991; Piluso et al, 1993). However, recent findings suggest that other opportunistic bacterial species, rather than the classical periodontal pathogens, may also be involved in HIV-1-associated periodontal disease (Paster et al, 2002; Aas et al, 2007; Goncalves Lde et al, 2007; Cobb et al, 2003; Botero et al, 2007). During the last decade, the potential association between periodontal disease and systemic disorders has generated substantial interest, and available data supports that the infectious/chronic responses in periodontal disease may have a role as risk modifiers of systemic conditions, including diabetes (Southerland et al, 2006), low-weight birth and preterm delivery (Bobetsis et al, 2006), atherosclerosis/heart failure (Hujoel et al, 2000), osteoporosis (Jeffcoat, 2005) and chronic obstructive pulmonary disease (Azarpazhooh and Leake, 2006). Periodontitis also has been studied in HIV-infected populations including those with AIDS, as an altered immune response would be predicted to modify the course of this oral inflammatory disease. In fact, recent and growing evidence shows significant immunological changes at oral level in HIV-1+ patients compared with healthy controls, which appear to be risk factors for more rapid progression of aggressive forms of periodontal disease in HIV-1-infected population (Alpagot et al, 2008, 2007). Importantly, severe forms of oral disease, including necrotizing ulcerative periodontitis, do appear to relate to the immunodeficiency of HIV infection, and correlate with a downhill progression of the viral infection and mortal opportunistic infections (Glick et al, 1994).
Table 2.
Microorganisms detected in supra- and sub-gingival plaque of HIV+ patients
| Microorganism | Reference |
|---|---|
| Porphyromonas gingivalis, P. intermedia, Fusobacterium nucleatum, Actinobacillus actinomycetemcomitans | Murray et al, 1989 |
| A. actinomycetemcomitans, Campylobacter rectus, Micromonas micros, P.intermedia, spirochetes | Rams et al, 1991 |
| P. intermedia, P. buccale, P. oralis, spirochetes | Piluso et al, 1993 |
| CMV, EBV type 1, HSV, HHV-6 | Contreras et al, 1997, 2001 |
| P. gingivalis, A. actinomycetemcomitans, Candida albicans, mycoplasma spp. | Chattin et al, 1999 |
| HHV-6, -7, -8 | Mardirossian et al, 2000 |
| C. dubliniensis, C. glabrata, C. parpsilosis | Jabra-Rizk et al, 2001 |
| Capnocytophaga spp., P. loescheii, Streptococcus sanguis, Lactobacillus spp., Fusobacterium spp. | Tsang and Samaranayake, 2001 |
| Bulleidia extructa, Dialister spp., Fusobaterium spp., Selenomonas spp., Peptostreptococcus spp., Veillonella spp. | Paster et al, 2002 |
| Spirochetes, yeasts | Cobb et al, 2003 |
| Combinations of P. nigrescens/C. rectus, P. nigrescens/P. gingivalis, P. nigrescens/Treponema denticola | Patel et al, 2003 |
| F. nucleatum, P. intermedia, A. actinomycetemcomitans | Alpagot et al, 2004 |
| Gemella spp., Dialister spp., Streptococcus spp., Veillonella spp., S. cerevisae, C. albicans | Aas et al, 2007 |
| Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae | Botero et al, 2007 |
HIV, human immunodeficiency virus; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HSV, herpes simplex virus; HHV, human herpes virus.
The immunosuppressive condition resulting from HIV infection has been associated with the appearance of varied opportunistic infections, e.g. tuberculosis, pneumonia and candidiasis. The oral cavity of HIV+ patients can also provide a great environment for seeding such infections by, for example, various fungi, including several strains of Candida, such as C. dubliniensis, C. glabrata and C. parapsilosis (Murray et al, 1989; Chattin et al, 1999; Jabra-Rizk et al, 2001), as well as Cryptococcus neoformans, Histoplasma capsulatum (McKinsey, 1998; Stansell, 1993), and viruses (HSV, herpes zoster virus and HPV). In addition to bacteria and fungi, higher detection frequency of viruses has been reported in the oral cavity of HIV+ patients, including human cytomegalovirus, human herpes viruses 6, 7, and Epstein–Barr virus, when compared with that in healthy controls (Contreras et al, 1997; Mardirossian et al, 2000; Contreras et al, 2001).
Several studies exploring the immunological characteristics of HIV-1+ patients have shown that the potential T-helper polarization in leucocytes from gingival tissues showed a trend towards a Th2 response, characterized by IL-4 synthesis (Gomez et al, 1997). It was hypothesized that this Th2 response may be correlated with a reduction in PMNs recruitment to crevicular fluid and enhances the potential colonization of Candida spp. in the subgingival sulcus, which would act in concert with certain periodontopathogens to increase inflammation and destruction in HIV+ patients (Lamster et al, 1998). Nevertheless, additional studies have reported a significant increase of Th1 inflammatory cytokines in crevicular fluid and saliva, such as IL-1β, IL-6, TNFα and IFNγ (Black et al, 2000; Spear et al, 2005). Among these cytokines, only IL-1β was correlated with high viral loads (Baqui et al, 2000b). The prevalence of pro-inflammatory cytokines in crevicular fluid is in agreement with the significant increased synthesis of the same cytokines by monocytes from HIV+ patients compared with that in healthy individuals following challenge of these cells with periodontopathogens and LPS (Baqui et al, 2000a). In addition, an altered apoptosis of mononuclear cells in gingival tissue from HIV+ patients correlates well with the Th1 gingival response in periodontal disease of these patients, because their prolonged presence at the periodontitis sites would guarantee a constant production of inflammatory Th1 cytokines that promotes more aggressive tissue destructions (Vieira et al, 2003).
