Abstract
Background
There is a need for safe, inexpensive and effective psoriasis therapies. Many anecdotal accounts of patients’ successful treatment with the alternative medicine curcumin exist.
Objective
To determine the safety and efficacy of oral curcumin in psoriasis patients.
Methods
A phase II, open-label, Simon’s two-stage trial of 4.5g/d of oral Curcuminoid C3 Complex® in plaque psoriasis patients. Endpoints included improvement in Physicians Global Assessment, Psoriasis Area and Severity Index, and safety endpoints throughout the study.
Results
The intention to treat analysis response rate was 2/12 (95% CI: 2%, 48%) and both responders achieved a PASI 75. There were no study-related adverse events that necessitated subject withdrawal.
Limitations
Small sample size and lack of placebo group.
Conclusion
The response rate was low and possibly due to a placebo effect or the natural history of psoriasis. Large placebo-controlled studies are necessary prior to recommending oral curcumin as a psoriasis treatment.
INTRODUCTION
Psoriasis is a common chronic inflammatory disease of the skin and joints, which affects about 2% of the general population1. Severe psoriasis is associated with significant decrements in health-related quality of life2, multiple co-morbidities3, and increased cardiovascular risk4 and mortality5. Treatment options for severe psoriasis are either time consuming (as in the case of UVB or PUVA therapy) or have the potential for organ toxicity with chronic use (methotrexate, acitretin, cyclosporine)1. Newer biologic therapies (infliximab, etanercept, adalimumab, efalizumab and alefacept) are immunosuppressive and theoretically could increase the risk of infections and malignancies with long-term use, and are limited by their high cost6, 7. Despite the advent of multiple systemic therapy options for severe psoriasis, many patients with this disease are unable to achieve effective long-term control8, 9. Given the chronic nature of psoriasis and the need for long-term treatment, there exists an unmet need for effective, non-toxic therapies that are also convenient and affordable.
Given the limitations of traditional pharmacological approaches in the treatment of psoriasis, patients frequently turn to complementary and alternative medicine therapies (CAM) to manage their disease. It is estimated that 51% of psoriasis patients use CAM to treat their skin despite limited or no scientific data on the safety and efficacy of these treatments10. Curcumin, (the active component of the Indian spice turmeric) is a CAM therapy that has been successfully used to treat psoriasis based on anecdotal reports11–13. A strong scientific rationale suggests that curcumin may in fact be promising for the treatment of psoriasis. In vitro and animal studies have demonstrated the inhibitory effect of curcuminoids (term interchangeable with curcumin) on immune pathways critical to the pathophysiology of psoriasis such as NFκB (Nuclear factor kappa B)14–18 and downstream, inflammatory gene products such as Th-1 type cytokines (i.e., TNF-α , IFN γ,) (45, 97–112)19–22. Furthermore, clinical trials of curcumin have been conducted for a variety of indications and it has been shown to be safe in oral doses of up to 12 grams23.
Based on the physiological effects of curcumin and the positive anecdotal reports of its benefit for psoriasis, we conducted a prospective, open label, clinical trial to assess the safety and efficacy of oral curcumin in the treatment of chronic psoriasis vulgaris.
METHODS
Study Patients
The institutional review board of the University of Pennsylvania approved the protocol and all patients gave written informed consent before any study-related procedures were performed. The study was conducted in accordance with the Declaration of Helsinki and was registered at ClinicalTrials.gov prior to study initiation. Patients were eligible if they were at least 18 years of age, had active but clinically stable plaque psoriasis that involved at least 6 percent of the body-surface area and was of moderate plaque thickness as defined by a thickness score of 2 on the Psoriasis Area Severity Index (PASI). Patients were included if they were using a medically acceptable method of contraception throughout the entire study period. Patients with guttate, erythrodermic, or pustular psoriasis were excluded as were patients who used systemic treatments for psoriasis (including methotrexate, cyclosporine, alefacept, adalimumab, efalizumab, infliximab, etanercept, etretinate, systemic steroids and PUVA) within 3 months prior to day 0 or at any time during the study. Patients were excluded if they had used topical treatments or phototherapy for their psoriasis within 14 days prior to day 0 or at any time during the study. Patients who were pregnant or nursing a child, had clinically significant laboratory abnormalities at screening or had significant uncontrolled comorbidities were excluded from the study. Subjects for whom the dose of clonidine, digoxin, beta-blockers, lithium, or anti-malarials had changed in the past month prior to enrollment were excluded from the study. Enrolled subjects were required to avoid prolonged exposure to sun or UV light and discontinue non-medicated emollients and medicated psoriasis shampoos 24 hours before each study visit.
