Abstract
Background
NIMH Project Accept (HPTN 043) was a cluster-randomized trial that tested whether a multicomponent, multi-level prevention strategy (community-based voluntary counselling and testing [CBVCT]) reduced HIV incidence compared to standard voluntary counselling and testing (SVCT).
Methods
Forty-eight communities were enrolled at five sites in South Africa, Tanzania, Zimbabwe, and Thailand. CBVCT was designed to make testing more accessible in communities, engage communities through outreach, and provide post-test support services. SVCT comprised standard VCT services established at existing facilities. Communities were randomized in matched pairs to 36 months of CBVCT or SVCT. Data were collected at baseline (n=14,567) and post-intervention (n=56,683) by cross-sectional random surveys of 18–32 year-old community residents. HIV incidence was estimated using a cross-sectional multi-assay algorithm. Thailand was excluded from incidence analyses due to low HIV prevalence.
Findings
The estimated incidence in the CBVCT was 1.52% vs. 1.81% in the SVCT with an estimated reduction in HIV incidence of 13·9% (relative risk [RR]=0·86; 95% confidence interval [CI]=0·725–1·023; p=0·08). Women older than 24 years had RR=0·70 (95% CI=0·54–0·90; p=0·009). CBVCT increased testing rates by 25% overall (95% CI=12%–39%; p=0·0003), by 45% among men and 15% among women. No overall effect on sexual risk behaviour was observed. However, among HIV-infected participants, CBVCT reduced the number of sexual partners by 8% (95% CI=1%–15%; p=0.03) and the proportion of multiple partnerships by 30% (95% CI=8%-46%; p=0.01). Social norms regarding HIV testing were improved in CBVCT communities.
Interpretations
The intervention was effective in increasing HIV testing, particularly among men, promoted positive social norms regarding testing, and reduced behavioural risk among HIV-infected participants. A modest reduction in HIV incidence was observed. This intervention focused primarily on HIV detection. Current and future studies that include strategies for HIV treatment and viral suppression should demonstrate further incidence reductions.
Keywords: HIV, incidence, Project Accept, Africa, HPTN 043
INTRODUCTION
Several interventions have demonstrated reduced HIV incidence in clinical trials, including early treatment of HIV infection,1,2 use of antiretroviral therapy (ART) to prevent mother-to-child transmission,3,4 chemoprophylaxis,5,6 and male circumcision.7–9 The challenge is to demonstrate community-level impact on the epidemic when efficacious interventions are taken to scale.10
NIMH Project Accept (HPTN 043) was the first cluster-randomized trial to test whether a theory-based, multi-component, multi-level social and behavioural prevention strategy could reduce HIV incidence within entire communities. The study hypothesis was that community-based voluntary counselling and testing (CBVCT), relative to standard voluntary counselling and testing (SVCT), would improve community norms about HIV testing, reduce HIV risk behaviours, reduce HIV stigma, promptly link HIV-infected individuals to available services, and lower HIV incidence. A major goal of the intervention was to reduce logistical barriers to HIV testing. The intervention was designed to adapt dynamically to changes occurring in the communities, using real-time performance feedback.
We have previously reported the baseline characteristics of the study population11 and the process measures describing the intervention components and their uptake.12 At three trial sites, the number of HIV tests performed by the VCT venues and the number of newly-detected HIV infections were higher in CBVCT communities.
In this paper, we report the analysis of HIV incidence, the primary outcome of Project Accept, and the analysis of secondary social and behavioural outcomes: sexual risk behaviour, HIV testing rates, social norms regarding testing, discussions about HIV, disclosure of HIV status, stigma associated with HIV, and HIV-related negative life events. All outcomes were evaluated at the community level, irrespective of participation in the intervention.
METHODS
Trial Design
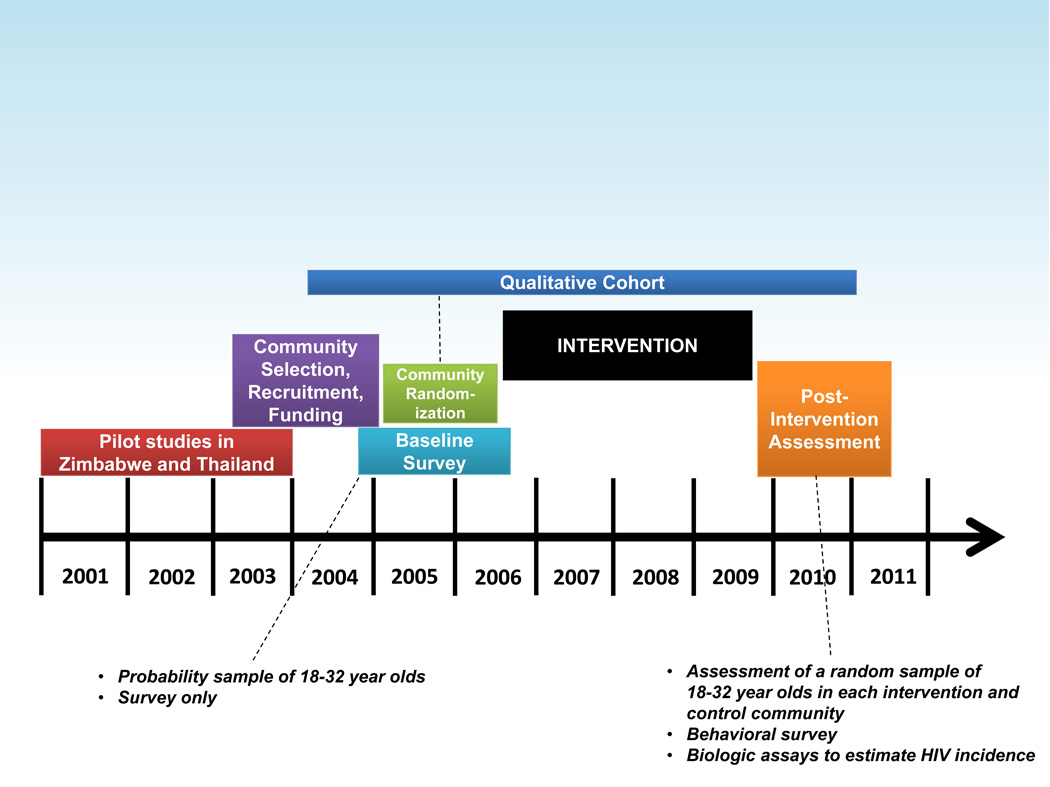
Project Accept was a cluster-randomized trial conducted in 34 communities at four sites in Africa (Soweto and KwaZulu-Natal, South Africa; Tanzania; Zimbabwe) and in 14 communities in Thailand. Baseline demographic, behavioural, and social data were collected on a random sample of community residents between 18 and 32 years of age,11 followed by community randomization and a 36-month intervention period13. The impact of the study intervention was assessed across entire communities in a single cross-sectional survey conducted at the end of the intervention period. Demographic and behavioural data and blood samples were collected from randomly-selected individuals 18 to 32 years of age. HIV incidence was estimated from cross-sectional data using a multi-assay algorithm (MAA).14 The study protocol, operation manuals, and standard operating procedures are publicly available.15 The timeline for the study is shown in Figure 1.
Figure 1.
Definition of Clusters and Randomization
The communities (clusters) were coherent geographical areas with well-defined boundaries.11 At the African sites, the communities were geographically close, but in most cases were not immediately adjacent to each other. The population size of communities varied from 5,000 to 15,000 at four sites; at the Soweto site, the typical population size was between 15,000 and 25,000. The communities were matched into pairs prior to randomization, based on socio-demographic, cultural, and infrastructure characteristics determined from formative research.17 Within each pair, one community was randomly assigned to receive CBVCT, the other received SVCT alone. Randomization was performed centrally by the Statistical Center18. Due to the nature of the intervention, the assignment was not blinded, except at the laboratories that analysed study samples.
Interventions
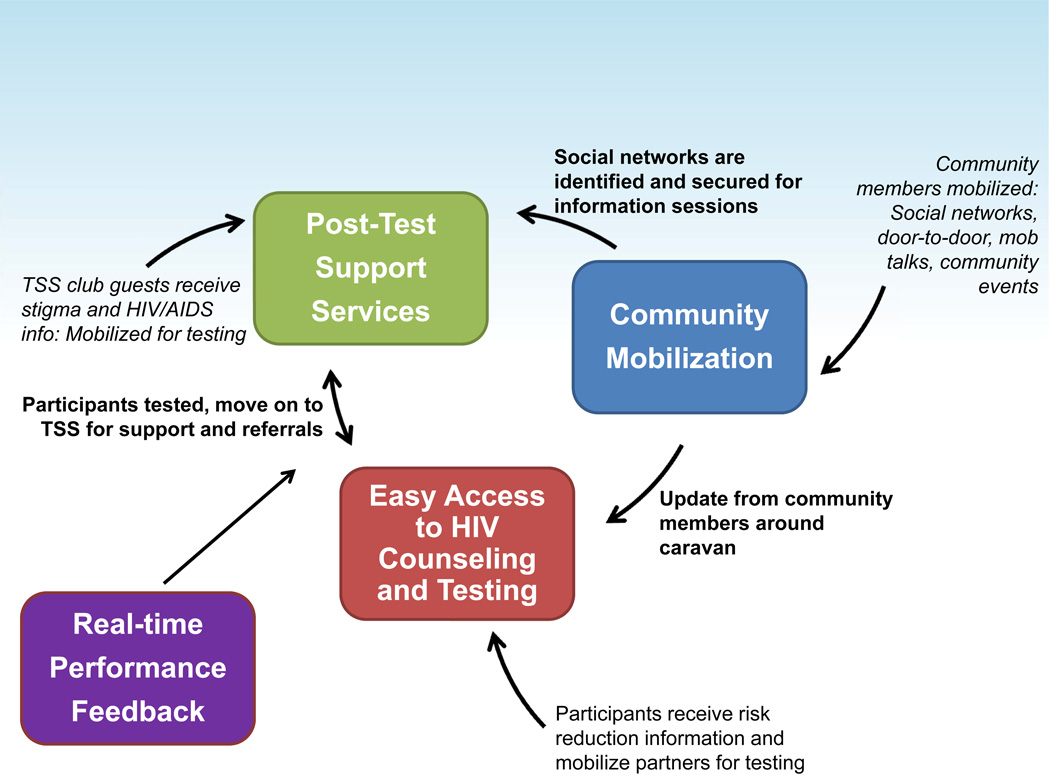
The CBVCT intervention was designed to change the context in which individuals and communities respond to HIV.13 The four main components of the CBVCT intervention are shown in Figure 2. The Community Mobilization component used outreach coordinators and early testers as outreach workers to modify norms for HIV testing, encourage discussion about HIV testing and disclosure of HIV status, increase the uptake of testing, and reduce stigma. The Easy Access to VCT component was designed to increase awareness of HIV status through easily accessible VCT services provided in mobile units. The Post-Test Support Services component provided peer-based social support groups for those who had been tested, regardless of test results. Topics included social benefits and harms, status disclosure, access to HIV-related services, advances in HIV treatment, and the risk of transmitting HIV. The Real-Time Performance Feedback component ensured that pre-set intervention goals were met.13 The SVCT intervention was comprised of VCT services established at existing district hospitals or local health care facilities. Such services were also available in CBVCT communities.
