Abstract
Being an atomically thin material, graphene is known to be extremely susceptible to its environment, including defects and phonons in the substrate on which it is placed as well as gas molecules that surround it. Thus, any device design using graphene has to take into consideration all surrounding components, and device performance needs to be evaluated in terms of environmental influence. However, no methods have been established to date to readily measure the density and distribution of external perturbations in a quantitative and non-destructive manner. Here, we present a rapid and non-contact method for visualizing the distribution of molecular adsorbates on graphene semi-quantitatively using terahertz time-domain spectroscopy and imaging. We found that the waveform of terahertz bursts emitted from graphene-coated InP sensitively changes with the type of atmospheric gas, laser irradiation time, and ultraviolet light illumination. The terahertz waveform change is explained through band structure modifications in the InP surface depletion layer due to the presence of localized electric dipoles induced by adsorbed oxygen. These results demonstrate that terahertz emission serves as a local probe for monitoring adsorption and desorption processes on graphene films and devices, suggesting a novel two-dimensional sensor for detecting local chemical reactions.
While the highly tunable conductivity of charge carriers through gating is one of the attractive features of graphene1, it also means that the conductivity of graphene can be adversely affected by accidental doping in a rather uncontrolled fashion. It has been shown that adsorption of environmental gas molecules (even a single molecule) can drastically affect graphene's DC2 and optical3 conductivities. For detection and identification of molecular adsorbates on graphene, it is necessary to develop a versatile and convenient method that probes local conductivity or doping but does not require the fabrication of electrical contacts. Raman spectroscopy is one of the most useful tools for characterizing basic properties of graphene samples4, including the defect density, the degree of strain, and the number of layers. However, it does not provide direct information on the effect of molecular adsorbates, and their spatial distribution, on the conductivity.
In the present study, we demonstrate visualization of the distribution of local molecular adsorbates on graphene using terahertz (THz) emission from a graphene-coated semiconductor surface. Specifically, we placed a large-area graphene film, grown by chemical vapor deposition5, on the (100) surface of InP, which is known to be an efficient source of coherent THz waves when excited by a femtosecond optical pulse6; see Fig. 1a for the experimental geometry. The waveform of the emitted THz burst sensitively changes due to the charge transfer between graphene and adsorbed molecules. According to classical electromagnetic theory, the electric field (E) of the generated THz wave is given by the time derivative of the transient current (J) induced by the optical pulse excitation, i.e., ETHz ∝ ∂J/∂t7. This equation indicates that the value and direction of the photoexcited current can be measured through the amplitude and polarity of the THz waveform, which are affected by the presence of molecular adsorbates.
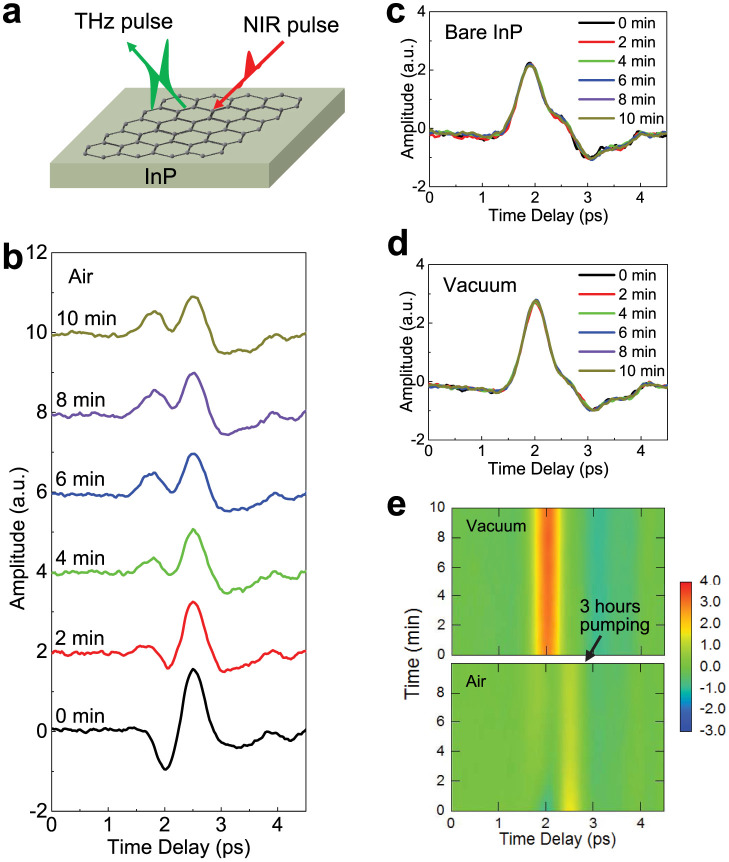
Figure 1. Time-domain waveforms of terahertz radiation emitted from graphene-coated InP under different conditions.