Study Drug
Curcuminoid C3 Complex® was provided by the Sabinsa Corporation (Piscataway, NJ) in capsules that contain 95% curcuminoids. Patients were given 500 mg capsules and were instructed to take three capsules three times a day by mouth.
Study Design
This was a phase II, single arm, single-dose, non-controlled, open label, modified Simon’s two-stage clinical trial of 4.5g/d Curcuminoid C3 Complex® administered orally in patients with chronic plaque psoriasis. The study took place at the University of Pennsylvania, Department of Dermatology in Philadelphia, PA. During this 16 week trial, subjects were treated with of 4.5g/day Curcuminoid C3 Complex® (3 pills of 500mg, three times daily) for the first 12 weeks followed by a 4 week observation period after discontinuing the study drug. Histological confirmation of psoriasis vulgaris was obtained for all enrolled subjects. Patients were seen at a screening visit and then at baseline and weeks 2, 4, 8, 12 and 16 for safety and efficacy evaluations. The first subject was enrolled in January 25, 2006 and all study procedures were concluded on May 22, 2007.
Efficacy End Points
The primary measure of efficacy was the proportion of patients who were classified as a responder using the Physician’s Global Assessment (PGA) of Change at week 12. A responder was defined as achieving a rating of good (50–74% improvement), excellent (75–99% improvement) or cleared (100% improvement) on the PGA compared to baseline. When necessary, baseline photographs were used in comparison to the current clinical examination in order to assess the PGA. Secondary endpoints included Psoriasis Area and Severity Index (PASI) scores, and health-related quality of life as measured by the Skindex 29 24, 25. Other outcome measures include PASI 75 and PASI 50 which correspond to 75% and 50% improvements in PASI scores from baseline, respectively and have been shown to represent a meaningful endpoint in psoriasis clinical trials26.
Safety Endpoints
All patients who received at least one dose of study drug were included in the safety analysis. Safety data was obtained by patient interview (and examination if necessary) at all study visits and by collecting laboratory data on blood count, serum chemistries, and liver function tests at week 4 and week 12 visits.
Statistical Analysis
To improve the efficiency of this early phase II study the investigators utilized a Simon’s two-stage design in which a planned interim analysis was preformed to determine if there was sufficient efficacy to warrant enrollment of additional subjects. The following assumptions were made: First, we predicted that at least 50% of patients would achieve a response as defined above. Second, we determined that a response rate of 20% or less would not be promising for clinical use and that further study of oral Curcuminoid C3 Complex® at the doses used in this protocol would be abandoned. This lower limit of efficacy is similar to what is seen in placebo controlled trials27. Third, we assumed a Type I error (significance level) of 0.05 and a type II error of 0.20. Thus, if the true response rate is less then 20%, the probability of recommending further investigation is limited to 5%, and if the true response rate is at least 50%, the probability of recommending further study is 80%.
The sample size calculations were preformed using a program downloaded from the NIH website (http://linus.nci.nih.gov/~brb/Opt.htm). The first stage of the trial enrolled 8 subjects. According to the parameters defined above, if 3 or more of the 8 subjects enrolled were classified as responders at week 12, this would justify enrolling an additional 10 subjects for stage 2.
The primary analysis included all subjects who were enrolled in the trial and received any study drug (intention to treat). Subjects who withdrew from the study were classified as non responders and PASI and Skindex scores were treated as the last outcome carried forward when available. . If no follow up data was obtained because the subject withdrew prior to these measurements, the subject was classified as having no change in baseline PASI and Skindex scores. A secondary as treated analysis (e.g. per protocol) was performed in subjects who completed the study up to week 12 and had taken at least 85% of their curcumin dose based on pill counts. The decision to continue or stop the trial after the first stage was determined by subjects who were compliant with all study procedures up to week 12 (e.g., per protocol analysis, selected as primary for the starting/stopping rules in the context of this early stage, exploratory study). Subjects were classified as responders based on week 12 PGA scores and a response rate was calculated with 95% exact confidence intervals. Median PASI and Skindex-29 scores were calculated at baseline and week 12 including interquartile ranges (IQR) and comparisons were tested with a Wilcoxon Rank-Sum for unpaired data and a Wilcoxon Signed Rank for paired data. Data analysis was performed using STATA version 10.