Figure 2.
Participation in all intervention activities and services was open to all individuals 16 years and older. The intervention and its adaptation to various specific settings have been described elsewhere.13,19–21
Baseline and Post-Intervention Assessments
Data were collected in random samples of community residents, regardless of their participation in intervention activities. The baseline assessment was conducted before randomization and did not include HIV testing.11 The post-intervention assessment (PIA) was conducted at the end of the intervention period, and was independent of the baseline assessment. Eligibility criteria for participation in the baseline assessment and PIA were: age 18–32 years, current residency in the community, and ability to provide informed consent. Households were selected with equal probability from a complete listing of community households. At baseline, one eligible subject was randomly selected from each household to provide detailed demographic and behavioural data. At the PIA, all eligible individuals from the selected households were invited to participate in a brief survey and collection of blood samples. In a subset of the households, one eligible subject was randomly selected to complete a detailed demographic and behavioural questionnaire.
Evaluation of HIV Incidence
HIV incidence was assessed by analysing cross-sectional samples collected during the PIA. Samples were tested in-country using HIV rapid tests; details of testing of samples are reported in Laeyendecker, et al.14 The final HIV status of study samples was determined at the HPTN Network Laboratory at Johns Hopkins University, as described. 14 HIV incidence was assessed using a MAA that included the BED capture immunoassay (BED-CEIA), an antibody avidity assay, CD4 cell count (obtained at study sites), and HIV viral load (obtained at the HPTN Network Laboratory for a subset of samples).14 The MAA was developed and validated using data obtained for 4,166 samples from 2,882 individuals with known duration of infection (from 1 month to >10 years) from seven African cohorts.22 The MAA was optimized to detect a difference in HIV incidence in southern African populations. An antiretroviral drug (ARV) screening assay was used to exclude individuals on ART from the incidence assessment.14
Behavioural Outcomes
Behavioural outcome measures were assessed using interviewer-administered questionnaires. HIV testing uptake was assessed as the proportion of participants who reported at least one HIV test over the past 12 months. In the PIA, testing in the prior 36 months was also assessed. The social norms score was calculated as the mean of scores (ranging from zero to three on a Likert scale) of participant’s responses to six statements assessing their opinion on prevailing community attitudes towards HIV testing (a higher value is associated with more positive attitudes). HIV behaviour risk score was assessed by self-reports of monthly number of unprotected sexual acts averaged over the past six months. Number of sexual partners was also examined in sub-analyses. Subjects who were not sexually active in the past six months were assigned a score of zero. Negative life events were assessed as the proportion of participants who reported any events related to partnership break-up, discrimination, estrangement, neglect, or violence. Discussions about HIV were measured by the proportion of participants who reported any HIV-related conversation in the past six months. Disclosure of HIV status was measured as the proportion of tested participants who disclosed their HIV test results to another person. HIV stigma score was calculated as the mean of scores (on a zero to four point Likert scale) assigned to validated 28 stigma-related scale items.23,24 A higher score was associated with more stigma.
Sample Size
The sample size (seven pairs in Thailand with 500 assessment participants per community; five pairs in Tanzania with 900 assessment participants per community; four pairs at the other sites with 1,430 assessment participants per community) was calculated by an adaptation of methods suggested by Hayes and Bennet25 to provide 80% power to detect a 35% reduction in HIV incidence. The assumptions behind the sample size calculation were: 30% prevalence, 3% annual incidence, follow-up duration 6 months, no misclassification of incident infections, and coefficient of variation 0·26. The power was recalculated after HIV prevalence in the communities was determined and the testing algorithm used for the HIV incidence assessment was developed and validated. Using a weighted t-test, the study provided over 90% power to detect a 35% reduction in HIV incidence.
Protocol Adaptations
During the study, several unexpected events warranted changes in the protocol. First, one community pair in Soweto was removed from the study soon after the start of the intervention due to threats of political violence. This community pair was replaced by another; a separate randomization was performed for the new pair and all study activities were conducted as prescribed. Data from the withdrawn community pair were not included in any analyses. Second, the original study plan was to determine the primary HIV incidence outcome using the BED-CEIA alone. The laboratory plan was revised because of serious concerns about the validity of this approach.26 Third, the HIV prevalence in the 14 Thai communities was very low (≈1%). Therefore, the incidence assessment was restricted to the 34 African communities. Fourth, quality assurance testing revealed that some stored samples from the Soweto site were cross-contaminated.14 The contaminated samples could not be assessed at the HPTN Network Laboratory. Finally, the standard paired t-test planned for the primary analysis was replaced by a weighted paired t-test.
Statistical Analysis
HIV incidence was estimated in each community using a MAA followed by an ARV screening assay; infections classified as acute or early were included in the incidence estimate.14 The intervention effect for each community pair was calculated as a log incidence ratio for the CBVCT vs. the SVCT community. The overall intervention effect was estimated by the weighted mean of pair-specific effects; the weights were proportional to the harmonic mean of the number of incident infections observed in the paired communities. The weighted paired t-test was used to test the hypothesis of no intervention effect at the two-sided level of 5%. CIs were based on the weighted paired t-statistic. The number of degrees of freedom of the reference t distribution was adjusted to take into account unequal weights (the primary analysis used 9·4 degrees of freedom in 17 pairs). The approximate degrees of freedom were calculated as the inverse of the sum of squared weights minus 1. If the weights are all equal, this yields a d.f. of 16 for the classical t-test. The test is asymptotic. We verified via simulations that the t distribution with approximated d.f. provides better results in small samples (correct level, correct C.I. coverage) than the t-distribution used with the classical t-test or the limiting standard normal distribution. Subgroup analyses by age and gender were performed by the same methods on a subset of the data; these analyses were pre-specified in the protocol.
For each behavioural outcome, community-specific means were calculated at baseline (if available) and in the PIA. For the outcomes that were measured on one randomly-selected household member, the means were weighted by inverse sampling probabilities to adjust for higher chance of inclusion of participants living in smaller households. Tests of intervention effects were performed by unweighted paired t-tests on logs of ratios of PIA community means to baseline community means. This approach adjusts the intervention effect for baseline differences between the communities. When the baseline mean was not available (36-month testing rates) or baseline data were too sparse (12-month testing rates), the test was done on log PIA means only (unadjusted for baseline). Estimates of overall and site-specific means and intervention effects were obtained by exponentiation of averages of community-specific means and intervention effects calculated on the log scale. Two-sided confidence intervals (CI) are based on the t-distribution on the log scale. Subgroup analyses were conducted by gender, and for selected outcomes also by HIV status determined by in-country HIV rapid testing.
Implementation
Study implementation was supervised and managed by Principal Investigators and Project Managers at the study sites. An 11-member Steering Committee (Coates[Chair], Celentano, Chariyalertsak, Chingono, Donnell, Gray, Kulich, Mbwambo, Morin, Richter, and Sweat) met monthly by conference call and semi-annually in person to design and approve all study procedures, monitor study progress, and approve all modifications and study publications. The NIMH constituted a Data and Safety Monitoring Board and Study Monitoring Committee (DSMB/SMC) that convened semi-annually to ensure that all study objectives were being met and that the safety of study participants was not compromised. Real-time performance feedback was provided monthly to each study team to ensure that study objectives were being met.
Ethical Considerations and Consent Procedures
The trial was conducted in close partnership with established community advisory boards and local government departments. Consent was obtained at the community level for trial participation and randomization. Participation in all intervention activities was voluntary. Participation in the post-intervention assessment required permission from the head of household for approaching household members. Consent was obtained from each participant for each component of data collection and for collection and testing of blood samples.
Ethical Review and the Role of the Funding Source
The study was approved by ethical committees for each site and by all participating academic institutions. NIMH funding for the project was through a cooperative agreement mechanism, allowing the NIMH Project Officer assigned to the study to participate in technical project activities. The NIMH project officer participated in Steering Committee meetings and reviewed DSMB/SMC reports before submission. None of the funding agencies had any role in the study design, data collection, analysis, and interpretation, or decision to submit for publication.