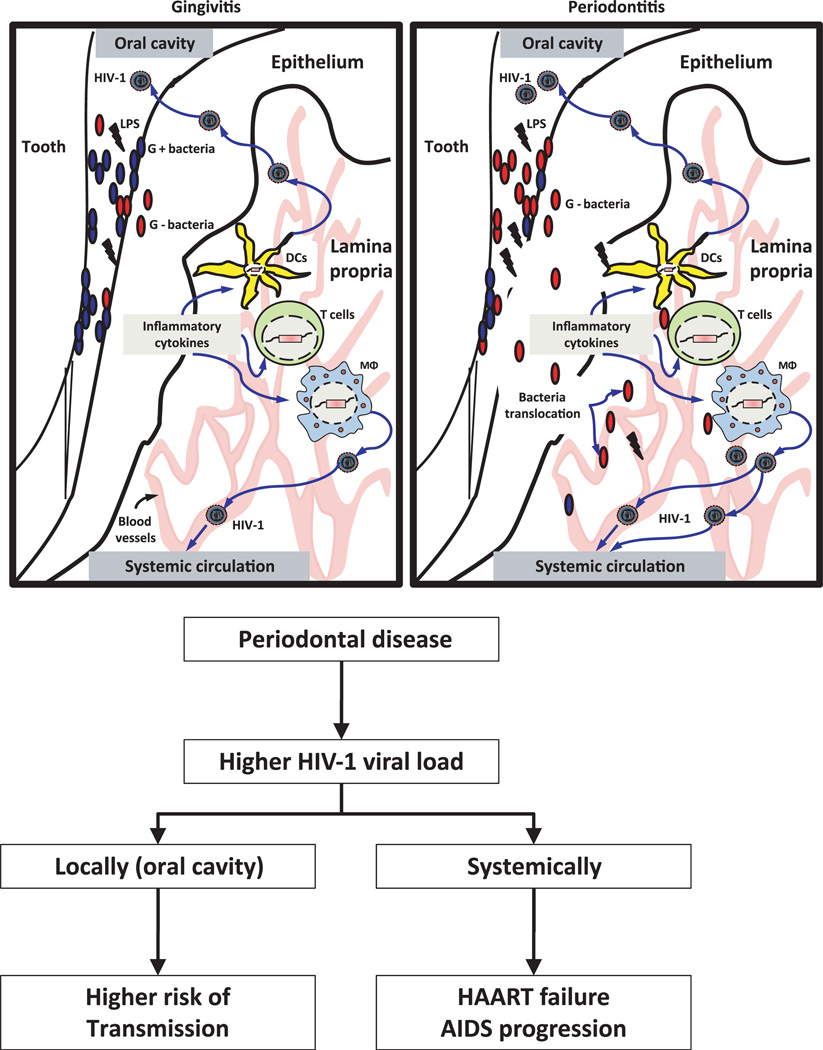
An important consideration in this review is that the biological underpinnings of the pathogenesis of periodontitis have been described with other infectious diseases that can reactivate HIV in latently infected cell reservoirs. This suggests that the inflammatory and infectious components of periodontal disease in HIV-1+ patients might have the capacity to induce viral reactivation. Based on these observations, it is tempting to hypothesize that latently infected cells either locally at gingival tissues or systemically, including CD4+ T lymphocytes, macrophages and dendritic cells, could be targeted either directly by oral common and/or opportunistic pathogens as well as their activating components (e.g. LPS), or indirectly by soluble mediators and cytokines/chemokines produced by immune and non-immune cells (fibroblasts and oral epithelial cells). The net result of this local chronic stimulation would contribute to HIV reactivation and potentially exacerbate local and/or systemic increases of viral load with undesirable clinical consequences for HIV+ patients (Figure 3). Attempting to develop biological evidence for a potential impact of oral infectious diseases and HIV reactivation, we have been able to demonstrate that oral bacteria associated with periodontal disease have the ability to trigger HIV-1 promoter reactivation in monocytes/macrophages, dendritic cells and T cells, using in vitro models (Huang CB, Emerson KA, Gonzalez OA and Ebersole JL unpublished data). Further in vitro and in vivo studies will be needed to provide a sufficient weight of evidence to better understand the magnitude of this contribution.
Figure 3.
Schematic of the concepts underlying the potential role of periodontal disease in human immunodeficiency virus (HIV) reactivation. Both gingivitis and periodontitis, like other mucosal infections, represent a chronic infection and inflammation that can provide host cell stimulation. HIV-infected patients demonstrating low viral loads following highly active antiretroviral (HAART) therapy contain long-life cells [e.g. macrophages, T cells and dendritic cells (DCs)] with provirus integrated into their genome. Interaction of these cells directly with oral bacteria and/or indirectly with soluble factors (e.g. cytokines/chemokines) from gingival resident cells would activate HIV production, which results in viral recrudescence. An increased reactivation of virus may have an impact both at local level in the oral cavity or systemically, which may be a risk modifier for HIV transmission as well as HAART failure and acquired immunodeficiency syndrome progression respectively
As the advent of HAART therapy in HIV infection has transitioned into a ‘chronic disease’ management strategy, there is growing evidence that co-infection and inflammation in HIV+ patients may have important public health implications in undermining HAART success. Therefore, the study of a potential role of periodontitis as a global co-infection contributing to HIV recrudescence as well as other oral infectious diseases is necessary. Thus, the adequate and efficient control of oral infections, including HIV-associated periodontal disease in HIV-1+ patients, would be expected to contribute to minimizing viral reactivation, which would marginalize the effect of HAART therapy and enabling opportunistic infections on this population.