RESULTS
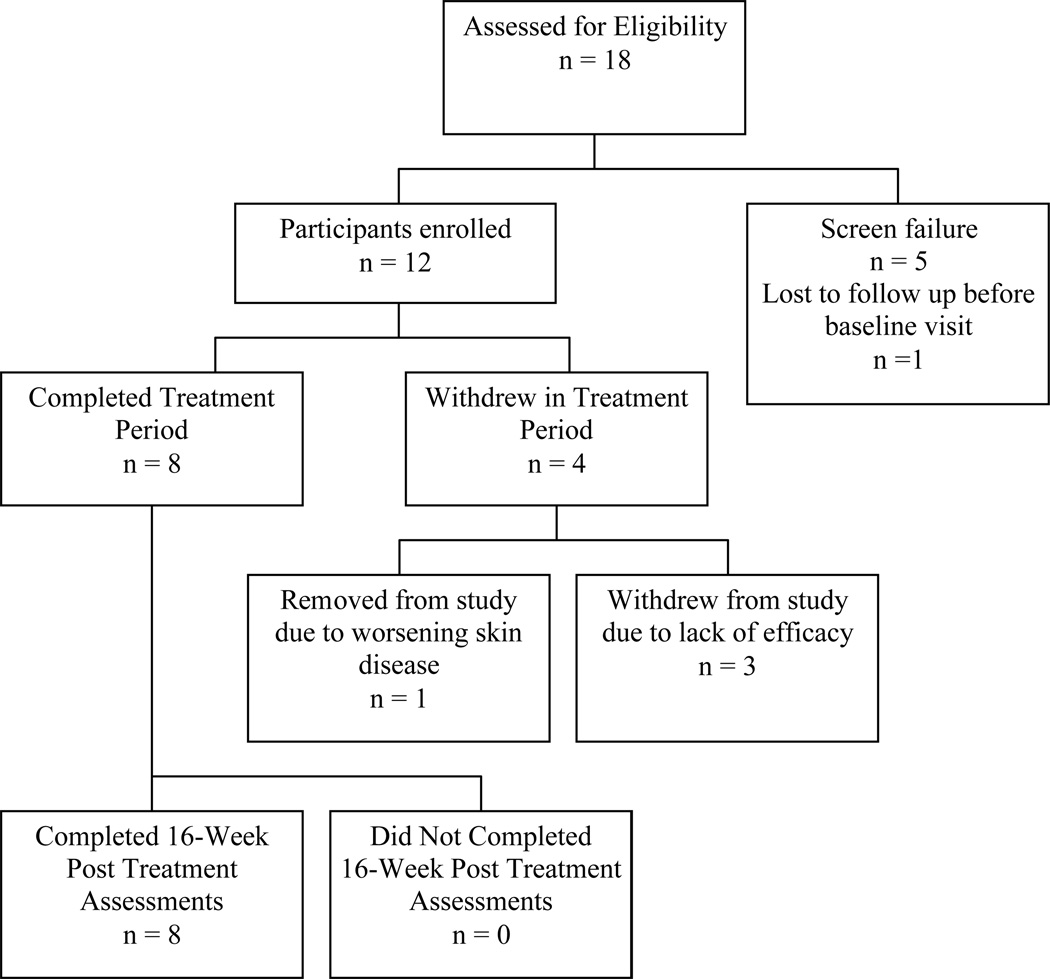
A total of 18 subjects were screened, 12 of whom were enrolled and received the investigational drug at day 0. Of the five subjects who did not receive drug, three were excluded because they did not have ≥6% of their body surface area covered with psoriasis and another subject was excluded because of anemia. One subject who was eligible for the study was lost to follow up before receiving any study drug. Eight subjects completed the trial up to week 16. Four of the 12 enrolled subjects did not complete the trial; one was withdrawn by the investigators due to worsening of her psoriasis and three withdrew prior to week 12 because of lack of efficacy (Figure 1). Subjects were instructed to take 3 pills 3 times a day for a total of 756 pills over 12 weeks. All subjects who completed the trial were at least 85% compliant with the treatment regimen as determined by patient diaries, patient interview, and pill counts and the median number of pills missed was 15 (IQR 0, 47.5). Although widely accepted, these clinical methods for measuring compliance may overestimate actual compliance. Subjects who failed to complete the trial had similar degrees of psoriasis severity as measured by PASI compared to patients who completed the trial, but non-completers had more impairment in health-related quality of life at baseline as measured by Skindex-29 (P= 0.04). Descriptive statistics and baseline data for enrolled subjects are summarized in Table 1.