(a), A schematic diagram of the experimental geometry. (b), Time evolution of THz waveforms from graphene-coated InP under continuous excitation by femtosecond near-infrared pulses from a Ti:sapphire laser. The curves are intentionally shifted vertically. (c), THz waveforms from InP (no graphene) under continuous excitation by femtosecond near-infrared pulses from a Ti:sapphire laser. The waveform remains the same even after ten minutes of continuous excitation. (d), THz waveforms from graphene-coated InP in vacuum under continuous excitation by femtosecond near-infrared pulses from a Ti:sapphire laser The waveform remains the same even after ten minutes of continuous excitation. (e), Time evolution of THz waveforms from graphene-coated InP under continuous excitation by femtosecond near-infrared pulses from a Ti:sapphire laser. The vacuum pump is turned on at 10 minutes and the waveform changes drastically.
Results
Figure 1b shows how the THz waveform evolves in time under continuous excitation by femtosecond optical pulses from a Ti:sapphire laser, which remove gas molecules from graphene. Initially, there is a dip at ~2.0 ps and a peak at ~2.5 ps. After ~4 minutes of Ti:sapphire excitation, the 2 ps dip vanishes, and a new positive peak appears at ~1.8 ps; at the same time, the amplitude of the 2.5 ps peak gradually decreases during 10 minutes of excitation, and the waveform does not change further. However, the waveform recovers its initial shape after the sample is exposed to air for a few hours without laser excitation, i.e., re-adsorption occurs. On the other hand, the waveform of THz radiation from a bare InP surface remains unchanged with time, as shown in Fig. 1c. Furthermore, the waveform of THz radiation from the graphene-coated InP (100) is also stable in time if the sample is placed in vacuum (~10−5 Torr), as demonstrated in Fig. 1d. Finally, Fig. 1e shows dramatic time evolution of THz radiation from graphene-coated InP. The vacuum pump is turned on at 10 minutes, and the 2 ps feature suddenly changes its sign. All these results indicate that the change in the THz waveform with time arises from adsorption and desorption of atmospheric gas molecules on graphene induced by femtosecond laser excitation.
In order to provide more insight into the origin of the THz waveform changes due to molecular adsorption and desorption, we performed THz emission experiments under various conditions. Figures 2a, 2b, and 2c show the time evolution of the waveform of THz radiation from graphene-coated InP (100) in air, vacuum, and oxygen, respectively. Figure 2d shows the case where the sample was baked at 120°C under vacuum for 1.5 hours to remove residual water at the interface between graphene and InP8,9 prior to exposing to oxygen. Before introducing gases, we kept the sample in a vacuum of 10−5 Torr. In all cases, after 10 minutes of THz emission measurement (Region I), we illuminated the sample by ultraviolet (UV) light (365 nm) for 5 minutes (Region II), motivated by the idea that UV illumination promotes oxidation of graphene10,11,12,13. In Region I, the THz waveforms from all samples are unchanged in different environmental gases and show similar characteristics, i.e., only the first peak can be seen. This result indicates that gas molecule adsorption dose not proceed much during 10-minute exposure to gases once molecules are desorbed through vacuum pumping. Indeed, drastic changes are seen as a result of UV illumination, especially in air (Fig. 2a) and oxygen (Fig. 2c); the first peak at 2.0 ps suddenly becomes negative and the second peak at 2.5 ps suddenly appears upon UV illumination. On the other hand, little change is observed in vacuum (Fig. 2b).
Figure 2. Time evolution of THz emission from graphene-coated InP in different environmental gases.