FINDINGS
Study Population
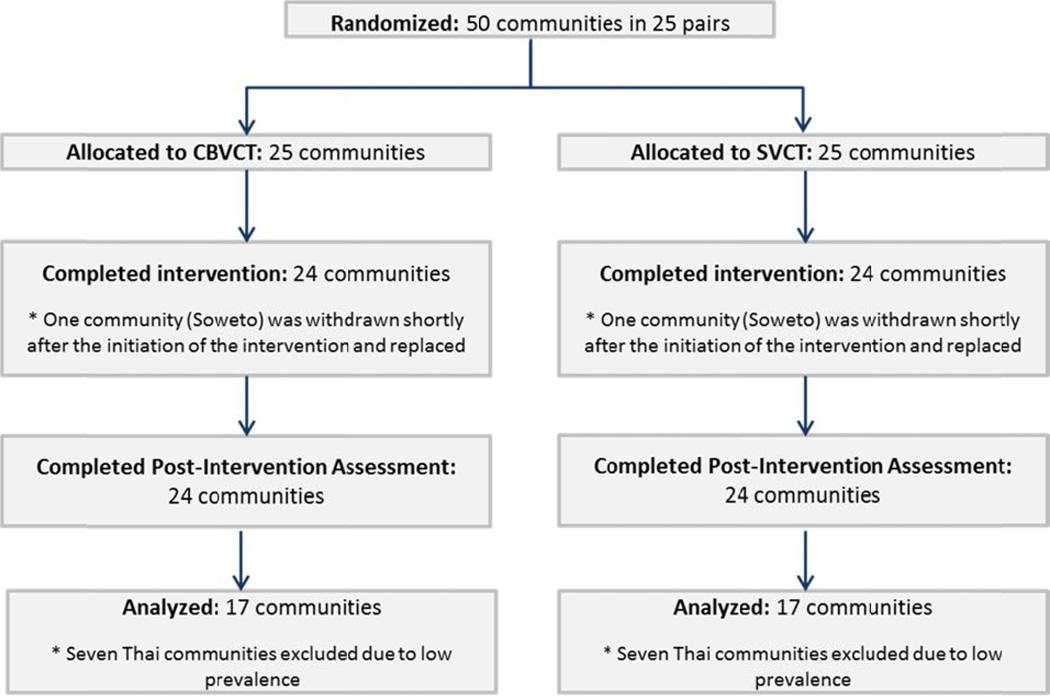
Fifty communities were enrolled, matched into pairs, and randomized; 48 of the communities received the assigned intervention and post-intervention assessment. Because of low HIV prevalence in the Thai site, only 34 African communities were included in the primary endpoint analysis (Figure 3). All 48 communities participated in secondary outcome analyses.
Figure 3.
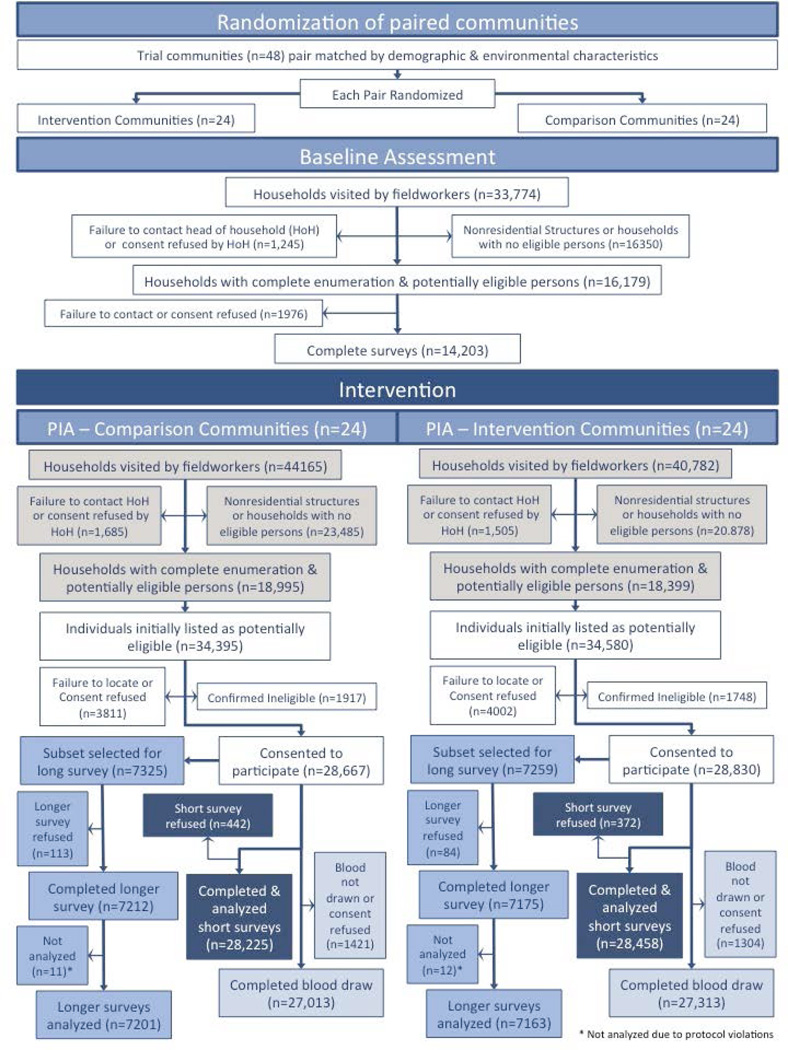
Community populations were well balanced between study arms (Table 1). During the intervention, the study teams organized 15,603 community mobilization activities, provided 71,842 VCT sessions, and 51,787 post-test support service sessions. Figure 4 describes the participant flow through the baseline assessment and PIA.A total of 84,947 potential housing structures were visited during the PIA. Among them, 9,535 (11.2%) were non-residential (e.g., business, storage, animal structures).Additional 34,828 (41.0%) households were found to include no eligible participants. Response rates for primary outcome assessment are summarized in Table 2. In African sites, nearly 60,000 potentially eligible participants lived in selected households. Of them, 7·9% could not be contacted, 3·7% refused participation, and 5·6% were ineligible. Among eligible participants, the response rate for blood sample collection was 94·1%. When failure to contact participants was taken into account, the overall response rate was 81·5%. There was no significant difference in the response rate by study arm. The PIA included a sizeable fraction of the whole community population: 29% of all eligible subjects in Soweto and 57–77% of all eligible subjects in the other three African sites. Study samples were analysed at the HPTN Network Laboratory to determine the final HIV status of study participants and to estimate HIV incidence.14 The results of the laboratory analyses are summarized in Table 3. A total of 46,693 blood samples were collected; 320 (0·7%) of those samples were excluded from incidence assessment (Table 3). The HIV incidence analysis included data from 39,012 HIV-uninfected participants and 7,361 HIV-infected participants enrolled at the African sites. A total of 445 samples were classified as potentially incident infections14 and formed the basis of the HIV incidence analysis (205 in the CBVCT arm and 240 in the SVCT arm); these samples included acute and early infections and MAA positive samples that did not have ARV drugs detected.
Table 1.
Characteristics of the participating communities (Post-Intervention Assessment)
| Thailand | Zimbabwe | Tanzania | KwaZulu-Natal | Soweto | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | |
| Site population (all ages) | 55,100 | 48,100 | 45,200 | 48,200 | 27,500 | 27,400 | 33,600 | 33,600 | 85,900 | 66,100 |
| Community population range (all ages) |
4,900- 10,900 |
6,300- 7,600 |
10,100- 12,500 |
8,600- 15,800 |
3,600- 7,700 |
3,400- 6,400 |
7,000- 9,400 |
7,700- 9,100 |
16,600- 24,400 |
5,100- 23,900 |
| Site population (18–32 years old) |
9,200 | 6,600 | 10,100 | 11,000 | 6,000 | 5,700 | 9,800 | 9,500 | 27,900 | 20,100 |
| Community population range (18–32 years old) |
800- 2,100 |
800- 1,200 |
2,200- 2,900 |
1,900- 3,800 |
700- 1,600 |
700- 1,300 |
2,100- 3,000 |
2,100- 2,500 |
5,300- 10,100 |
1,500- 7,300 |
| Number of households per community (mean) |
2,471 | 2,223 | 2,888 | 3,024 | 1,570 | 1,587 | 2,588 | 2,604 | 4,665 | 3,642 |
| Number of households per community (range) |
1,626- 3,276 |
1,910- 2,478 |
2,605- 3,189 |
2,233- 3,712 |
950- 1,949 |
881- 1,920 |
2,306- 2,900 |
1,801- 3,134 |
3,395- 6,178 |
1,649- 5,263 |
| Median household size | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
Figure 4.
Participant Flow Diagram
Table 2.
Summary of blood sample collection at the post-intervention assessment
| Thailand | Zimbabwe | Tanzania | KwaZulu-Natal | Soweto | African sites | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | |
| Post-intervention assessment participants | ||||||||||||
| All subjects | 4,607 | 4,639 | 8,061 | 7,975 | 5,856 | 5,631 | 6,789 | 6,797 | 9,267 | 9,360 | 29,973 | 29,763 |
| Failure to contact |
347 (7•5%) |
316 (6•8%) |
653 (8•1%) |
479 (6•0%) |
168 (2•9%) |
177 (3•1%) |
175 (2•6%) |
122 (1•8%) |
1,425 (15•4%) |
1,515 (16•2%) |
2,421 (8•1%) |
2,293 (7•7%) |
| Refused to participate |
125 (2•7%) |
117 (2•5%) |
753 (9•3%) |
725 (9•1%) |
61 (1•0%) |
80 (1•4%) |
61 (0•9%) |
55 (0•8%) |
234 (2•5%) |
227 (2•4%) |
1,109 (3•7%) |
1,087 (3•7%) |
| Ineligible | 146 (3•2%) |
154 (3•3%) |
373 (4•6%) |
387 (4•9%) |
485 (8•3%) |
542 (9•6%) |
474 (7•0%) |
560 (8•2%) |
270 (2•9%) |
274 (2•9%) |
1,602 (5•3%) |
1,763 (5•9%) |
| No blood collection consent |
198 (4•3%) |
217 (4•7%) |
344 (4•3%) |
414 (5•2%) |
455 (7•8%) |
465 (8•3%) |
120 (1•8%) |
93 (1•4%) |
187 (2•0%) |
232 (2•5%) |
1,106 (3•7%) |
1,204 (4•0%) |
| Blood sample unavailable |
4 (0•1%) |
3 (0•1%) |
15 (0•2%) |
13 (0•2%) |
3 (0•1%) |
10 (0•2%) |
46 (0•7%) |
37 (0•5%) |
153 (1•6%) |
181 (1•9%) |
217 (0•7%) |
241 (0•8%) |
| Blood sample available |
3,787 (82•2%) |
3,832 (82•6%) |
5,923 (73•5%) |
5,957 (74•7%) |
4,684 (80•0%) |
4,357 (77•4%) |
5,913 (87•1%) |
5,930 (87•2%) |
6,998 (75•5%) |
6,931 (74•0%) |
23,518 (78•5%) |
23,175 (77•9%) |
| Response rates | ||||||||||||
| Stepwisea | 95•0% | 94•6% | 94•3% | 93•4% | 90•1% | 89•1% | 97•3% | 97•9% | 95•5% | 94•6% | 94•3% | 93•8% |
| Cumulativeb | 83•3% | 84•5% | 73•4% | 75•3% | 84•8% | 83•1% | 93•5% | 94•8% | 74•9% | 72•4% | 81•6% | 81•4% |
Response rate for obtaining a sufficient blood sample from successfully contacted eligible subjects
Cumulative response rate takes into account failure to contact the selected household or individual and confirm eligibility.