Figure 1.
Flowchart of Subject Enrollment
Table 1.
Baseline Demographic Information
| Subjects | Age (median, IQR) |
Sex (N, %) male |
Median (IQR)# of prior systemic agents/phototherapy used |
Race | Baseline PASI (median, IQR) |
Baseline Skindex (median IQR) |
|---|---|---|---|---|---|---|
| Completed trial | ||||||
| N=8 | 50.5 | N=7 | 1.5 (1, 2.5) | N=7 White | 13.7 | 34.6 |
| (45, 55) | (87.5%) | N=1 Asian | (9.7, 17.2) | (18.5, 50.9) | ||
| male | ||||||
| Did not complete trial | ||||||
| N=4 | 50 | N=2 | 1.5 (0.5, 2.5) | N=2 White | 14.6 | 63.2 |
| (38.5, 62.5) | (50%) | N=1 Black | (5.4, 8.3) | (52, 79.9) | ||
| male | N=1 Other | |||||
EFFICACY AND QUALITY OF LIFE ENDPOINTS
Intention-to-treat analysis, in which all subjects who withdrew from the trial were classified as non-responders, showed a response rate of 16.7% (95% CI: 2%, 48%). The secondary as treated analysis, which included only subjects who competed the trial up to week 12, had a response rate of 25% (95% CI: 3, 65%). The study was terminated based on lack of sufficient evidence of efficacy as only 2 subjects who completed the trial achieved a response at week 12,.
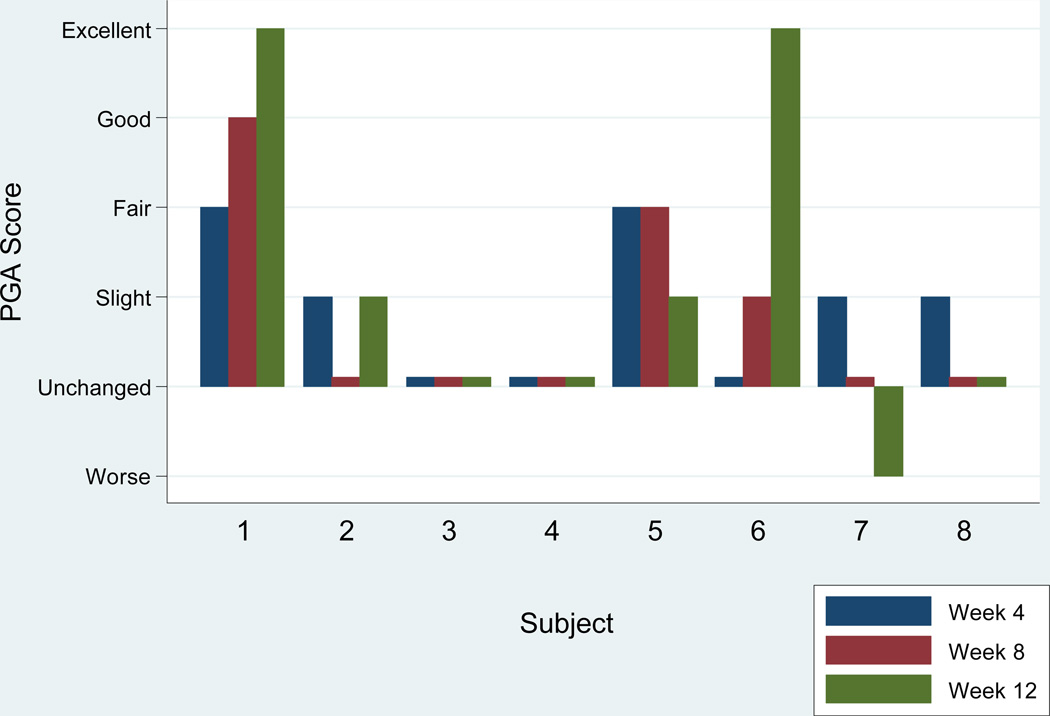
These two subjects who were classified as responders achieved a score of excellent on the PGA and were seen during winter months and were not exposed to sunlight during the trial based on patient report and physical examinations. No patients received a score of “cleared,” “good” or “fair” at week 12 while two subjects received a PGA score of “slight,” three of “unchanged,” and one of “worse” at week 12. Both responders achieved a PASI 75 at week 12, while no other subjects achieved a PASI 75 or a PASI 50. Four weeks after discontinuation of curcumin therapy the responders maintained an excellent response based on PGA and PASI 75 at week 16. In those subjects who completed the trial, the median Skindex 29 score was reduced by 0.35 (IQR 5.5, 5.0) (a lower score signifies improvement in quality of life). In subgroup analysis, the two responders had a median reduction in Skindex scores of 16 (IQR 5.1, 26.9) (n=2) whereas non-responders had a median increase (worsening) in Skindex scores of 2.5 (IQR 0, 5.6) (n=6).
SAFETY ENDPOINTS