Acknowledgements
This work was supported by U.S.P.H.S. grant from the National Institute for Research Resources P20 RR020145 and funds from the University of Kentucky, College of Dentistry.
Footnotes
Author contributions
O. Gonzalez analyzed the data, wrote the manuscript draft and developed the schematics. J. Ebersole advised the authors on data analysis, interpretation and manuscript preparation. C. Huang directed the study, finalized the manuscript and served as the corresponding author.
References
- Aas JA, Barbuto SM, Alpagot T, Olsen I, Dewhirst FE, Paster BJ. Subgingival plaque microbiota in HIV positive patients. J Clin Periodontol. 2007;34:189–195. doi: 10.1111/j.1600-051X.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- Alpagot T, Font K, Lee A. Longitudinal evaluation of GCF IFN-gamma levels and periodontal status in HIV + patients. J Clin Periodontol. 2003;30:944–948. doi: 10.1034/j.1600-051x.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- Alpagot T, Duzgunes N, Wolff LF, Lee A. Risk factors for periodontitis in HIV patients. J Periodontal Res. 2004;39:149–157. doi: 10.1111/j.1600-0765.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- Alpagot T, Suzara V, Bhattacharyya M. The associations between gingival crevice fluid matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and periodontitis in human immunodeficiency virus-positive patients. J Periodontal Res. 2006;41:491–497. doi: 10.1111/j.1600-0765.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- Alpagot T, Remien J, Bhattacharyya M, Konopka K, Lundergan W, Duzgunes N. Longitudinal evaluation of prostaglandin E2 (PGE2) and periodontal status in HIV + patients. Arch Oral Biol. 2007;52:1102–1108. doi: 10.1016/j.archoralbio.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpagot T, Konopka K, Bhattacharyya M, Gebremedhin S, Duzgunes N. The association between gingival crevicular fluid tgf-beta1 levels and periodontal status in HIV-1(+) patients. J Periodontol. 2008;79:123–130. doi: 10.1902/jop.2008.070312. [DOI] [PubMed] [Google Scholar]
- Amici C, Belardo G, Rozera C, Bernasconi D, Santoro MG. Inhibition of herpesvirus-induced HIV-1 replication by cyclopentenone prostaglandins: role of IkappaB kinase (IKK) AIDS. 2004;18:1271–1280. doi: 10.1097/00002030-200406180-00005. [DOI] [PubMed] [Google Scholar]
- Ankel H, Turriziani O, Antonelli G. Prostaglandin A inhibits replication of human immunodeficiency virus during acute infection. J Gen Virol. 1991;72(Pt 11):2797–2800. doi: 10.1099/0022-1317-72-11-2797. [DOI] [PubMed] [Google Scholar]
- Ayash-Rashkovsky M, Chenine AL, Steele LN, et al. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian-human immunodeficiency virus clade C infection. Infect Immun. 2007;75:1751–1756. doi: 10.1128/IAI.01703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465–1482. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Equils O, Sher A. The induction of toll-like receptor tolerance enhances rather than suppresses HIV-1 gene expression in transgenic mice. J Leukoc Biol. 2004;75:460–466. doi: 10.1189/jlb.0803388. [DOI] [PubMed] [Google Scholar]
- Baqui AA, Jabra-Rizk MA, Kelley JI, Zhang M, Falkler WA, Jr, Meiller TF. Enhanced interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha production by LPS stimulated human monocytes isolated from HIV+ patients. Immunopharmacol Immunotoxicol. 2000a;22:401–421. doi: 10.3109/08923970009026002. [DOI] [PubMed] [Google Scholar]
- Baqui AA, Meiller TF, Jabra-Rizk MA, Zhang M, Kelley JI, Falkler WA., Jr Enhanced interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in gingival crevicular fluid from periodontal pockets of patients infected with human immunodeficiency virus 1. Oral Microbiol Immunol. 2000b;15:67–73. doi: 10.1034/j.1399-302x.2000.150201.x. [DOI] [PubMed] [Google Scholar]
- Bernstein MS, Tong-Starksen SE, Locksley RM. Activation of human monocyte – derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Invest. 1991;88:540–545. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P, Poli G, Kinter AL, et al. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med. 1992;176:739–750. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KP, Merrill KW, Jackson S, Katz J. Cytokine profiles in parotid saliva from HIV-1-infected individuals: changes associated with opportunistic infections in the oral cavity. Oral Microbiol Immunol. 2000;15:74–81. doi: 10.1034/j.1399-302x.2000.150202.x. [DOI] [PubMed] [Google Scholar]
- Blanchard A, Montagnier L, Gougeon ML. Influence of microbial infections on the progression of HIV disease. Trends Microbiol. 1997;5:326–331. doi: 10.1016/S0966-842X(97)01089-5. [DOI] [PubMed] [Google Scholar]
- Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- Bobetsis YA, Barros SP, Offenbacher S. Exploring the relationship between periodontal disease and pregnancy complications. J Am Dent Assoc. 2006;137(Suppl):7S–13S. doi: 10.14219/jada.archive.2006.0403. [DOI] [PubMed] [Google Scholar]