Table 3.
Summary of blood sample analysis
| Thailand | Zimbabwe | Tanzania | KwaZulu-Natal | Soweto | African Sites | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | |
| Initial HIV status (rapid testing) | ||||||||||||
| Negative | 3,727 (98•4%) |
3,775 (98•5%) |
5,126 (86•5%) |
5,187 (87•1%) |
4,284 (91•5%) |
4,028 (92•4%) |
4,041 (68•3%) |
4,107 (69•3%) |
6,116 (87•4%) |
5,806 (83•8%) |
19,567 (83•2%) |
19,128 (82•5%) |
| Positive | 44 (1•2%) |
34 (0•9%) |
788 (13•3%) |
760 (12•8%) |
290 (6•2%) |
252 (5•8%) |
1,851 (31•3%) |
1,798 (30•3%) |
874 (12•5%) |
1,111 (16•0%) |
3,803 (16•2%) |
3,921 (16•9%) |
| Discordant | 16 (0•4%) |
23 (0•6%) |
9 (0•2%) |
10 (0•2%) |
110 (2•3%) |
77 (1•8%) |
21 (0•4%) |
25 (0•4%) |
8 (0•1%) |
14 (0•2%) |
148 (0•6%) |
126 (0•5%) |
| Final HIV status | ||||||||||||
| Uninfected | 3,743 (98•8%) |
3,797 (99•1%) |
5,146 (86•9%) |
5,202 (87•3%) |
4,400 (93•9%) |
4,105 (94•2%) |
4,063 (68•7%) |
4,134 (69•7%) |
6,134 (87•7%) |
5,828 (84•1%) |
19,743 (83•9%) |
19,269 (83•1%) |
| Infecteda | 44 (1•2%) |
35 (0•9%) |
777 (13•1%) |
755 (12•7%) |
284 (6•1%) |
252 (5•8%) |
1,850 (31•3%) |
1,795 (30•3%) |
712 (10•2%) |
941 (13•6%) |
3,623 (15•4%) |
3,743 (16•2%) |
| Unknownb | 0 (0•0%) |
0 (0•0%) |
0 (0•0%) |
0 (0•0%) |
0 (0•0%) |
0 (0•0%) |
0 (0•0%) |
1 (0•0%) |
152 (2•2%) |
162 (2•3%) |
152 (0•7%) |
163 (0•7%) |
| MAA status (among samples classified as HIV infected) | ||||||||||||
| MAA negative | 43 (97•7%) |
33 (94•3%) |
750 (96•5%) |
711 (94•2%) |
251 (88•4%) |
228 (90•5%) |
1,733 (93•7%) |
1,667 (92•8%) |
668 (93•8%) |
879 (93•4%) |
3,402 (93•9%) |
3,485 (93•1%) |
| MAA positive, acute, and early infections |
1 (2•3%) |
2 (5•7%) |
27 (3•5%) |
43 (5•7%) |
33 (11•6%) |
24 (9•5%) |
115 (6•2%) |
127 (7•1%) |
44 (6•2%) |
61 (6•5%) |
219 (6•0%) |
255 (6•8%) |
| Not evaluateda | 0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (0.1%) |
0 (0.0%) |
0 (0.0%) |
2 (0.1%) |
1 (0.1%) |
0 (0.0%) |
1 (0.1%) |
2 (0•1%) |
3 (0•1%) |
| Results of ARV drug testing (among samples classified as MAA positive, acute infection or early infection) | ||||||||||||
| ARV drugs detected | 0 (0•0%) |
0 (0•0%) |
2 (7•4%) |
1 (2•3%) |
6 (18•2%) |
4 (16•7%) |
5 (4•3%) |
7 (5•5%) |
1 (2•3%) |
3 (4•9%) |
14 (6•4%) |
15 (5•9%) |
| No ARV drugs detected c | 1 (100•0%) |
2 (100•0%) |
25 (92•6%) |
42 (97•7%) |
27 (81•8%) |
20 (83•3%) |
110 (95•7%) |
120 (94•5%) |
43 (97•7%) |
58 (95•1%) |
205 (93•6%) |
240 (94•1%) |
Five of the 7,366 participants who were confirmed to be HIV-infected were excluded from the incidence analysis because of missing CD4 cell count data (not evaluated by the MAA).
Three-hundred fifteen participants who had at least one reactive HIV rapid test were excluded from the analysis because it was not possible to confirm their HIV status. This included 292 participants who were excluded because of sample contamination and 23 participants who were excluded for other reasons.12
This group includes samples from participants with acute and early HIV infection (those samples were not tested for the presence of ARV drugs) and samples that did not have enough plasma remaining for ARV testing.12
Table 4 presents the demographic and behavioural characteristics of the 46,378 study participants with known final HIV status. A total of 54·3% of these participants were women; a slight majority of men and women were 18–24 years old. The South African sites had on average more years of education and lower marriage levels than the other sites.
Table 4.
Summary of demographic and behavioral characteristics among subjects with known final HIV status.
| Thailand | Zimbabwe | Tanzania | KwaZulu-Natal | Soweto | Africa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | |
| 3,787 | 3,832 | 5,923 | 5,957 | 4,684 | 4,357 | 5,913 | 5,929 | 6,846 | 6,769 | 23,366 | 23,012 | |
| Gender | ||||||||||||
| Men | 1,947 (51•4%) |
1,960 (51•2%) |
2,826 (47•7%) |
2,924 (49•1%) |
2,143 (45•8%) |
2,016 (46•3%) |
2,483 (42•1%) |
2,488 (42•1%) |
3,143 (45•9%) |
3,159 (46•7%) |
10,595 (45•4%) |
10,587 (46•0%) |
| Women | 1,840 (48•6%) |
1,871 (48•8%) |
3,094 (52•3%) |
3,033 (50•9%) |
2,541 (54•2%) |
2,340 (53•7%) |
3,420 (57•9%) |
3,428 (57•9%) |
3,698 (54•1%) |
3,607 (53•3%) |
12,753 (54•6%) |
12,408 (54•0%) |
| Age (Men) | ||||||||||||
| 18–24 years | 925 (47•5%) |
926 (47•3%) |
1,465 (51•9%) |
1,530 (52•4%) |
936 (43•7%) |
858 (42•6%) |
1,636 (66•7%) |
1,665 (67•7%) |
1,685 (53•7%) |
1,802 (57•1%) |
5,722 (54•2%) |
5,855 (55•5%) |
| 25–32 years | 1,022 (52•5%) |
1,033 (52•7%) |
1,360 (48•1%) |
1,392 (47•6%) |
1,205 (56•3%) |
1,157 (57•4%) |
815 (33•3%) |
796 (32•3%) |
1,454 (46•3%) |
1,352 (42•9%) |
4,834 (45•8%) |
4,697 (44•5%) |
| Age (Women) | ||||||||||||
| 18–24 years | 851 (46•3%) |
853 (45•6%) |
1,467 (47•5%) |
1,471 (48•5%) |
1,129 (44•4%) |
986 (42•2%) |
2,006 (59•2%) |
2,039 (60•0%) |
1,894 (51•2%) |
1,951 (54•2%) |
6,496 (51•1%) |
6,447 (52•1%) |
| 25–32 years | 988 (53•7%) |
1,018 (54•4%) |
1,624 (52•5%) |
1,560 (51•5%) |
1,411 (55•6%) |
1,353 (57•8%) |
1,383 (40•8%) |
1,361 (40•0%) |
1,803 (48•8%) |
1,650 (45•8%) |
6,221 (48•9%) |
5,924 (47•9%) |
| Education | ||||||||||||
| <5 years | 1,299 (34•3%) |
794 (20•7%) |
160 (2•7%) |
173 (2•9%) |
1,066 (22•8%) |
1,071 (24•6%) |
121 (2•1%) |
132 (2•2%) |
54 (0•8%) |
60 (0•9%) |
1,401 (6•0%) |
1,436 (6•3%) |
| 5–8 years | 781 (20•6%) |
787 (20•5%) |
1,456 (24•6%) |
1,413 (23•8%) |
2,788 (59•6%) |
2,693 (61•9%) |
388 (6•6%) |
503 (8•5%) |
245 (3•6%) |
241 (3•6%) |
4,877 (20•9%) |
4,850 (21•1%) |
| 9–12 years | 1,423 (37•6%) |
1,811 (47•3%) |
3,932 (66•5%) |
3,976 (66•8%) |
781 (16•7%) |
547 (12•6%) |
5,098 (86•4%) |
4,971 (84•2%) |
5,295 (77•5%) |
5,143 (76•2%) |
15,106 (64•8%) |
14,637 (63•8%) |
| >12 years | 284 (7•5%) |
439 (11•5%) |
366 (6•2%) |
387 (6•5%) |
46 (1•0%) |
41 (0•9%) |
293 (5•0%) |
299 (5•1%) |
1,235 (18•1%) |
1,305 (19•3%) |
1,940 (8•3%) |
2,032 (8•9%) |
| Marital status | ||||||||||||
| Single | 1,045 (27•6%) |
1,299 (33•9%) |
1,895 (32•0%) |
1,849 (31•1%) |
1,938 (41•4%) |
1,687 (38•8%) |
5,730 (97•6%) |
5,744 (97•6%) |
6,085 (89•3%) |
6,200 (91•9%) |
15,648 (67•2%) |
15,480 (67•5%) |
| Married | 2,411 (63•7%) |
2,201 (57•4%) |
3,381 (57•1%) |
3,522 (59•2%) |
2,436 (52•0%) |
2,378 (54•6%) |
131 (2•2%) |
127 (2•2%) |
677 (9•9%) |
509 (7•5%) |
6,625 (28•4%) |
6,536 (28•5%) |
| Separated | 329 (8•7%) |
332 (8•7%) |
641 (10•8%) |
577 (9•7%) |
310 (6•6%) |
287 (6•6%) |
11 (0•2%) |
15 (0•3%) |
54 (0•8%) |
39 (0•6%) |
1,016 (4•4%) |
918 (4•0%) |
| Sexual activity in the last 6 months | ||||||||||||
| Active | 2,770 (82•5%) |
2,673 (80•7%) |
4,088 (79•6%) |
4,208 (80•4%) |
3,146 (73•1%) |
3,087 (76•1%) |
3,767 (74•2%) |
3,558 (70•9%) |
5,073 (80•3%) |
4,963 (80•3%) |
16,074 (77•2%) |
15,816 (77•2%) |
Primary Outcome
The overall HIV prevalence in the African sites was 16·5%. The highest HIV prevalence was observed in KwaZulu-Natal (Table 6): 30·8% for all subjects, over 50% of subjects in the age range 25–32 years; over 60% of women 25–32 years old were HIV infected. The other sites had lower HIV prevalence: Soweto 14·1%, Zimbabwe 12·9%, and Tanzania 5·9%. At all sites, HIV prevalence in women was more than twice as high as in men, and HIV prevalence in the older age group (25–32 years) was about three times higher than in the younger age group (18–24 years).