Ten out of 12 patients who received study drug reported an adverse event for a total of 18 adverse events. The adverse events that were possibly related to the study drug were all mild and were either gastrointestinal upset or heat intolerance/hot flashes. Other adverse events that were mild to moderate in intensity and judged unlikely to be related to the study drug were respiratory (n=2), musculoskeletal (n=3), and neurological (n=1) in nature. One subject experienced worsening of her psoriasis at week 2 and developed near erythrodermic psoriasis prior to week 4. The patient had previously used extensive topical steroids which were discontinued 2 weeks prior to the start of the study drug. The investigator withdrew the patient so she could be treated with standard of care. Another subject underwent a kidney stone ablation procedure while enrolled in the study. The stone was diagnosed prior to the subject’s enrollment in the study and the subject experienced the only serious adverse event when during a transurethral ablation procedure she experienced hypertension and respiratory distress and was hospitalized overnight for monitoring. Her symptoms resolved completely and she was discharged the following day. She was also the only subject to experience a significant laboratory aberration, which was a mild elevation in her liver enzymes at week 12 (AST 54, ALT 59) from baseline levels (AST 26, ALT 31) and the elevation in liver enzymes persisted at week 16 (AST 40, ALT 45).
DISCUSSION
As expected, 4.5g per day of oral curcumin was well tolerated and safe in subjects with psoriasis. All adverse events possibly related to the study drug were mild in nature and limited to gastrointestinal upset and heat intolerance or hot flashes. No subjects were removed from the study due to study-related adverse events.
The efficacy of the study drug was low with an intention to treat response rate of 16.7% (95% CI: 2%, 48%).and did not justify continuation of the study based on our a priori termination rules. However, those who did respond achieved excellent responses of 83% and 88% improvement in PASI scores at week 12. The confidence interval for the 16.7% response rate is wide due to the small sample size and therefore we cannot exclude the possibility that the response observed is due to a placebo effect or the natural history of skin disease in these subjects rather than efficacy of the drug itself. The lack of any evidence of meaningful response (e.g., PASI 50) in all other subjects argues that either the true response rate is very low, limited to a small subset of psoriasis patients, or not due to curcumin but rather other factors which cannot be accounted for in an uncontrolled study.