- Borghi MO, Panzeri P, Shattock R, Sozzani S, Dobrina A, Meroni PL. Interaction between chronically HIV-infected promonocytic cells and human umbilical vein endothelial cells: role of proinflammatory cytokines and chemokines in viral expression modulation. Clin Exp Immunol. 2000;120:93–100. doi: 10.1046/j.1365-2249.2000.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero JE, Arce RM, Escudero M, Betancourth M, Jaramillo A, Contreras A. Frequency of detection of periodontopathic and superinfecting bacteria in HIV-positive patients with periodontitis. J Int Acad Periodontol. 2007;9:13–18. [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Broder S, Gallo RC. A pathogenic retrovirus (HTLV-III) linked to AIDS. N Engl J Med. 1984;311:1292–1297. doi: 10.1056/NEJM198411153112006. [DOI] [PubMed] [Google Scholar]
- Bush CE, Donovan RM, Markowitz NP, Kvale P, Saravolatz LD. A study of HIV RNA viral load in AIDS patients with bacterial pneumonia. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:23–26. doi: 10.1097/00042560-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Caselli E, Galvan M, Cassai E, Caruso A, Sighinolfi L, Di Luca D. Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood. 2005;106:2790–2797. doi: 10.1182/blood-2005-04-1390. [DOI] [PubMed] [Google Scholar]
- Chattin BR, Ishihara K, Okuda K, Hirai Y, Ishikawa T. Specific microbial colonizations in the periodontal sites of HIV-infected subjects. Microbiol Immunol. 1999;43:847–852. doi: 10.1111/j.1348-0421.1999.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Caplan DJ, Slade GD, Shugars DC, Tien HC, Patton LL. Risk indicators for oral candidiasis and oral hairy leukoplakia in HIV-infected adults. Community Dent Oral Epidemiol. 2005;33:35–44. doi: 10.1111/j.1600-0528.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- Chermann JC, Barre-Sinoussi F, Dauguet C, et al. Isolation of a new retrovirus in a patient at risk for acquired immunodeficiency syndrome. Antibiot Chemother. 1983;32:48–53. doi: 10.1159/000409704. [DOI] [PubMed] [Google Scholar]
- Chou LL, Epstein J, Cassol SA, West DM, He W, Firth JD. Oral mucosal Langerhans’ cells as target, effector and vector in HIV infection. J Oral Pathol Med. 2000;29:394–402. doi: 10.1034/j.1600-0714.2000.290805.x. [DOI] [PubMed] [Google Scholar]
- Cobb CM, Ferguson BL, Keselyak NT, Holt LA, MacNeill SR, Rapley JW. A TEM/SEM study of the microbial plaque overlying the necrotic gingival papillae of HIV-seropositive, necrotizing ulcerative periodontitis. J Periodontal Res. 2003;38:147–155. doi: 10.1034/j.1600-0765.2003.02011.x. [DOI] [PubMed] [Google Scholar]
- Collman RG, Perno CF, Crowe SM, Stevenson M, Montaner LJ. HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: new answers yield new questions. J Leukoc Biol. 2003;74:631–634. doi: 10.1189/jlb.0703357. [DOI] [PubMed] [Google Scholar]
- Condos R, Rom WN, Weiden M. Lung-specific immune response in tuberculosis. Int J Tuberc Lung Dis. 2000;4:S11–S17. [PubMed] [Google Scholar]
- Contreras A, Falkler WA, Jr, Enwonwu CO, et al. Human Herpesviridae in acute necrotizing ulcerative gingivitis in children in Nigeria. Oral Microbiol Immunol. 1997;12:259–265. doi: 10.1111/j.1399-302x.1997.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Contreras A, Mardirossian A, Slots J. Herpesviruses in HIV-periodontitis. J Clin Periodontol. 2001;28:96–102. doi: 10.1034/j.1600-051x.2001.280115.x. [DOI] [PubMed] [Google Scholar]
- Coogan MM, Greenspan J, Challacombe SJ. Oral lesions in infection with human immunodeficiency virus. Bull World Health Organ. 2005;83:700–706. [PMC free article] [PubMed] [Google Scholar]
- Demonte D, Quivy V, Colette Y, Van Lint C. Administration of HDAC inhibitors to reactivate HIV-1 expression in latent cellular reservoirs: implications for the development of therapeutic strategies. Biochem Pharmacol. 2004;68:1231–1238. doi: 10.1016/j.bcp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Devadas K, Hardegen NJ, Wahl LM, et al. Mechanisms for macrophage-mediated HIV-1 induction. J Immunol. 2004;173:6735–6744. doi: 10.4049/jimmunol.173.11.6735. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- Dumais N, Barbeau B, Olivier M, Tremblay MJ. Prostaglandin E2 Up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. J Biol Chem. 1998;273:27306–27314. doi: 10.1074/jbc.273.42.27306. [DOI] [PubMed] [Google Scholar]
- Equils O, Faure E, Thomas L, Bulut Y, Trushin S, Arditi M. Bacterial lipopolysaccharide activates HIV long terminal repeat through Toll-like receptor 4. J Immunol. 2001;166:2342–2347. doi: 10.4049/jimmunol.166.4.2342. [DOI] [PubMed] [Google Scholar]