Table 6.
Summary of site-specific incidence results for all participants and by gender and age.
| Site | Prevalence | # Incident samples | Incidence | Effect | Site weight |
|||
|---|---|---|---|---|---|---|---|---|
| CBVCT | SVCT | CBVCT | SVCT | CBVCT | SVCT | |||
| All participants | ||||||||
| Zimbabwe | 13•1 | 12•7 | 25 | 42 | 0•68 | 1•13 | 0•63 | 13•9 |
| Tanzania | 6•1 | 5•8 | 27 | 20 | 0•86 | 0•68 | 1•23 | 10•1 |
| KwaZulu-Natal | 31•3 | 30•3 | 110 | 120 | 3•76 | 4•03 | 0•93 | 53•8 |
| Soweto | 12•3 | 15•9 | 43 | 58 | 1•18 | 1•63 | 0•74 | 22•2 |
| Females | ||||||||
| Zimbabwe | 17•5 | 17•4 | 15 | 27 | 0•83 | 1•51 | 0•55 | 13•2 |
| Tanzania | 8•1 | 8•2 | 22 | 16 | 1•32 | 1•05 | 1•18 | 10•9 |
| KwaZulu-Natal | 40•9 | 39•0 | 77 | 75 | 5•26 | 4•96 | 1•06 | 53•2 |
| Soweto | 17•3 | 22•4 | 28 | 43 | 1•53 | 2•38 | 0•67 | 22•8 |
| Males | ||||||||
| Zimbabwe | 8•3 | 7•7 | 10 | 15 | 0•54 | 0•78 | 0•99 | 15•5 |
| Tanzania | 3•6 | 3•0 | 5 | 4 | –* | –* | –* | 4•9 |
| KwaZulu-Natal | 18•0 | 18•3 | 33 | 45 | 2•26 | 3•08 | 0•73 | 61•5 |
| Soweto | 6•4 | 8•4 | 15 | 15 | 0•82 | 0•84 | 0•89 | 18•1 |
| Age 18–24 years | ||||||||
| Zimbabwe | 5•8 | 5•7 | 7 | 19 | 0•36 | 0•94 | 0•41 | 7•8 |
| Tanzania | 2•4 | 2•3 | 8 | 4 | –* | –* | –* | 3•3 |
| KwaZulu-Natal | 18•7 | 18•9 | 90 | 79 | 4•21 | 3•65 | 1•14 | 69•4 |
| Soweto | 6•1 | 8•2 | 20 | 32 | 1•08 | 1•52 | 0•72 | 19•5 |
| Age 25–32 years | ||||||||
| Zimbabwe | 20•3 | 19•7 | 18 | 23 | 1•06 | 1•36 | 0•87 | 21•0 |
| Tanzania | 8•9 | 8•3 | 19 | 16 | 1•12 | 0•98 | 1•10 | 19•7 |
| KwaZulu-Natal | 52•7 | 50•1 | 20 | 41 | 2•68 | 5•26 | 0•51 | 32•0 |
| Soweto | 19•1 | 25•4 | 23 | 25 | 1•38 | 1•79 | 0•79 | 27•3 |
Not enough incident samples to calculate incidence reliably
Prevalence: HIV prevalence in % of population.
Incident samples: This indicates the number of samples from HIV-infected individuals that were classified as MAA positive (excluding samples with antiretroviral drugs detected), as well as samples from HIV-infected individuals with acute or early infection.
Incidence: Annual rate in %; calculated across the whole site.
Effect: Relative risk of HIV infection (CBVCT vs. SVCT); weighted average of incidence ratios across community pairs at the site.
Site weight: % contribution of the site to the overall weighted analysis.
We observed an overall 13.9% reduction in HIV incidence in the CBVCT intervention communities compared to the SVCT control communities (Table 5). The 95% CI for the relative risk (RR) was 0·73–1·02, p=0·082. The reduction in incidence was 12% among women and 19% among men, neither of which attained significance. Little change in incidence was observed in the younger age group (RR=0·98); the older age group had a 25% reduction (95% CI for RR 0·54–1·04; p=0·078). The intervention effect was significant among women in the older age group (RR=0·70; 95% CI 0·54–0·90; p=0·009).
Table 5.
Summary of incidence results across all African sites.
| Subgroup | # Incident samples | Incidence | Intervention Effect |
95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| CBVCT | SVCT | CBVCT | SVCT | ||||
| All participants | 205 | 240 | 1•52 | 1•81 | 0•86 | 0•73–1•02 | 0•082 |
| Analysis by gender | |||||||
| Women | 142 | 161 | 2•06 | 2•42 | 0•88 | 0•73–1•06 | 0•17 |
| Men | 63 | 79 | 0•95 | 1•19 | 0•81 | 0•57–1•15 | 0•19 |
| Analysis by age | |||||||
| 18–24 years | 125 | 134 | 1•65 | 1•76 | 0•98 | 0•80–1•22 | 0•86 |
| 25–32 years | 80 | 105 | 1•38 | 1•90 | 0•75 | 0•54–1•04 | 0•078 |
| Analysis by gender and age | |||||||
| Women, age 18–24 years | 96 | 96 | 2•50 | 2•55 | 1•00 | 0•78–1•28 | 0•98 |
| Men, age 18–24 years | 29 | 38 | 0•76 | 0•98 | 0•95 | 0•64–1•40 | 0•69 |
| Women, age 25–32 years | 46 | 65 | 1•54 | 2•29 | 0•70 | 0•54–0•90 | 0•0085 |
| Men, age 25–32 years | 34 | 40 | 1•20 | 1•46 | 0•78 | 0•41–1•47 | 0•39 |
Incident samples: This indicates the number of samples from HIV-infected individuals that were classified as MAA positive (excluding samples with antiretroviral drugs detected), as well as samples HIV-infected individuals with acute or early infection.
Incidence: Annual rate in %; calculated across the African sites.
Intervention effect: Relative risk of HIV infection (CBVCT vs. SVCT); weighted average of incidence ratios for 17 community pairs.
95% CI: 95% confidence interval for intervention effect.
p-value: p-value for the hypothesis of no intervention effect on incidence.
We repeated the analysis using a more stringent MAA, as well as using a standard paired t-test rather than weighted t-test. In both cases, we obtained very similar intervention effects, but with larger standard errors and wider confidence intervals (data not shown).
Site-specific HIV incidence and prevalence results are presented in Table 6. Overall, the intervention appeared to reduce incidence at all sites except Tanzania. The reduction was quite small in KwaZulu-Natal, the site with the highest HIV incidence. In KwaZulu-Natal, the intervention did not appear to reduce HIV incidence in the younger subgroup or among all women, but in the older subgroup of women incidence was reduced by more than 40%.
Secondary Outcomes
The intervention increased HIV testing uptake significantly, and was especially effective in increasing testing among men. Overall 12-month testing rates (Table 7) were 25% higher in the CBVCT arm than in the SVCT arm (95% CI 12%-39%, p=0·0003). The largest effect on testing uptake was observed in Thailand (56%, see Table S1 in the Online Supplement). Testing rates over 36 months were 27% higher in the CBVCT arm (95% CI 15%-41%, p<0·0001). Overall, 49% of the entire 18–32 years old population living in CBVCT communities was tested over the duration of the study, compared to 39% in SVCT communities.
Table 7.