Although in vitro curcumin has been shown to block pathways necessary to develop psoriasis, it is possible that oral administration will not produce a desired clinical effect due to low bioavailability. Orally administered curcumin has been shown to have low bioavailability in both animals and humans28, 29. This relatively low bioavailability can be explained by the observation that curcumin is extensively reduced and conjugated in the intestinal tract. Animal experiments suggest that independent of dose, 40–90% of orally administered curcumin is excreted in stool30. We administered the highest dose of oral curcumin (e.g. 4.5 grams) we felt would be acceptable to patients based on the number and size of pills they would be required to swallow as well as recommendations from previous phase I trials31. This dose is substantially higher than what is achieved in diets high in turmeric which corresponds to 60–100mg of curcumin32. It is possible that administering curcumin at higher doses or combining oral curcumin with agents which may enhance its absorption may be result in better efficacy. Doses of curcumin of up to 8 grams daily for up to 12 months have been safe in humans and therefore, higher doses may be considered for future studies23. Furthermore, new liposomal formulations of curcumin may enhance oral bioavailability and should be considered for future trials33. Finally, curcumin may have efficacy when applied topically as demonstrated in one trial, however, it stains the skin yellow and therefore may not be acceptable to patients34.
In conclusion, oral curcumin was well tolerated by patients with psoriasis. The overall response rate was low and we cannot rule out that the responses were due to a placebo effect or a natural disease remission. Nevertheless, excellent responses were observed in two patients and therefore, large, placebo controlled trials will be necessary to definitively prove or disprove oral curcumin as a potential therapeutic agent for psoriasis. For example, a placebo-controlled trial would need to enroll 254 subjects in order to have statistical power of 80% to differentiate the response rate observed in our study from an expected PASI75 response rate of 5% in the placebo group[DoD1]. Until such trials are performed, oral curcumin should not be recommended for the treatment of psoriasis given lack of proven efficacy. The results of our study further emphasize the need for rigorous prospective studies in assessing treatments for psoriasis.
Figure 2. PGA Score by Month.
PGA scores by month for 8 subjects who completed the trial. Week 12 PGA scores of “Good,” “Excellent” and “Cleared” are classified as responders.
Table 2.
Summary of Efficacy Endpoints at week 12*
| Efficacy Outcome | Results of subjects who completed trial (n=8) |
Results of intention to treat analysis (n=12)* |
|---|---|---|
| Response rate based on achieving at least a PGA of “good” | 25%, 95% CI (3%, 65%) | 16.7%, 95% CI (2%, 48%) |
| PGA at week 12 (median, IQR) | Unchanged-Slight improvement (Unchanged, Fair-Good) |
Unchanged (Worse-Unchanged, Slight Improvement) |
| PASI 75 at week 12 | 25%, 95% CI (3%, 65%) | 16.7%, 95% CI (2%, 48%) |
| Change in PASI (baseline-week 12) (median, IQR) | 5.4 (0.65, 7.6) p= 0.04 | 0.65 (−1.25, 6.5) p=0.26 |
| Change in Skindex**(median, IQR) | 0.35 (IQR −5.5, 5.0) p= 0.9 | 0.0 (−2.55, 4.95) p=0.63 |
data taken from latest visit and carried forward for subjects who withdrew.
positive values indicate an increase in Skindex scores, suggesting a decrease in QOL
Acknowledgments
Funding Source/Role of Sponsors: Supported by departmental funds from the Hospital of the University of Pennsylvania Department of Dermatology, NIH/NIAMS grant K23AR051125 (JMG), NIH/NCCAM Ruth L. Kirschstein National Research Service Award T32-AT00600 (NS), matching funds University of Pennsylvania School of Medicine (NS), NIH K30 Center for Clinical Epidemiology and Biostatistics institutional funds (NS), contributions from various pharmaceutical manufacturers to the pharmacoepidemiology training program at the Center for Clinical Epidemiology and Biostatistics of the University of Pennsylvania (SKK), the Doris Duke Charitable Foundation (SKK) and the Clinical and Translational Research Center (CTRC) (Research Grant UL1RR024134 from the National Center For Research Resources) at the University of Pennsylvania. Study drug was provided by the Sabinsa Corporation in Piscataway, NJ.
Footnotes
Financial Disclosures/ Conflicts of Interest (Potentially Relevent): Dr. Gelfand has received grant support from Biogenidec, AMGEN, Astellas, and Centocor. He has been a consultant for Genentech, Novartis, Warner-Chilcott, AMGEN, Centocor, and Wyeth. Ms. Kurd is supported by contributions from various pharmaceutical manufacturers to the pharmacoepidemiology training program at the Center for Clinical Epidemiology and Biostatistics of the University of Pennsylvania and the Doris Duke Charitable Foundation. Dr. Badmaev is an employee of Sabinsa Corporation. Dr. Van Voorhees has received grant support from Amgen, Astellas, and Genentech and has been a consultant for Abbott, Amgen, Centocor, Genentech, and Warner-Chilcott.
