Abstract
Synergistic molecular vulnerabilities enhancing hypomethylating agents in myeloid malignancies have remained elusive. RNA-interference drug modifier screens identified antiapoptotic BCL-2 family members as potent 5-Azacytidine-sensitizing targets. In further dissecting BCL-XL, BCL-2 and MCL-1 contribution to 5-Azacytidine activity, siRNA silencing of BCL-XL and MCL-1, but not BCL-2, exhibited variable synergy with 5-Azacytidine in vitro. The BCL-XL, BCL-2 and BCL-w inhibitor ABT-737 sensitized most cell lines more potently compared with the selective BCL-2 inhibitor ABT-199, which synergized with 5-Azacytidine mostly at higher doses. Ex vivo, ABT-737 enhanced 5-Azacytidine activity across primary AML, MDS and MPN specimens. Protein levels of BCL-XL, BCL-2 and MCL-1 in 577 AML patient samples showed overlapping expression across AML FAB subtypes and heterogeneous expression within subtypes, further supporting a concept of dual/multiple BCL-2 family member targeting consistent with RNAi and pharmacologic results. Consequently, silencing of MCL-1 and BCL-XL increased the activity of ABT-199. Functional interrogation of BCL-2 family proteins by BH3 profiling performed on patient samples significantly discriminated clinical response versus resistance to 5-Azacytidine-based therapies. On the basis of these results, we propose a clinical trial of navitoclax (clinical-grade ABT-737) combined with 5-Azacytidine in myeloid malignancies, as well as to prospectively validate BH3 profiling in predicting 5-Azacytidine response.
Keywords: 5-Azacytidine, BH3 profiling, myeloid malignancies
Introduction
Conventional therapies for poor-risk, elderly and secondary acute myeloid leukemia (AML), relapsed/refractory high-risk myelodysplastic syndrome (MDS) and accelerated and transformed (leukemic blast phase) myeloproliferative neoplasm (MPN) have limited benefit.1 Response and long-term outcome with cytotoxic chemotherapies are poor.2 Hypomethylating agents, 5-Azacytidine (5-Aza) and decitabine, demonstrate substantial single-agent activity in ∼25–50% of MDS3, 4 and MPN,5 and are active in ∼15–25% of AML.6, 7, 8 Although these single-agent activities are encouraging, substantial room for improvement remains.9, 10 The development of rational combinations with hypomethylating agents has been hampered by a lack of precise mechanistic understanding. Furthermore, although promising predictive biomarkers for 5-Aza have recently been reported,11, 12, 13 there is no clinically validated assay or biomarker predicting response to hypomethylating agents. Therefore, we sought to identify therapeutically exploitable molecular vulnerabilities to design rational combinations with 5-Aza, and, in parallel, to develop biomarkers of response to 5-Aza.
As a mechanistically unbiased starting point to identify targets that modulate the antileukemic activity of 5-Aza, we performed replicate RNA-interference (RNAi) sensitizer screens of 861 kinase, cell cycle, apoptosis and other cancer-associated genes in myeloid cell lines in combination with 5-Aza treatment. Primary and secondary RNAi screen results indicated that inhibition of antiapoptotic BCL-2 family proteins constitutes an important concept for modulating 5-Aza antileukemic activity. Antiapoptotic BCL-2 family members have been proposed as targets in myeloid malignancies, particularly MCL-1, BCL-2 and BCL-XL, and therapeutic agents for these targets are currently in preclinical or clinical development. However, there is no direct comparison of these antiapoptotic BCL-2 family members as targets in myeloid malignancies. Therefore, we focused on further studies to dissect how inhibition of BCL-XL, BCL-2 and MCL-1, with siRNA and pharmacological inhibitors, modulates the antileukemic activity of 5-Aza. These functional studies are complemented by proteomic data analyzing BCL-XL, BCL-2 and MCL-1 in 577 primary AML specimens, demonstrating that expression of antiapoptotic family members is heterogeneous within AML FAB subtypes and overlapping across subtypes, suggesting functional redundancy. We present additional evidence that the overall balance of BCL-2 family proteins, assessed with a functional BH3-profiling assay, predicts clinical response to 5-Aza. The results presented herein have important implications for the design of clinical trials with BCL-2 family-targeting agents in myeloid malignancies, the future development of novel BCL-2 family-targeted agents with distinct inhibitory profiles and the clinical application of 5-Aza in myeloid malignancies through the potential development of a predictive biomarker of response to 5-Aza.