Summary of behavioural outcome results.
| BASELINE ASSESSMENT | POST-INTERVENTION ASSESSMENT | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | CBVCT | SVCT | Ratio | CBVCT | SVCT | Effect | 95% CI | P-value |
| 12 month testing uptake | 0·14 | 0·16 | 0·87 | 0·32 | 0·26 | 1·25 | 1·12 – 1·39 | 0·0003 |
| Men | 0·09 | 0·08 | 1·13 | 0·24 | 0·16 | 1·45 | 1·25 – 1·69 | < 0·0001 |
| Women | 0·19 | 0·22 | 0·86 | 0·39 | 0·34 | 1·15 | 1·03 – 1·28 | 0·013 |
| 36 month testing uptake | Data not available | 0·49 | 0·39 | 1·27 | 1·15 – 1·41 | < 0·0001 | ||
| Men | Data not available | 0·37 | 0·25 | 1·48 | 1·29 – 1·69 | < 0·0001 | ||
| Women | Data not available | 0·59 | 0·50 | 1·17 | 1·07 – 1·29 | 0·0019 | ||
| Sexual risk behaviour | 3·97 | 3·76 | 1·06 | 4·39 | 4·27 | 0·97 | 0·89 – 1·06 | 0·53 |
| Social norms regarding testing | 1·26 | 1·25 | 1·01 | 1·38 | 1·29 | 1·06 | 1·03 – 1·09 | 0·0001 |
| Discussions about HIV | 0·46 | 0·46 | 0·99 | 0·39 | 0·39 | 1·03 | 0·92 – 1·16 | 0·56 |
| Disclosure of HIV status | 0·81 | 0·83 | 0·98 | 0·87 | 0·89 | 0·98 | 0·95 – 1·02 | 0·29 |
| HIV-related stigma | 1·39 | 1·37 | 1·02 | 1·22 | 1·21 | 0·99 | 0·96 – 1·03 | 0·74 |
| Negative life events | 0·30 | 0·29 | 1·01 | 0·31 | 0·30 | 1·02 | 0·87 – 1·20 | 0·80 |
CBVCT: mean outcome in CBVCT communities
SVCT: mean outcome in SVCT communities
Ratio: mean baseline ratio of CBVCT vs. SVCT communities
Effect: intervention effect, increase in mean CBVCT/SVCT ration since baseline (except for testing uptake and disclosure, where the effect is PIA CBVCT/SVCT ratio)
95% CI: 95% confidence interval for intervention effect
p-value: p-value for the hypothesis of no intervention effect on outcome
Testing uptake measured by proportion who reported HIV test; sexual risk behaviour measured by self-reported monthly number of unprotected sexual acts; social norms measured by scores ranging from 0 to 3, higher values corresponding to more favourable social norms; discussions about HIV measured by proportion who reported a discussion in the last month; disclosure of HIV status measured by proportion of tested participants who disclosed their last test result; stigma measured by scores ranging from 0 to 4, higher values corresponding to more stigma; negative life events measured by proportion who reported any events related to partnership break-up, discrimination, estrangement, neglect, or violence.
Annual testing rates in men reached 16% after the intervention period in the SVCT arm, and 24% in the CBVCT arm (Table 7), showing a 45% increase over SVCT arm (95% CI 25%–69%, p<0·0001). At baseline, 12-month testing rates in women (around 20%) were more than twice as large as in men (<10%), probably due to antenatal testing. After the interventions were concluded, testing rates among women increased to 34% (SVCT) and 39% (CBVCT), which corresponds to a 15% intervention effect on testing (p=0·0134). Among the individual sites, only Thailand had a meaningful effect on testing in women. Soweto, South Africa was the only site that did not show a clear effect on testing rates in men (Table S2 in the Online Supplement).
The intervention had a significant effect on community social norms regarding HIV testing (Table 7 and Table S3 in the Online Supplement). The mean social norms score was 6% higher in the CBVCT communities than in the SVCT communities after adjusting for baseline differences (95% CI 3%-9%, p=0·0001). The positive change in social norms was greater in men (8% increase) than in women (4% increase), but the intervention effect was significant in both subgroups.
There was no intervention effect on sexual risk behaviour measured by the number of unprotected sexual acts (Table 7 and Table S4 in the Online Supplement). However, among HIV-infected individuals, a significant reduction of high-risk sexual behaviour was observed in the CBVCT arm. The number of sexual partners of HIV-infected participants was reduced by 8% (95% CI 1–15%, p=0·034) and HIV-infected men reduced the number of partners by 18% (95% CI 5%-28%, p=0·009). Additionally, the proportion of HIV-infected participants reporting multiple partners in the six months prior to interview was 30% lower in the CBVCT communities compared to the SVCT communities (95% CI 8%-46%, p=0·014). This effect was stronger among HIV-infected men: 36% reported multiple partners in the SVCT communities compared to 26% in the CBVCT communities (reduction by 29%, 95% CI 11%-43%, p=0·006). Among HIV-infected women, self-reported multiple partnerships were rare.
The intervention did not affect the proportion of participants who reported having experienced negative life events or having a conversation about HIV in the past six months (Table 7). About 80%–90% of participants who were tested for HIV reported disclosing their status to at least one person and the proportion did not vary by intervention arm. HIV-related stigma also was not affected by the intervention. Baseline mean stigma scores were low (1·37 and 1·39 in the comparison and intervention arm, respectively), with slight declines at PIA (1·21 and 1·22, respectively).
DISCUSSION
Project Accept demonstrated that a multi-component, multi-level social and behavioural intervention can produce modest reductions in HIV incidence, especially among older women. The intervention did not appear to reduce HIV incidence in younger people; the reduction in incidence among the older subgroup (25–32 years, both genders) was more pronounced, but did not reach statistical significance. The 30% reduction in HIV incidence among older women was consistent in nearly all community pairs and was highly significant. The intervention improved HIV testing rates in the peak age range for HIV infection (18 to 32 years), especially among men; increased the number of people who knew their HIV status; and reduced HIV risk behaviours among people with HIV who might otherwise transmit the virus to others. The effectiveness of the intervention was tested among all community residents within the selected age range, rather than only among those subjects who directly participated in intervention activities.
It is not clear why the intervention failed to reduce HIV incidence among younger individuals and why most of the effect was concentrated among older women. We can only speculate as to the reason for lack of statistically significant reduction in HIV incidence, and these might include exclusion of Thailand from the analysis, insufficient penetration of the intervention in key HIV-1 transmission groups and/or the need to provide more services (e.g., active referral to and maintenance in treatment) than was provided in this community intervention. The most important behavioural change occurred among HIV-infected men, who reduced the number of sexual partners and occurrence of multiple partnerships. This change could perhaps protect the primary partners of these men, most likely women older than 25 years. However, a more in-depth analysis is needed to verify this hypothesis. During the course of the trial, ART became widely available at most sites. The increased testing rates in CBVCT communities should have improved treatment coverage and led to a reduction in incidence. However, there may not have been sufficient time to see community-wide reduction in incidence resulting from increased ART uptake. A laboratory assessment is planned to assess ART uptake and to evaluate the possible role of ART in the intervention effect.
This is the first documentation that we could find of programs effective in reaching men, and increasing their HIV testing and reducing their risk behaviour to a greater degree than among women. We believe that the increased testing among men, relative to women, was due to more than the higher baseline among women because of more frequent use of the healthcare system and thus more routine testing. There is a growing literature that men in sub-Saharan Africa are less likely to access testing, and more likely to present for treatment later in their illness and to die sooner from HIV. We believe that a Project Accept model, that takes testing to the individual rather than having the person come to testing, might be important for testing hard-to-reach populations such as men. Reaching men in this way might have been important in the reductions in risk behaviour we observed among HIV+ men in the CBVCT communities.
In Project Accept we demonstrated community-wide effects from an intervention focused on mobilization, testing, and support. The behavioural results were clearly more significant than the declines in HIV incidence. Inclusion of accessible VCT is likely to be a key component of an integrated combination approach to HIV prevention and care. Our results also suggest that community-wide testing plus treatment programs can be both safe and feasible. High testing rates are essential for any prevention strategy to be successful. Project Accept thus sets a benchmark for evaluating the success of ongoing and future combination prevention trials that include a broader range of interventions, including increased provision of treatment for HIV.
This was the first cluster-randomized trial with stigma reduction as a secondary endpoint. However, stigma levels were low at baseline and had little room to decline further, possibly due to social desirability bias. Similarly a recent trial investigating changes in stigma through provision of home-based VCT in Zambia27 showed no effect and observed an overall reduction in stigmatizing attitudes from baseline to follow-up. Further work is needed to adequately measure the impact of stigma reduction efforts.
HIV incidence was estimated from a cross-sectional survey performed at the end of the intervention period using a MAA developed and validated for this purpose. This was a novel approach in HIV prevention research. It was not possible to measure incidence at baseline or to use cohort follow-up to estimate incidence because either of those activities would have interfered with the study intervention. Therefore, we could not adjust for baseline HIV incidence or match the communities on HIV prevalence. Our validation studies suggest that the MAA provided better precision than 6 months of cohort follow-up.22 The validation studies also showed that the MAA had a negligible bias for estimating the intervention effect and provided valid tests and confidence intervals. The incidence estimates we obtained were consistent with those reported in cohort studies performed in regions with similar HIV prevalence.28 Use of ART was addressed by excluding infections from the incidence estimate if samples contained ARV drugs.14
Limitations
With the exception of the KwaZulu-Natal site, the observed HIV incidence and prevalence at the African sites was lower than anticipated. Further, it was unfortunate that the Thailand site had to be excluded due to very low prevalence. Much higher prevalence in Thailand was anticipated, reflecting widespread injection drug use. In some communities, participation rates were lower than desired, possibly influencing outcomes. It would also have been helpful to have full information on the number of tests provided in both the CBVCT SVCT communities, and to be able to track tests in CBVCT and SVCT communities. In South Africa, there were no data on testing in the control communities and, at all sites, we could not gather data on all testing that took place. We had no control over the variety of approaches and alternative opportunities for HIV testing that might have been available, and therefore we had no alternative but to use self-reported testing data to ensure comparability. It should be noted, however, that the self-reports were collected from random probability samples of the community members who may or may not have known that their community was part of a study. The data were collected by assessors who had no knowledge of the participant’s serostatus, thus reducing the potential for self-report bias.