References
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51:704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Kurd S, Lewis J, Troxel A, et al. Patients with severe psoriasis have an increased risk of mortality. Journal of Investigative Dermatology. 2007;127:S54. [Google Scholar]
- 6.Kurd S, Richardson S, Gelfand J. An Update on the Epidemiology and Systemic Treatment of Psoriasis. Expert Review of Clinical Immunology. 2007;3:171–185. doi: 10.1586/1744666X.3.2.171. [DOI] [PubMed] [Google Scholar]
- 7.Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581–588. doi: 10.1056/NEJM199503023320907. [DOI] [PubMed] [Google Scholar]
- 8.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–139. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM. Long-term treatment for severe psoriasis: we're halfway there, with a long way to go. Arch Dermatol. 2007;143:1191–1193. doi: 10.1001/archderm.143.9.1191. [DOI] [PubMed] [Google Scholar]
- 10.Fleischer AB, Jr, Feldman SR, Rapp SR, Reboussin DM, Exum ML, Clark AR. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216–220. [PubMed] [Google Scholar]
- 11.Turmeric Eases Suffering From Psoriasis. Peoples Pharmacy. Available at: http://www.healthcentral.com/peoplespharmacy/408/60710.html.
- 12.Turmeric or curcumin for psoriasis? http://www.psoriasis.org/forum/archive/index.php/t-15803.html.
- 13.Turmeric eases psoriasis. The Peoples Pharmacy. Available at: http://www.buffalonews.com/opinioncolumns/columns/otherlife/story/191877.html.
- 14.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 15.Brennan P, O'Neill LA. Inhibition of nuclear factor kappaB by direct modification in whole cells--mechanism of action of nordihydroguaiaritic acid, curcumin and thiol modifiers. Biochem Pharmacol. 1998;55:965–973. doi: 10.1016/s0006-2952(97)00535-2. [DOI] [PubMed] [Google Scholar]
- 16.Han SS, Keum YS, Seo HJ, Surh YJ. Curcumin suppresses activation of NF-kappaB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J Biochem Mol Biol. 2002;35:337–342. doi: 10.5483/bmbrep.2002.35.3.337. [DOI] [PubMed] [Google Scholar]
- 17.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 18.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 19.Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 20.Jang MK, Sohn DH, Ryu JH. A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-alpha release from Curcuma zedoaria. Planta Med. 2001;67:550–552. doi: 10.1055/s-2001-16482. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda H, Tewtrakul S, Morikawa T, Nakamura A, Yoshikawa M. Anti-allergic principles from Thai zedoary: structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem. 2004;12:5891–5898. doi: 10.1016/j.bmc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Gulcubuk A, Altunatmaz K, Sonmez K, et al. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49–54. doi: 10.1111/j.1439-0442.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 23.Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 25.Abeni D, Picardi A, Pasquini P, Melchi CF, Chren MM. Further evidence of the validity and reliability of the Skindex-29: an Italian study on 2,242 dermatological outpatients. Dermatology. 2002;204:43–49. doi: 10.1159/000051809. [DOI] [PubMed] [Google Scholar]
- 26.Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol. 2004;50:859–866. doi: 10.1016/j.jaad.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 28.Grant KL, Schneider CD. Turmeric. Am J Health Syst Pharm. 2000;57:1121–1122. [PubMed] [Google Scholar]
- 29.Ireson CR, Jones DJ, Orr S, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 30.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 31.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 32.Plus M. Turmeric (Curcuma longa Linn.) and Curcumin. Drugs and Supplements. Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-turmeric.html.
- 33.Huang MT, Ma W, Lu YP, et al. Effects of curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion. Carcinogenesis. 1995;16:2493–2497. doi: 10.1093/carcin/16.10.2493. [DOI] [PubMed] [Google Scholar]
- 34.Heng MC, Song MK, Harker J, Heng MK. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937–949. doi: 10.1046/j.1365-2133.2000.03767.x. [DOI] [PubMed] [Google Scholar]