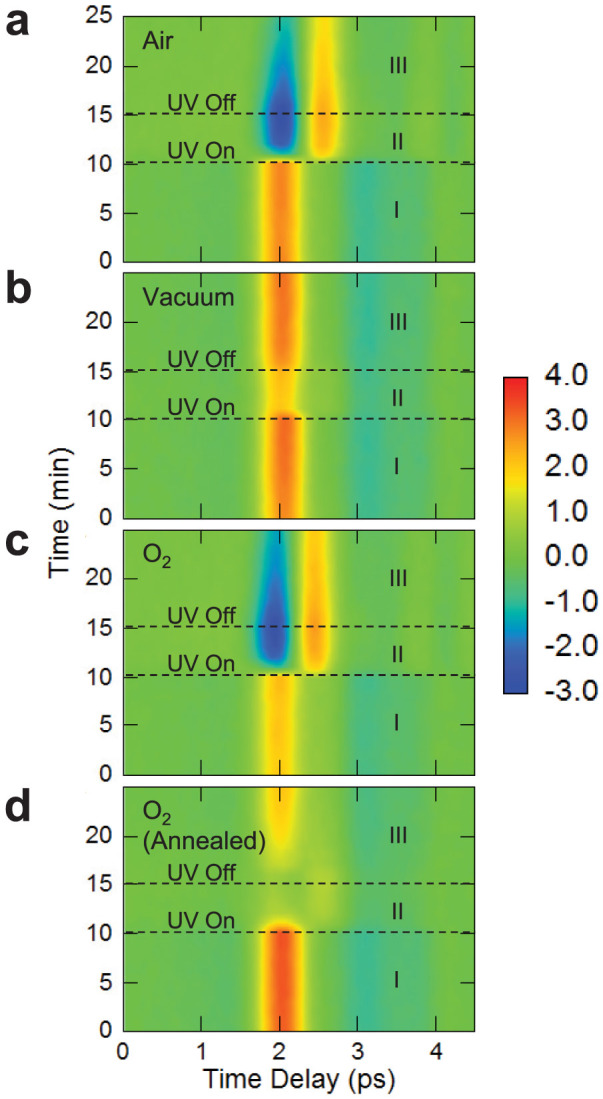
(a), Air, (b), vacuum, (c), oxygen, and (d), oxygen. In (d), the sample was pre-annealed to remove water before exposure to oxygen. In all cases, after 10 minutes of continuous THz emission measurements (Region I), the sample was illuminated by ultraviolet UV light (365 nm) for 5 minutes (Region II). After the UV light is switched off (Region III), the THz waveform slowly tends towards the original form.
After the UV light is switched off (Region III), the THz waveform in air and oxygen slowly returns towards the original shape but does not completely recover the initial waveform in the time frame of the measurements. Basically no similar change is observed when the UV light is turned off in the vacuum case (Fig. 2b). These results suggest that the existence of oxygen molecules is necessary for changes to occur in the waveform of THz radiation emitted from graphene-coated InP (100) upon UV illumination. Finally, for the pre-annealed sample measured in the presence of oxygen (Fig. 2d), the THz waveform has one peak in Region I and shows a smaller change under UV light illumination than the case without pre-annealing (Fig. 2c). In Region III in Fig. 2d, the waveform completely recovers the initial waveform whereas in Fig. 2c the waveform does not recover the original. These results show that the dramatic UV response is due to the co-existence of oxygen and water adsorbates on graphene, consistent with previous results reported by Mitoma et al.10. Moreover, adsorbed oxygen molecules, after UV illumination in the presence of water, are not easily removable by femtosecond laser excitation (Region III in Figs. 2a and 2c).
Discussion
The emission mechanism of THz radiation from an InP surface is primarily the current surge effect due to the built-in electric field in the surface depletion layer6. We believe that the observed changes in THz waveforms are caused by the existence of electric dipoles induced by adsorbed oxygen molecules, which modify the surface electric field. The schematic band diagrams in Fig. 3 show the expected flow of photoexcited carriers both in the (a) absence and (b) presence of adsorbed oxygen. The Fermi level of bulk semi-insulating InP (100) is located 0.75 eV below the conduction band bottom while at the surface it is pinned at 0.45 eV below the conduction band bottom6,14. Thus, the band bends downward toward the surface, and the photoexcited current driven by the surface field flows toward the inside of the InP (100) substrate (Fig. 3a). Judging from the fact that the THz waveform is identical between bare InP (100) and graphene-coated InP (100) under vacuum, it can be assumed that pristine graphene alone, without adsorbates, does not change the surface band bending. On the other hand, exposure to air for an extended period results in a charge transfer between graphene and adsorbates9,15,16,17. Theoretical calculations suggest that, while graphene is hydrophobic, it is still capable of adsorbing small amounts of oxygen and water molecules18,19,20,21,22,23. Moreover, it has been reported that the shift of the Fermi level induced by gas adsorption strongly depends on the substrate17,19,24. Therefore, it is likely that adsorbed gas molecules predominantly exist between graphene and InP (100), as shown in Fig. 3b, forming local electric dipoles at the InP surface, which induce changes in the waveform of THz radiation. Specifically, the electric field generated by the local dipole between graphene and InP increases the surface potential of InP (100), which tends to bend the band upward toward the surface. As a result, the photoexcited current flows toward the surface (Fig. 3b), opposite to the case without molecules (Fig. 3a).