- Equils O, Schito ML, Karahashi H, et al. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J Immunol. 2003;170:5159–5164. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- Equils O, Madak Z, Liu C, Michelsen KS, Bulut Y, Lu D. Rac1 and Toll-IL-1 receptor domain-containing adapter protein mediate Toll-like receptor 4 induction of HIV-long terminal repeat. J Immunol. 2004;172:7642–7646. doi: 10.4049/jimmunol.172.12.7642. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol. 2006;2000:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Folks TM, Clouse KA, Justement J, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed EO. HIV-1 and the host cell: an intimate association. Trends Microbiol. 2004;12:170–177. doi: 10.1016/j.tim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Freed EO, Mouland AJ. The cell biology of HIV-1 and other retroviruses. Retrovirology. 2006;3:77. doi: 10.1186/1742-4690-3-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Reis LF, Watanabe N, Kimura Y, Taniguchi T, Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci USA. 1989;86:9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS ONE. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Gaynor RB. The human immunodeficiency virus type-1 long terminal repeat and its role in gene expression. Prog Nucleic Acid Res Mol Biol. 1994;49:157–196. doi: 10.1016/s0079-6603(08)60050-1. [DOI] [PubMed] [Google Scholar]
- Garcia M, Yu XF, Griffin DE, Moss WJ. In vitro suppression of human immunodeficiency virus type 1 replication by measles virus. J Virol. 2005;79:9197–9205. doi: 10.1128/JVI.79.14.9197-9205.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman AE, LaRosa DF, Zhang J, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick M, Muzyka BC, Salkin LM, Lurie D. Necrotizing ulcerative periodontitis: a marker for immune deterioration and a predictor for the diagnosis of AIDS. J Periodontol. 1994;65:393–397. doi: 10.1902/jop.1994.65.5.393. [DOI] [PubMed] [Google Scholar]
- Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication role of immune activation. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- Gomez RS, de Souza PE, da Costa JE, Araujo NS. CD30+ lymphocytes in chronic gingivitis from HIV-positive patients: a pilot study. J Periodontol. 1997;68:881–883. doi: 10.1902/jop.1997.68.9.881. [DOI] [PubMed] [Google Scholar]
- Goncalves Lde S, Ferreira SM, Silva A, Jr, et al. Association of T CD4 lymphocyte levels and subgingival microbiota of chronic periodontitis in HIV-infected Brazilians under HAART. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:196–203. doi: 10.1016/j.tripleo.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Goncalves Lde S, Soares Ferreira SM, Souza CO, Souto R, Colombo AP. Clinical and microbiological profiles of human immunodeficiency virus (HIV)-seropositive Brazilians undergoing highly active antiretroviral therapy and HIV-seronegative Brazilians with chronic periodontitis. J Periodontol. 2007;78:87–96. doi: 10.1902/jop.2007.060040. [DOI] [PubMed] [Google Scholar]
- Gornitsky M, Clark DC, Siboo R, et al. Clinical documentation and occurrence of putative periodontopathic bacteria in human immunodeficiency virus-associated periodontal disease. J Periodontol. 1991;62:576–585. doi: 10.1902/jop.1991.62.9.576. [DOI] [PubMed] [Google Scholar]
- Greenspan D, Komaroff E, Redford M, et al. Oral mucosal lesions and HIV viral load in the Women’s Interagency HIV Study (WIHS) J Acquir Immune Defic Syndr. 2000;25:44–50. doi: 10.1097/00042560-200009010-00006. [DOI] [PubMed] [Google Scholar]
- Greenspan D, Gange SJ, Phelan JA, et al. Incidence of oral lesions in HIV-1-infected women: reduction with HAART. J Dent Res. 2004;83:145–150. doi: 10.1177/154405910408300212. [DOI] [PubMed] [Google Scholar]
- Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun. 2005;73:4155–4160. doi: 10.1128/IAI.73.7.4155-4160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Ylisastigui L, Margolis DM. The regulation of HIV-1 gene expression: the emerging role of chromatin. DNA Cell Biol. 2002;21:697–705. doi: 10.1089/104454902760599672. [DOI] [PubMed] [Google Scholar]
- Henderson AJ, Zou X, Calame KL. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol. 1995;69:5337–5344. doi: 10.1128/jvi.69.9.5337-5344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Honda Y, Rogers L, Nakata K, et al. Type I interferon induces inhibitory 16-kD CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med. 1998;188:1255–1265. doi: 10.1084/jem.188.7.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Nakata K, Hoshino S, et al. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med. 2002;195:495–505. doi: 10.1084/jem.20011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Hoshino S, Gold JA, et al. Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J Infect Dis. 2007;195:1303–1310. doi: 10.1086/513438. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–1410. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- Imamichi T, Yang J, Huang DW, et al. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS. 2008;22:39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- Jabra-Rizk MA, Ferreira SM, Sabet M, Falkler WA, Merz WG, Meiller TF. Recovery of Candida dubliniensis and other yeasts from human immunodeficiency virusassociated periodontal lesions. J Clin Microbiol. 2001;39:4520–4522. doi: 10.1128/JCM.39.12.4520-4522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat M. The association between osteoporosis and oral bone loss. J Periodontol. 2005;76:2125–2132. doi: 10.1902/jop.2005.76.11-S.2125. [DOI] [PubMed] [Google Scholar]