Conclusions
Project Accept demonstrates what can happen when multi-component mobilization, testing, and support services are implemented well. Even if treatment as prevention is proven efficacious, the likelihood of implementing it well in most jurisdictions is low. We believe, as well, that our results are sufficiently robust, especially taking the primary and secondary outcomes together, to recommend a combination of mobilization, mobile testing, post-test support services, and monitoring and evaluation of service providers as routine components of public health practice. The results of Project Accept demonstrate that modest reductions in HIV incidence can be achieved using a multi-component, multi-level social and behavioural intervention alone, in the absence of scale-up of other services and implementation of structural and biomedical interventions. Project Accept also demonstrated an effective method for increasing HIV testing and reducing HIV risk behaviour. High testing rates are essential for any prevention strategy to be successful, and are an essential first step in the implementation of any prevention strategy, especially treatment-as-prevention. Project Accept thus sets a benchmark for evaluating the success of on-going and future combination prevention trials that include a broader range of study interventions, including increased provision of ART. It seems likely that the judicious combination and application of behavioural, social, and biomedical interventions should achieve greater reductions in HIV incidence in entire communities.
Supplementary Material
PANEL: RESEARCH IN CONTEXT.
Systematic review
An extraordinarily thorough systematic review and meta-analysis of community-based voluntary HIV testing and counseling was published in August 2013 by Suthar et al.* For this review, PubMed, clinical trial registries, Embase, and the World Health Organization Global Index Medicus were searched for studies that included community-based HIV testing and counseling (HTC). Both randomized controlled trials and observational studies qualified if they included uptake, proportion receiving their first HIV test, CD4 value at diagnosis, linkage to care, HIV positivity rate, HTC coverage, HIV incidence, and/or cost per person tested as outcomes. Eleven community-based HTC strategies were reviewed. The reviewers then employed the Newcastle-Ottawa Quality Assessment Scale and the Cochrane Collaboration's "risk of bias" tool to assess the risk of bias in studies with a comparator arm included in pooled estimates. They found that 117 studies, which included 864,651 participants completing HTC, met the inclusion criteria.
We also conducted separate searches on each secondary outcome we report in this paper. We searched PubMed for relevant articles on experimental studies, and we placed no restriction on date, or language. We searched the literature for experimental studies using uptake of HIV testing as a primary or secondary outcome, using the following terms (“HIV testing” OR “VCT”) AND (“utilization” OR “uptake”) AND (“trial” OR “intervention”). Seventeen studies (11 randomized trials and six other experimental designs), not including our own study, were found to assess the effect of an intervention on uptake of HIV testing. For experimental studies aiming to effect social norms as related to HIV testing we searched the following terms: (“HIV testing” OR “VCT”) AND (“social norms” OR “community norms” OR “testing norms”) AND (“trial” OR “intervention”). Our trial is the only experimental study we found in the literature, which aimed to change social/community norms related to HIV testing. For experimental studies aiming to affect HIV risk behavior through HIV testing and counseling we searched the following terms: (“HIV testing” OR “VCT”) AND (“HIV risk” OR “risk behaviors”) AND (“trial” OR “intervention”). Nine studies (7 randomized and 2 other experimental designs), not including our own study, were found to assess the effect of HIV testing and counseling on HIV risk behavior. For studies aiming to affect HIV related discussions as related to HIV testing and counseling we searched the following terms (“HIV testing” OR “VCT”) AND (“HIV discussions” OR “HIV communication”) AND (“trial” OR “intervention”). One randomized trial was found to have assessed the effect of HIV testing and counseling on HIV related discussions. For studies aiming to affect HIV stigma as related to HIV testing and counseling we searched the following terms: (“HIV testing” OR “VCT”) AND (“HIV stigma” OR “stigma”) AND (“trial” OR “intervention”). One randomized trial and one experimental study (non-randomized) was found to have assessed the effect of an intervention on HIV stigma as related to HIV testing and counseling.
Interpretation
The reviewers found that mobile HTC uptake among key populations (men who have sex with men, people who inject drugs, female sex workers, and adolescents) ranged from 9% to 100% (among 41,110 participants across studies), with heterogeneity related to how HIV testing was offered. They found that community-based approaches increased HTC uptake (relative risk [RR] 10.65, 95% confidence interval [CI] 6.27–18.08), the proportion of first-time testers (RR 1.23, 95% CI 1.06–1.42), and the proportion of participants with CD4 counts above 350 cells/µl (RR 1.42, 95% CI 1.16–1.74), and obtained a lower positivity rate (RR 0.59, 95% CI 0.37–0.96), relative to facility-based approaches. They found that 80% (95% CI 75%-85%) of 5,832 community-based HTC participants obtained a CD4+ T-cell measurement following diagnosis of HIV, and 73% (95% CI 61%-85%) of 527 community-based HTC participants started antiretroviral therapy following a CD4+ T-cell measurement signifying eligibility. Data on linking HIV-negative participants to prevention services were limited. No studies reported harm as a result of having been tested. In these studies, community-based HTC achieved high rates of HTC uptake, reached people with high CD4+ T-cell counts, and linked people to care. It also obtained a lower HIV positivity rate relative to facility-based approaches. Numerous studies, including Project Accept, have documented substantial benefit when using community-based HCT. None have demonstrated an effect in lowering HIV incidence and few have documented the effects on special populations at risk (eg, men), on HIV risk behavior, and on community social norms regarding HIV. While numerous studies have tested the effectiveness of single interventions on the uptake of HIV testing, few experimental studies have tested the effect of combining social, behavioral and structural interventions to address barriers to HIV testing or change in community norms that lead to decreased HIV transmission.
* Suthar AB, Ford N, Bachanas PJ, et al. Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches. PLOS Medicine. 2013 Aug; 10(8): e1001496.
Acknowledgments
We thank the communities that partnered with us in conducting this research, and all study participants for their contributions. We also thank study staff and volunteers at all participating institutions for their work and dedication.
Funding: This research was sponsored by the U.S. National Institute of Mental Health as a cooperative agreement, through contracts U01-MH066687 (Johns Hopkins University – David Celentano, PI); U01MH066688 (Medical University of South Carolina – Michael Sweat, PI); U01MH066701 (University of California, Los Angeles – Thomas J. Coates, PI); and U01MH066702 (University of California, San Francisco – Stephen F. Morin, PI). In addition, this work was supported as HPTN Protocol 043 through grants U01AI068613/UM1AI068613 (HPTN Network Laboratory – Susan Eshleman, PI); U01AI068617/ UM1AI068617 (SCHARP – Deborah Donnell, PI); and U01AI068619/UM1AI068619 (HPTN Leadership and Operations Center – Sten Vermund/Wafaa El-Sadr, PIs) of the Division of AIDS of the U.S. National Institute of Allergy and Infectious Diseases; and by the Office of AIDS Research of the U.S. National Institutes of Health, and the Division of Intramural Research (Oliver Laeyendecker), National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- ART
Antiretroviral Therapy
- ARV
Antiretroviral Drug
- CI
Confidence Interval
- CBVCT
Community-Based Voluntary Counseling and Testing
- DSMB/SMC
Data and Safety Monitoring Board and Study Monitoring Committee
- HPTN
HIV Prevention Trial Network
- MAA
Multi-Assay Algorithm
- NIMH
US National Institute of Mental Health
- PIA
Post-Intervention Assessment
- RR
Relative risk
- SVCT
Standard Voluntary Counseling and Testing
- VCT
Voluntary Counseling and Testing
NIMH Project Accept (HPTN 043) Study Team
Salim Abdool Karim, MBChB, PhD1
Laurie Abler, MPH2
Christopher Bamanyisa, MA, AD3
Chris Beyrer, MD, MPH4
Adam W. Carrico, PhD5
David D. Celentano, ScD, MHS4
Chonlisa Chariyalertsak, MPHM6
Suwat Chariyalertsak, MD, DrPH6
Alfred Chingono, MSc7
Lillianne Chovenye, MA3
Thomas J. Coates, PhD8
Kathryn Curran, MHS9
Deborah Donnell, PhD10
Susan H. Eshleman MD, PhD11
Agnès Fiamma, MIPH8
Katherine Fritz, PhD, MPH12
Janet Frohlich, Dcur1
Becky Genberg, MPH4
Glenda Gray, MBBCH, FCPaeds(SA)13
Amy Gregowski, MHS12
Harry Hausler, MD, MPH14
Zdenek Hlavka, PhD15
Daniel Hlubinka, PhD15
Nora Margaret Hogan, PsyD3
LeTanya Johnson-Lewis, MT, BS11
Philip Joseph1
Tendayi Jubenkanda, Bsc Hons (Psychology)7
Sebastian Kevany, MPH5
Gertrude Khumalo-Sakutukwa, MSW, MMS5
G.P. Kilonzo, MD, FRCP, Mmed, MBChB, BA3
Michal Kulich, PhD15
Oliver Laeyendecker, PhD, MS, MBA11,16
Tim Lane, PhD, MPH5
Florence P. Lema, MSc, MPH3
Benjamin Link, MPH, MSW4
Tserayi Machinda, BSC Admin ACCA, MBA(wip)7
Suzanne Maman, PhD2
Jessie K. K. Mbwambo, MD3
Nuala McGrath, ScD, MSc, BSc17
James McIntyre, MBChB, MRCOG18
Sakhile Mhlongo13
Joanne Mickalian, MA5
Precious Modiba, MA(SW)13
Simon Morfit, MPH, BA5
Stephen F. Morin, PhD5
Khalifa M. Mrumbi, MSc. PhD3
Marta I. Mulawa, MHS9
Oliver Murima, MSc7
Thulani Ngubani, BTh, Hons1
Audrey Pettifor, PhD, MPH2
Estelle Piwowar-Manning, BS MT(ASCP)SI11
Linda Richter, PhD1
Gavin Robertson, MEd13
Andrew M. Sadowski9
Memory Sendah, MSc7
Basant Singh, PhD, Bsc, Msc9
Kriengkrai Srithanaviboonchai MD, MPH6
Wayne Steward, PhD5
Michael Sweat, PhD9
Greg Szekeres, BA8
Andrew Timbe, Med7
Heidi van Rooyen, PhD1
Surasing Visrutaratna, PhD6
Godfrey Woelk, PhD, MCOMMH, BSc7
Carla E. Zelaya, PhD, MSc4
1 Human Sciences Research Council, South Africa
2 University of North Carolina at Chapel Hill
3 Muhimbili University of Health and Allied Sciences
4 Johns Hopkins Bloomberg School of Public Health
5 University of California, San Francisco
6 Chiang Mai University, Research Institute for Health Sciences
7 University of Zimbabwe
8 University of California, Los Angeles
9 Medical University of South Carolina
10 Fred Hutchinson Cancer Research Center, Statistical Center for HIV/AIDS Research & Prevention
11 Johns Hopkins University School of Medicine
12 International Center for Research on Women
13 University of the Witwatersrand/Chris Hani Baragwanath Hospital
14 TB/HIV Care Association, Cape Town, South Africa
15 Charles University, Faculty of Mathematics and Physics
16 National Institute of Allergy and Infectious Diseases
17 University of Southampton, Southampton, UK
18 Anova Health Institute, South Africa
Footnotes
DISCLAIMER
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health (NIH). Use of trade names is for identification purposes only and does not constitute endorsement by the NIH. Views expressed are those of the authors, and not necessarily those of sponsoring agencies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
PRIOR PRESENTATION
Some of the information in this report was presented at the 20th Conference on Retroviruses and Opportunistic Infections, March 3–6, 2013; Atlanta, GA. Abstract 30.