Figure 3. Band diagrams explaining the sign change of THz amplitude due to adsorbed oxygen molecules.
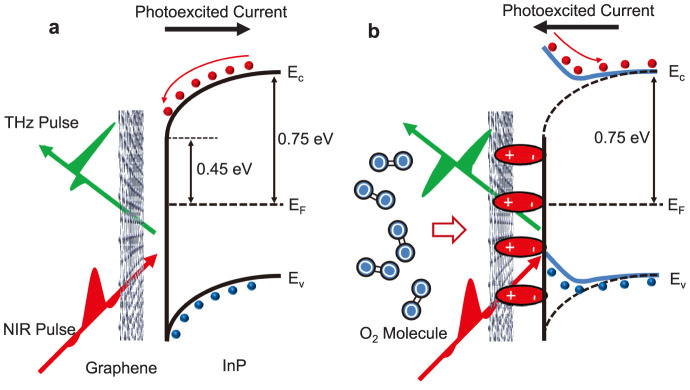
(a), Band diagram for pristine graphene on InP. The photo excited current flows towards the substrate. (b), Band diagram for graphene on InP with adsorbed oxygen molecules between them. The charge transfer between graphene and oxygen creates dipoles, which modify the band bending. The photoexicted current now flows towards the surface.
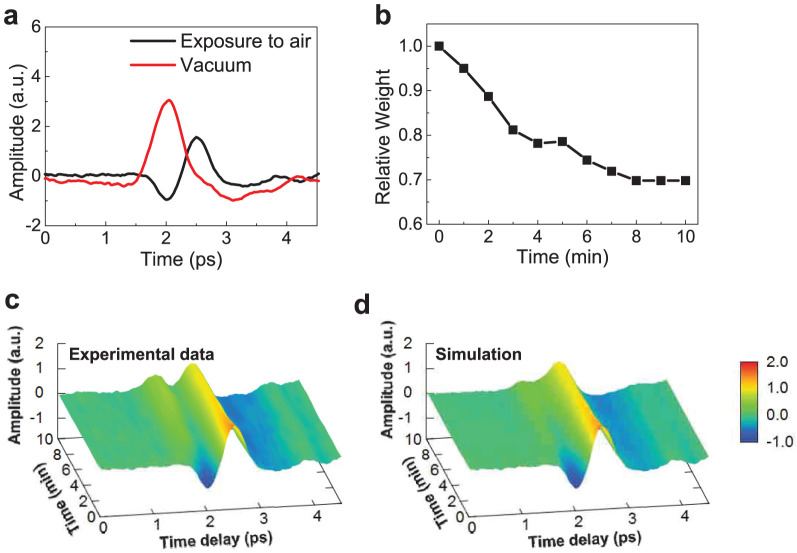
In general, the waveform of THz radiation emitted from graphene-coated InP should be a superposition of two waveforms representing, respectively, the band diagrams in Figs. 3a and 3b. The relative weights of the two components should depend on the density of local dipoles within the focal spot of the excitation laser beam, which gradually decreases with time under laser excitation. We explain our theoretical model in a semi-quantitative manner with experimental data in Fig. 4. Figure 4a shows two different waveforms – the one taken under high vacuum (red line, representing Fig. 3a) and the other taken after exposure to air for a few days (black line, representing Fig. 3b). The observed THz waveforms (Fig. 4c) for graphene-coated InP in air at different times are simulated with waveforms consisting of a superposition of these two observed waveforms in Fig. 4a at different relative weights. Figure 4b shows the relative weights of the waveforms taken after exposure to air used for the simulation of experimental data. The simulated waveforms (Fig. 4d) agree with the observed waveforms very well, with appropriately changing relative weights in the superposition. Furthermore, the UV-induced changes in THz waveforms can also be explained by our model. UV light promotes the oxidation of graphene in the presence of oxygen and water molecules10,11,12,13; this reaction is rapid and creates tightly-bonded chemisorbed oxygen. Therefore, desorption of oxygen molecules via femtosecond laser excitation becomes more difficult, and the THz waveform does not fully recover the initial one in Region III (Figs. 2a and 2c).