- Jotwani R, Cutler CW. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J Dent Res. 2003;82:736–741. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- Jotwani R, Muthukuru M, Cutler CW. Increase in HIV receptors/co-receptors/alpha-defensins in inflamed human gingiva. J Dent Res. 2004;83:371–377. doi: 10.1177/154405910408300504. [DOI] [PubMed] [Google Scholar]
- Juhl M, Stoltze K, Reibel J. Distribution of Langerhans cells in clinically healthy human gingival epithelium with special emphasis on junctional epithelium. Scand J Dent Res. 1988;96:199–208. doi: 10.1111/j.1600-0722.1988.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Maerz A, Warby T, et al. Granulocyte-macrophage colony-stimulating factor inhibits HIV-1 replication in monocyte-derived macrophages. AIDS. 2000;14:1739–1748. doi: 10.1097/00002030-200008180-00008. [DOI] [PubMed] [Google Scholar]
- Khan NU, Pulford KA, Farquharson MA, et al. The distribution of immunoreactive interferon-alpha in normal human tissues. Immunology. 1989;66:201–206. [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Nag P, Landay AL, et al. Saliva can mediate HIV-1-specific antibody-dependent cell-mediated cytotoxicity. FEMS Immunol Med Microbiol. 2006;48:267–273. doi: 10.1111/j.1574-695X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- King MD, Reznik DA, O’Daniels CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34:641–648. doi: 10.1086/338637. [DOI] [PubMed] [Google Scholar]
- Koziel H, Kim S, Reardon C, et al. Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1999;160:2048–2055. doi: 10.1164/ajrccm.160.6.9902099. [DOI] [PubMed] [Google Scholar]
- Kuritzkes DR. HIV resistance: frequency, testing, mechanisms. Top HIV Med. 2007;15:150–154. [PubMed] [Google Scholar]
- Lamster IB, Grbic JT, Mitchell-Lewis DA, Begg MD, Mitchell A. New concepts regarding the pathogenesis of periodontal disease in HIV infection. Ann Periodontol. 1998;3:62–75. doi: 10.1902/annals.1998.3.1.62. [DOI] [PubMed] [Google Scholar]
- Lane BR, Lore K, Bock PJ, et al. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol. 2001;75:8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10:525–531. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- Liu X, Zha J, Chen H, et al. Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. J Virol. 2003;77:3470–3476. doi: 10.1128/JVI.77.6.3470-3476.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maek ANW, Buranapraditkun S, Klaewsongkram J, Ruxrungtham K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007;20:66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]
- Mardirossian A, Contreras A, Navazesh M, Nowzari H, Slots J. Herpesviruses 6, 7 and 8 in HIV- and non-HIV-associated periodontitis. J Periodontal Res. 2000;35:278–284. doi: 10.1034/j.1600-0765.2000.035005278.x. [DOI] [PubMed] [Google Scholar]
- Maticic M, Poljak M, Kramar B, et al. Proviral HIV-1 DNA in gingival crevicular fluid of HIV-1-infected patients in various stages of HIV disease. J Dent Res. 2000;79:1496–1501. doi: 10.1177/00220345000790071101. [DOI] [PubMed] [Google Scholar]
- McKinsey DS. Histoplasmosis in AIDS: advances in management. AIDS Patient Care STDS. 1998;12:775–781. doi: 10.1089/apc.1998.12.775. [DOI] [PubMed] [Google Scholar]
- McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- Montaner LJ, Bailer RT, Gordon S. IL-13 acts on macrophages to block the completion of reverse transcription, inhibit virus production, and reduce virus infectivity. J Leukoc Biol. 1997;62:126–132. doi: 10.1002/jlb.62.1.126. [DOI] [PubMed] [Google Scholar]
- Moore JS, Rahemtulla F, Kent LW, et al. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virol. 2003;313:343–353. doi: 10.1016/s0042-6822(03)00283-6. [DOI] [PubMed] [Google Scholar]
- Moriuchi H, Moriuchi M, Mizell SB, Ehler LA, Fauci AS. In vitro reactivation of human immunodeficiency virus 1 from latently infected, resting CD4+ T cells after bacterial stimulation. J Infect Dis. 2000;181:2041–2044. doi: 10.1086/315496. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H, Mon HM, Kanbara H. Dichotomous effects of Plasmodium falciparum antigens on expression of human immunodeficiency virus (HIV) coreceptors and on infectability of CD4 cells by HIV. J Infect Dis. 2002;186:1194–1197. doi: 10.1086/343814. [DOI] [PubMed] [Google Scholar]
- Murray PA, Grassi M, Winkler JR. The microbiology of HIV-associated periodontal lesions. J Clin Periodontol. 1989;16:636–642. doi: 10.1111/j.1600-051x.1989.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Naif H, Ho-Shon M, Chang J, Cunningham AL. Molecular mechanisms of IL-4 effect on HIV expression in promonocytic cell lines and primary human monocytes. J Leukoc Biol. 1994;56:335–339. doi: 10.1002/jlb.56.3.335. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- Nakata K, Weiden M, Harkin T, Ho D, Rom WN. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol Med. 1995;1:744–757. [PMC free article] [PubMed] [Google Scholar]
- Nordone SK, Ignacio GA, Su L, et al. Failure of TLR4-driven NF-kappa B activation to stimulate virus replication in models of HIV type 1 activation. AIDS Res Hum Retroviruses. 2007;23:1387–1395. doi: 10.1089/aid.2007.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WA, Grovit-Ferbas K, Namazi A, et al. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- Oguariri RM, Brann TW, Imamichi T. Hydroxyurea and interleukin-6 synergistically reactivate HIV-1 replication in a latently infected promonocytic cell line via SP1/SP3 transcription factors. J Biol Chem. 2007;282:3594–3604. doi: 10.1074/jbc.M608150200. [DOI] [PubMed] [Google Scholar]