Conflicts of Interest
We declare that we have no conflicts of interest.
Contributor Information
Thomas J. Coates, University of California, Los Angeles, UCLA Center for World Health, 10833 Le Conte Avenue; CHS 12-105, Los Angeles, CA 90095; USA, +1-310-367-9044, tcoates@mednet.ucla.edu.
Michal Kulich, Charles University, Faculty of Mathematics and Physics, Sokolovska 83, 186 75 Prague 8; Czech Republic.
David D. Celentano, Johns Hopkins Bloomberg School of Public Health, 615 North Wolfe Street, Suite W6041, Baltimore, MD 21205; USA.
Carla E. Zelaya, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, 615 N. Wolfe Street, E6535, Baltimore, MD, 21205; USA.
Suwat Chariyalertsak, Chiang Mai University, Research Institute for Health Sciences, 110 Inthavaroros Road, Maung District, Chiang Mai; Thailand 50200.
Alfred Chingono, University of Zimbabwe, College of Health Sciences, PO Box A178, Avondale, Harare; Zimbabwe.
Glenda Gray, University of the Witwatersrand/Chris Hani Baragwanath Hospital, Faculty of Health Sciences, Perinatal HIV Research Unit, PO Box 114, Diepkloof, Soweto, 1864; South Africa.
Jessie K. K. Mbwambo, Muhimbili University of Health and Allied Sciences, Muhimbili University Teaching Hospital, PO Box 65466, Dar es Salaam; Tanzania.
Stephen F. Morin, University of California, San Francisco, Center for AIDS Prevention Studies, 50 Beale Street, Suite 1300, San Francisco, CA 94105; USA.
Linda Richter, Human Sciences Research Council, Private Bag X07, Dalbridge 4014; South Africa.
Michael Sweat, Medical University of South Carolina, Family Services Research Center, 176 Croghan Spur Road; Suite 104, Charleston, SC 29407; USA.
Heidi van Rooyen, Human Sciences Research Council, 750 Francois Road, Durban; South Africa 4001.
Nuala McGrath, University of Southampton, Southampton General Hospital, Mailpoint 805, South Academic Block, Level C Room AC23, Tremona Road, Southampton, SO16 6YD; UK.
Agnès Fiamma, University of California, Los Angeles, UCLA Center for World Health, 10833 Le Conte Avenue; CHS 12-105, Los Angeles, CA 90095; USA.
Oliver Laeyendecker, Johns Hopkins University School of Medicine, Department of Medicine, And the National Institutes of Health, 855 North Wolfe Street, Rangos Building, Room 538A, Baltimore MD, 21205; USA.
Estelle Piwowar-Manning, Johns Hopkins University School of Medicine, 600 North Wolfe Street; Pathology Room 306, Baltimore, MD 21287; USA.
Greg Szekeres, University of California, Los Angeles, UCLA Center for World Health, 10833 Le Conte Avenue; CHS 12-105, Los Angeles, CA 90095; USA.
Deborah Donnell, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M2-C200, Seattle, WA 98109; USA.
Susan H. Eshleman, Johns Hopkins University School of Medicine, Department of Pathology, 720 Rutland Avenue; Ross Building, Room 646, Baltimore, MD 21205; USA.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994 Nov 3;331(18):1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 4.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003 Sep 13;362(9387):859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum in: PLoS Med. 2006 May;3(5):e298. [Google Scholar]
- 8.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 Feb 24;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 9.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007 Feb 24;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 10.Holtgrave DR, Maulsby C, Wehrmeyer L, Hall HI. Behavioral Factors in Assessing Impact of HIV Treatment as Prevention. AIDS Behav. 2012;16(5):1085–1091. doi: 10.1007/s10461-012-0186-1. [DOI] [PubMed] [Google Scholar]
- 11.Genberg BL, Kulich M, Kawichai S, et al. HIV risk behaviors in sub-Saharan Africa and Northern Thailand: baseline behavioral data from Project Accept. J Acquir Immune Defic Syndr. 2008 Nov 1;49(3):309–319. doi: 10.1097/QAI.0b013e3181893ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011 Jul;11(7):525–532. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khumalo-Sakutukwa G, Morin SF, Fritz K, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008 Dec 1;49(4):422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laeyendecker O, Piwowar-Manning E, Fiamma A, et al. Estimation of HIV incidence in a large, community-based, randomized clinical trial: NIMH Project Accept (HIV Prevention Trials Network 043) PLoS One. 2013 Jul 11;8(7):e68349. doi: 10.1371/journal.pone.0068349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Project Accept (HPTN 043) [(accessed September 15, 2013)]; Web site: http://www.cbvct.med.ucla.edu/
- 16.Maman S, Lane T, Ntogwisangu J, et al. Using participatory mapping to inform a community-randomized trial of HIV counseling and testing. Field Methods. 2009 Nov;21:368–387. doi: 10.1177/1525822X09341718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirowodza AC, Sikotoyi SV, Joseph P, et al. Using community ethnography and geographical information systems in a community based intervention trial in Vulindlela, South Africa (Project Accept-HPTN 043) J Community Psychol. 2009;37(1):41–57. doi: 10.1002/jcop.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chingono A, Lane T, Chitumba A, Kulich M, Morin S. Balancing science and community concerns in resource-limited settings: Project Accept in rural Zimbabwe. Clin Trials. 2008;5(3):273–276. doi: 10.1177/1740774508091576. [DOI] [PubMed] [Google Scholar]
- 19.Kevany S, Khumalo-Sakutukwa G, Murima O, et al. Health diplomacy and adapting global health interventions to local needs: findings from project accept (HPTN 043), a community-based intervention to reduce HIV incidence in populations at risk in Sub-Saharan Africa and Thailand. BMC Public Health. 2012 Jun 20;12:459. doi: 10.1186/1471-2458-12-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedrow VA, Zelaya CE, Kennedy CE, et al. No "magic bullet": exploring community mobilization strategies used in a multi-site community based randomized controlled trial: Project Accept (HPTN 043) AIDS Behav. 2012 Jul;16(5):1217–1226. doi: 10.1007/s10461-011-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawichai S, Celentano D, Srithanaviboonchai K, et al. NIMH Project Accept (HPTN 043) HIV/AIDS community mobilization (CM) to promote mobile HIV voluntary counseling and testing (MVCT) in rural communities in northern Thailand: Modifications by experience. AIDS Behav. 2012 Jul;16(5):1227–1237. doi: 10.1007/s10461-011-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laeyendecker O, Kulich M, Donnell D, et al. Development of methods for cross-sectional incidence HIV estimation in a large community-randomized trial. PLoS One. 2013 doi: 10.1371/journal.pone.0078818. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genberg BL, Hlavka Z, Konda KA, et al. A comparison of HIV/AIDS-related stigma in four countries: negative attitudes and perceived acts of discrimination towards people living with HIV/AIDS. Soc Sci Med. 2009 Jun;68(12):2279–2287. doi: 10.1016/j.socscimed.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelaya CE, Sivaram S, Johnson SC, et al. HIV/AIDS stigma: reliability and validity of a new measurement instrument in Chennai, India. AIDS Behav. 2008;12(5):781–788. doi: 10.1007/s10461-007-9331-7. [DOI] [PubMed] [Google Scholar]
- 25.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28(2):319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 26.UNAIDS Reference Group on Estimates, Modeling, and Projections: Statement on the use of the BED assay for estimation of HIV-1 incidence or epidemic monitoring. Weekly Epidemiol Rec. 2006;81:33–40. [PubMed] [Google Scholar]
- 27.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Group ZS. Effects of home-based voluntary counselling and testing on HIV-related stigma: findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81:18–25. doi: 10.1016/j.socscimed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Rehle T, Shisana O, Pillay V, et al. National HIV incidence measures--new insights into the South African epidemic. S Afr Med J. 2007 Mar;97(3):194–199. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.