Figure 4. Laser-induced desorption dynamics of oxygen molecules from graphene observed through THz waveforms.
(a), Two kinds of waveforms emitted from graphene-coated InP, one taken under high vacuum (red line) and the other taken after exposure to air for several days (black line). (b), The time dependent relative weights of the waveforms taken after exposure to air used for the simulation of experimental data. (c), THz waveforms for graphene-coated InP in air at different times. (d), Simulated waveforms consisting of a superposition of two observed waveforms of different relative weights. The simulated waveforms agree with the observed waveforms very well, with appropriately changing relative weights in the superposition.
Finally, Fig. 5a shows a map of the amplitude of THz radiation emitted from graphene-coated InP (100) after exposure to air for a few days. The amplitude of the first peak greatly varies, exhibiting both positive and negative values; a reference InP substrate without graphene shows almost constant values (see Supplementary Information). The THz waveforms corresponding to Point 1 and Point 2 in the map (Fig. 5a) as well as that for a bare InP (100) substrate are shown in Fig. 5b. The waveform at Point 1 is similar to that from a highly polarized surface due to adsorption of oxygen molecules, and the waveform at Point 2 is qualitatively the same as that from the bare InP. These results indicate that this method using THz emission is a valuable tool for measuring the distribution of local dipoles induced by oxygen adsorption.
Figure 5. THz imaging of adsorbed oxygen on graphene.
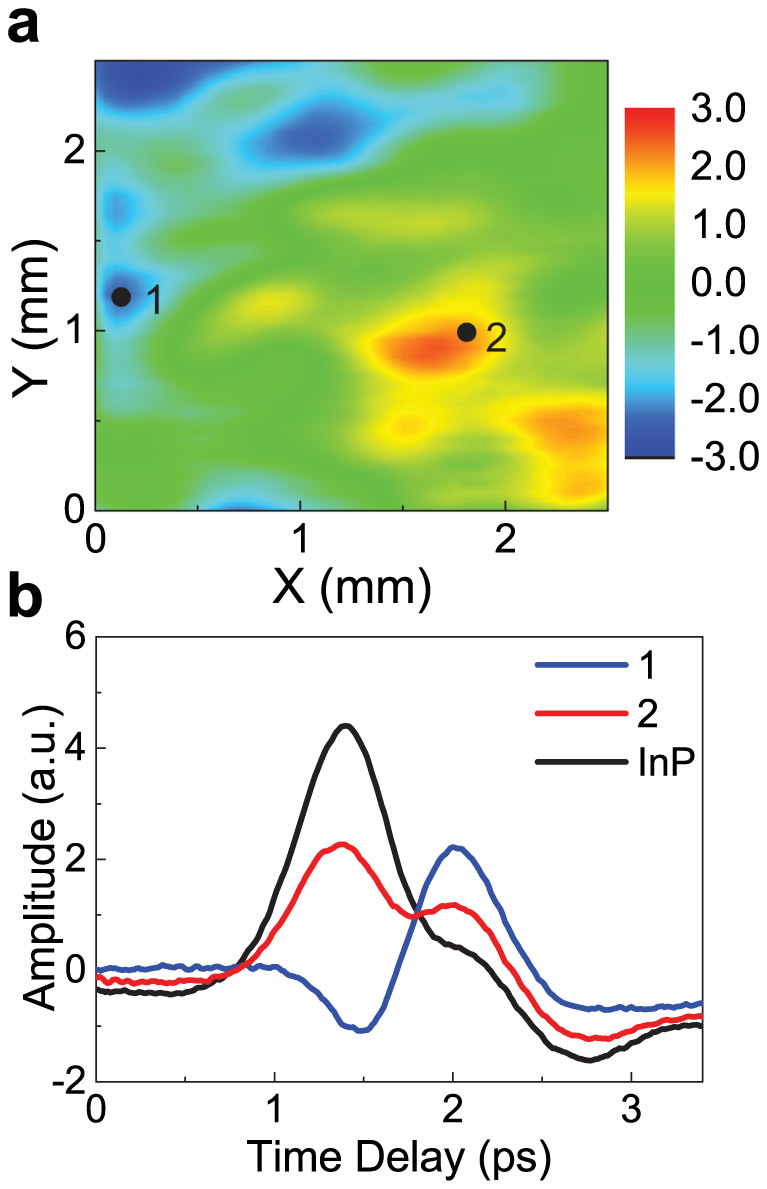
(a), Amplitude mapping of THz radiation emitted from graphene-coated InP after exposure to air for a few weeks. (b), THz waveforms corresponding to Point 1 and Point 2 indicated in the map as well as that for a bare InP substrate. The waveform at Point 1 indicates a highly polarized surface due to adsorption of oxygen molecules, and the waveform at Point 2 is qualitatively the same as that from the bare InP.