- Osiecki K, Xie L, Zheng JH, Squires R, Pettoello-Mantovani M, Goldstein H. Identification of granulocyte-macrophage colony-stimulating factor and lipopolysaccharide-induced signal transduction pathways that synergize to stimulate HIV type 1 production by monocytes from HIV type 1 transgenic mice. AIDS Res Hum Retroviruses. 2005;21:125–139. doi: 10.1089/aid.2005.21.125. [DOI] [PubMed] [Google Scholar]
- Ott DE. Potential roles of cellular proteins in HIV-1. Rev Med Virol. 2002;12:359–374. doi: 10.1002/rmv.367. [DOI] [PubMed] [Google Scholar]
- Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Russell MK, Alpagot T, et al. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann Periodontol. 2002;7:8–16. doi: 10.1902/annals.2002.7.1.8. [DOI] [PubMed] [Google Scholar]
- Patel M, Coogan M, Galpin JS. Periodontal pathogens in subgingival plaque of HIV-positive subjects with chronic periodontitis. Oral Microbiol Immunol. 2003;18:199–201. doi: 10.1034/j.1399-302x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Patton LL. Sensitivity, specificity, and positive predictive value of oral opportunistic infections in adults with HIV/AIDS as markers of immune suppression and viral burden. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:182–188. doi: 10.1067/moe.2000.108799. [DOI] [PubMed] [Google Scholar]
- Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- Pereira LA, Bentley K, Peeters A, Churchill MJ, Deacon NJ. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res. 2000;28:663–668. doi: 10.1093/nar/28.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piluso S, Ficarra G, Orsi A, Gaglioti D, Pierotti P, Orlando S. Clinical aspects and microbiology of HIV-associated periodontal lesions. Minerva Stomatol. 1993;42:301–309. [PubMed] [Google Scholar]
- Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Kinter AL, Vicenzi E, Fauci AS. Cytokine regulation of acute and chronic HIV infection in vitro: from cell lines to primary mononuclear cells. Res Immunol. 1994;145:578–582. doi: 10.1016/s0923-2494(05)80036-7. [DOI] [PubMed] [Google Scholar]
- Pugliese A, Gennero L, Vidotto V, Speranza F, Tambini R, Torre D. Interleukin-18 enhances HIV-1 production in a human chronically-infected T cell line (H9-V) Cell Biochem Funct. 2002;20:333–337. doi: 10.1002/cbf.981. [DOI] [PubMed] [Google Scholar]
- Quinones-Mateu ME, Lederman MM, Feng Z, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- Rabbi MF, Finnegan A, Al-Harthi L, Song S, Roebuck KA. Interleukin-10 enhances tumor necrosis factor-alpha activation of HIV-1 transcription in latently infected T cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:321–331. doi: 10.1097/00042560-199812010-00002. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amador V, Ponce-De-Leon S, Sierra-Madero J, Soto-Ramirez L, Esquivel-Pedraza L, Anaya-Saavedra G. Synchronous kinetics of CD4+ lymphocytes and viral load before the onset of oral candidosis and hairy leukoplakia in a cohort of Mexican HIV-infected patients. AIDS Res Hum Retroviruses. 2005;21:981–990. doi: 10.1089/aid.2005.21.981. [DOI] [PubMed] [Google Scholar]
- Rams TE, Andriolo M, Jr, Feik D, Abel SN, McGivern TM, Slots J. Microbiological study of HIV-related periodontitis. J Periodontol. 1991;62:74–81. doi: 10.1902/jop.1991.62.1.74. [DOI] [PubMed] [Google Scholar]
- Redpath S, Angulo A, Gascoigne NR, Ghazal P. Immune checkpoints in viral latency. Annu Rev Microbiol. 2001;55:531–560. doi: 10.1146/annurev.micro.55.1.531. [DOI] [PubMed] [Google Scholar]
- Reznik DA. Oral manifestations of HIV disease. Top HIV Med. 2005;13:143–148. [PubMed] [Google Scholar]
- Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. Interleukin-8-mediated heterologous receptor internalization provides resistance to HIV-1 infectivity. Role of signal strength and receptor desensitization. J Biol Chem. 2003;278:15867–15873. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Inigo E, Jimenez E, Bartolome J, et al. Detection of human immunodeficiency virus type 1 RNA by in situ hybridization in oral mucosa epithelial cells from anti-HIV-1 positive patients. J Med Virol. 2005;77:17–22. doi: 10.1002/jmv.20409. [DOI] [PubMed] [Google Scholar]
- Rohr O, Marban C, Aunis D, Schaeffer E. Regulation of HIV-1 gene transcription: from lymphocytes to microglial cells. J Leukoc Biol. 2003;74:736–749. doi: 10.1189/jlb.0403180. [DOI] [PubMed] [Google Scholar]