Methods
Terahertz time-domain spectroscopy
The graphene-coated InP (100) and the reference bare InP crystals were optically excited by near-infrared pulses with a duration of ~100 fs and photon energy of 1.55 eV generated by a Ti:sapphire laser. The excitation laser beam was delivered to the sample surface at an incident angle of 45°. The power of the excitation laser beam was set at 20 mW. The diameter of excitation laser spot was ~100 µm, and the fluence was three orders of magnitude lower than the damage threshold previously reported25. THz radiation was mainly emitted from the surface of the InP (100) substrate through ultrafast surface surge-currents with the range of the fluence in this experimet6,26. THz radiation was emitted into free space and then focused onto a dipole-shaped low-temperature-grown GaAs photoconductive switch via a pair of off-axis parabolic mirrors. The output of the photoconductive antenna was fed into a lock-in amplifier, referenced to a 2.0 kHz optical chopper signal. THz radiation images were obtained by raster-scanning of an x-y mechanical stage on which the sample was mounted. For UV light illumination, a light emitting diode with 365 nm wavelength was utilized. The spot diameter was ~8 mm, and the power was 36 mW. The gases used in the THz emission measurements were ambient air, dry air, 99.9995% pure N2, and 99.999% pure O2. All measurements in atmospheric gases were performed at 1 atm.
Sample fabrication
The studied graphene samples were synthesized on copper (Cu) foil using hexane as a liquid precursor in a chemical vapor deposition system5. After growth, a thin poly-methyl-meth-acrylate (PMMA) film was deposited on the graphene/Cu substrate as supporting layer for the graphene transfer. The underlying Cu substrate was then dissolved in dilute HNO3 and washed several times in deionized water. The floating graphene/PMMA film was transferred onto an InP substrate. The sample was immersed into acetone solvent to remove PMMA, and then dried in critical point dryer to avoid damage of the partially deposited graphene layer by the local capillary forces arising in conventional drying processes.
Author Contributions
I.K., J.K. and M.T. designed the work. Y.S., M.T. and K.A.S. carried out the terahertz measurements, and M.W., R.V. and P.M.A. grew and processed the graphene samples. All authors discussed the results. Y.S., I.K., J.K. and M.T. wrote the paper and R.V., P.M.A. and H.M. commented on the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by Grant-in-Aid 25630149 and 26107524, Core-to-Core program, MEXT/JSPS, the Program for promoting international joint research, Osaka University, the Murata Science Foundation, the Robert A. Welch Foundation through Grant No. C-1509, and the National Science Foundation through Grant No. OISE-0968405.