- Rotchford K, Strum AW, Wilkinson D. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex Transm Dis. 2000;27:243–248. doi: 10.1097/00007435-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol. 1997;2000:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Sgarbanti M, Borsetti A, Moscufo N, et al. Modulation of human immunodeficiency virus 1 replication by interferon regulatory factors. J Exp Med. 2002;195:1359–1370. doi: 10.1084/jem.20010753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgarbanti M, Remoli AL, Marsili G, et al. IRF-1 is required for full NF-kappaB transcriptional activity at the human immunodeficiency virus type 1 long terminal repeat enhancer. J Virol. 2008;82:3632–3641. doi: 10.1128/JVI.00599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugars DC, Alexander AL, Fu K, Freel SA. Endogenous salivary inhibitors of human immunodeficiency virus. Arch Oral Biol. 1999;44:445–453. doi: 10.1016/s0003-9969(99)00003-5. [DOI] [PubMed] [Google Scholar]
- Shugars DC, Patton LL, Freel SA, et al. Hyperexcretion of human immunodeficiency virus type 1 RNA in saliva. J Dent Res. 2001;80:414–420. doi: 10.1177/00220345010800020301. [DOI] [PubMed] [Google Scholar]
- Smith D. The long-term consequences of antiretroviral therapy. J HIV Ther. 2006;11:24–25. [PubMed] [Google Scholar]
- Southerland JH, Taylor GW, Moss K, Beck JD, Offenbacher S. Commonality in chronic inflammatory diseases: periodontitis, diabetes, and coronary artery disease. Periodontol. 2006;2000:130–143. doi: 10.1111/j.1600-0757.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- Spear GT, Alves ME, Cohen MH, Bremer J, Landay AL. Relationship of HIV RNA and cytokines in saliva from HIV-infected individuals. FEMS Immunol Med Microbiol. 2005;45:129–136. doi: 10.1016/j.femsim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Stanley SK, Ostrowski MA, Justement JS, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- Stansell JD. Pulmonary fungal infections in HIV-infected persons. Semin Respir Infect. 1993;8:116–123. [PubMed] [Google Scholar]
- Staprans SI, Hamilton BL, Follansbee SE, et al. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, De Clercq E, Balzarini J. The regulation of HIV-1 transcription: molecular targets for chemotherapeutic intervention. Med Res Rev. 2006;26:595–625. doi: 10.1002/med.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkanuparph S, Vibhagool A, Mootsikapun P, Chetchotisakd P, Tansuphaswaswadikul S, Bowonwatanuwong C. Opportunistic infections after the initiation of highly active antiretroviral therapy in advanced AIDS patients in an area with a high prevalence of tuberculosis. AIDS. 2003;17:2129–2131. doi: 10.1097/00002030-200309260-00018. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Hoshino Y, Gold J, et al. Interleukin-10 induces inhibitory C/EBPbeta through STAT-3 and represses HIV-1 transcription in macrophages. Am J Respir Cell Mol Biol. 2005;33:406–411. doi: 10.1165/rcmb.2005-0140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer VM, Rajadhyaksha A, Babin J, Bina M. NF-IL6-mediated transcriptional activation of the long terminal repeat of the human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:7298–7302. doi: 10.1073/pnas.90.15.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen CT, Kilroe B, Martin DJ. Interleukin-8 fails to induce human immunodeficiency virus-1 expression in chronically infected promonocytic U1 cells but differentially modulates induction by proinflammatory cytokines. Immunology. 2000;101:140–146. doi: 10.1046/j.1365-2567.2000.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toossi Z, Sierra-Madero JG, Blinkhorn RA, Mettler MA, Rich EA. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177:1511–1516. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong MJ, Darcissac EC, Hermann E, Dewulf J, Capron A, Bahr GM. Interleukin-16 inhibits human immunodeficiency virus type 1 entry and replication in macrophages and in dendritic cells. J Virol. 1999;73:7008–7013. doi: 10.1128/jvi.73.8.7008-7013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CS, Samaranayake LP. Predominant cultivable subgingival microbiota of healthy and HIV-infected ethnic Chinese. APMIS. 2001;109:117–126. doi: 10.1034/j.1600-0463.2001.d01-113.x. [DOI] [PubMed] [Google Scholar]
- Vieira BJ, de Souza AR, Aarestrup FM. Tumor necrosis factor-alpha expression and detection of apoptosis at the site of chronic periodontitis in AIDS patients. J Periodontal Res. 2003;38:606–610. doi: 10.1034/j.1600-0765.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- Wahl SM, Greenwell-Wild T, Peng G, Ma G, Orenstein JM, Vazquez N. Viral and host cofactors facilitate HIV-1 replication in macrophages. J Leukoc Biol. 2003;74:726–735. doi: 10.1189/jlb.0503220. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rice AP. Interleukin-10 inhibits HIV-1 LTR-directed gene expression in human macrophages through the induction of cyclin T1 proteolysis. Virol. 2006;352:485–492. doi: 10.1016/j.virol.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Wang FX, Xu Y, Sullivan J, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest. 2005;115:128–137. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden M, Tanaka N, Qiao Y, et al. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol. 2000;165:2028–2039. doi: 10.4049/jimmunol.165.4.2028. [DOI] [PubMed] [Google Scholar]
- Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nakata K, Weiden M, Rom WN. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;95:2324–2331. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]