References
- Ren L. et al. Terahertz and infrared spectroscopy of gated large-area graphene. Nano Lett. 12, 3711–3715 (2012). [DOI] [PubMed] [Google Scholar]
- Schedin F. et al. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 6, 652–655 (2007). [DOI] [PubMed] [Google Scholar]
- Docherty C. J. et al. Extreme sensitivity of graphene photoconductivity to environmental gases. Nat. Commun. 3, 1228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A. C. & Basko D. M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nano. 8, 235–246 (2013). [DOI] [PubMed] [Google Scholar]
- Srivastava A. et al. Novel liquid precursor-based facile synthesis of large-area continuous, single, and few-layer graphene films. Chem. Mater. 22, 3457–3461 (2010). [Google Scholar]
- Nakajima M., Hangyo M., Ohta M. & Miyazaki H. Polarity reversal of terahertz waves radiated from semi-insulating InP surfaces induced by temperature. Phys. Rev. B 67, 195308 (2003). [Google Scholar]
- Auston D. H., Cheung K. P. & Smith P. R. Picosecond photoconducting Hertzian dipoles. Appl. Phys. Lett. 45, 284–286 (1984). [Google Scholar]
- Ni Z. H. et al. The effect of vacuum annealing on graphene. J. Raman Spectrosc. 41, 479–483 (2010). [Google Scholar]
- Cheng Z. et al. Toward intrinsic graphene surfaces: A systematic study on thermal annealing and wet-chemical treatment of SiO2-supported graphene devices. Nano Lett. 11, 767–771 (2011). [DOI] [PubMed] [Google Scholar]
- Mitoma N., Nouchi R. & Tanigaki K. Photo-oxidation of graphene in the presence of water. J. Phys. Chem. C 117, 1453–1456 (2013). [Google Scholar]
- Liu L. et al. Graphene oxidation: Thickness-dependent etching and strong chemical doping. Nano Lett. 8, 1965–1970 (2008). [DOI] [PubMed] [Google Scholar]
- Zhao S., Surwade S. P., Li Z. & Liu H. Photochemical oxidation of CVD-grown single layer graphene. Nanotechnology 23, 355703 (2012). [DOI] [PubMed] [Google Scholar]
- Mulyana Y., Horita M., Ishikawa Y., Uraoka Y. & Koh S. Thermal reversibility in electrical characteristics of ultraviolet/ozone-treated graphene. Appl. Phys. Lett. 103, 063107 (2013). [Google Scholar]
- Spicer W. E., Chye P. W., Skeath P. R., Su C. Y. & Lindau I. New and unified model for Schottky barrier and III-V insulator interface states formation. J. Vac. Sci. Technol. 16, 1422–1433 (1979). [Google Scholar]
- Ryu S. et al. Atmospheric oxygen binding and hole doping in deformed graphene on a SiO2 substrate. Nano Lett. 10, 4944–4951 (2010). [DOI] [PubMed] [Google Scholar]
- Yang Y. & Murali R. Binding mechanisms of molecular oxygen and moisture to graphene. Appl. Phys. Lett. 98, 093116 (2011). [Google Scholar]
- Levesque P. L. et al. Probing charge transfer at surfaces using graphene transistors. Nano Lett. 11, 132–137 (2011). [DOI] [PubMed] [Google Scholar]
- Leenaerts O., Partoens B. & Peeters F. M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 77, 125416 (2008). [Google Scholar]
- Wehling T. O., Lichtenstein A. I. & Katsnelson M. I. First-principles studies of water adsorption on graphene: The role of the substrate. Appl. Phys. Lett. 93, 202110 (2008). [Google Scholar]
- Sque S. J., Jones R. & Briddon P. R. The transfer doping of graphite and graphene. phys. stat. sol. (a) 204, 3078–3084 (2007). [Google Scholar]
- Dai J. & Yuan J. Adsorption of molecular oxygen on doped graphene: Atomic, electronic, and magnetic properties. Phys. Rev. B 81, 165414 (2010). [Google Scholar]
- Leenaerts O., Partoens B. & Peeters F. M. Water on graphene: Hydrophobicity and dipole moment using density functional theory. Phys. Rev. B 79, 235440 (2009). [Google Scholar]
- Ma J. et al. Adsorption and diffusion of water on graphene from first principles. Phys. Rev. B 84, 033402 (2011). [Google Scholar]
- Lafkioti M. et al. Graphene on a hydrophobic substrate: Doping reduction and hysteresis suppression under ambient conditions. Nano Lett. 10, 1149–1153 (2010). [DOI] [PubMed] [Google Scholar]
- Currie M. et al. Quantifying pulsed laser induced damage to graphene. Appl. Phys. Lett. 99, 211909 (2011). [Google Scholar]
- Nakajima M., Oda Y. & Suemoto T. Competing terahertz radiation mechanisms in semi-insulating InP at high-density excitation. Appl. Phys. Lett. 85, 2694–2696 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information