Materials and methods
Cells, culture conditions and reagents
Cell lines were obtained from ATCC or DSMZ, and MDS-L was kindly provided by Professor Kaoru Tohyama (Kawasaki Medical School, Kurashiki, Japan).14 Primary specimens were obtained in accordance with Institutional Review Board-approved protocols, separated by Ficoll gradient centrifugation and/or treated with ACK lysis buffer before short-term ex vivo culture. All cells were cultured in RPMI-1640 with 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C/5% CO2. 5-Azacytidine was obtained from Sigma-Aldrich (St Louis, MO, USA), ABT-737 from ChemieTek (Indianapolis, IN, USA), and ABT-199 from SeleckChem (Houston, TX, USA).
siRNA drug-dose-response assays
Selected candidates from 5-Aza RNAi sensitizer screens were validated with siRNA drug-dose-response (siDDR) assays as described previously for primary RNAi screens.15 All siRNA were obtained from Qiagen, except for two additional validation BCL-2 siRNA sequences, IDs: s224526 and s194310 (Silencer Select, Ambion, Carlsbad, CA, USA). Four different siRNA sequences for each selected target, nonsilencing negative control siRNA, universal lethal positive control siRNA and buffer-transfection reagent were included on each 384-well siDDR assay plate.
Drug-dose-response experiments and CalcuSyn analysis
For ABT-737 and ABT-199 combination experiments with 5-Aza, compounds were added simultaneously and relative cell number was determined at 96 h with CTG. Prism Version 5.03 software (Prism Software Corporation, Irvine, CA, USA) was used to calculate 5-Aza EC50 values at various concentrations of ABT-737 and ABT-199. Synergy was assessed by calculating combination index values with CalcuSyn Version 2.1 software (Biosoft, Cambridge, UK) according to the Chow and Talalay model.16
Cleaved caspase-3 analysis
Cells were processed according to the Cell Signaling Technology protocol. TF-1 cells were treated for 24 h with 625 nM ABT-737 before addition of 1.0 μM 5-Aza and fixed at 72 h total. HL-60 was dosed with 500 nM ABT-737 simultaneously with 1.0 μM 5-Aza, before fixation at 8, 24 and 48 h. Cells were incubated for 1 h with cleaved caspase-3 (Asp175)-Alexa Fluor 488 antibody conjugate (Cell Signaling Technology, Danvers, MA, USA) at 1:50 dilution. Fluorescence intensity was measured on a CyAn flow cytometer (Beckman Coulter, Pasadena, CA, USA) and data analyzed with Summit Version 4.3 software (DAKO, Carpinteria, CA, USA).
Protein expression/reverse phase protein array (RPPA)
Proteomic profiling was performed on primary AML specimens using validated methods described previously.17, 18 Primary specimens were printed in five serial dilutions onto slides with normalization and expression controls. Slides were probed with validated primary antibodies (Cell Signaling Technology; Epitomics, Burlingame, CA, USA) at 1:500 dilution and secondary antibody to amplify the signal at 1:15 000 dilution. Stained slides were analyzed using Vigene Tech Microvigene Version 3.4 software (Carlisle, MA, USA) to produce quantified data as previously described.19
mRNA expression from public data sets
Data from public data sets GEO accession numbers GSE19429, GSE6891, GSE12417 were MAS5 transformed using Expression Console Software (Affymetrix, Santa Clara, CA, USA) and subsequently median normalized. The number of cases were as follows: CD34+ (17), MDS (50), M0 (16), M1 (95), M2 (104), M3 (23), M4 (23), M5 (104), M6 (6). The ANOVA test statistical analysis was performed across all groups, thus the P-value corresponds to simultaneous analysis of all French-American-British (FAB) classifications. The Valk and Metzeler2 data sets were obtained from Oncomine.
BH3-profiling assay
Viably frozen patient specimens (N=22) consisted of 11 bone marrow and 11 peripheral blood mononuclear cells. For in vitro experiments, identical cell line passages were used for BH3-profiling assays and 5-Aza drug-dose-response experiments, performed simultaneously with the same lot of freshly prepared 5-Aza.
AML specimens suspended in 1% FBS, 2 mM EDTA-PBS were stained with primary antibodies CD45-V450 (BD Biosciences, Franklin Lakes, NJ, USA), CD3-Biotin (BD Biosciences) and CD20-Biotin (eBiosciences, San Diego, CA, USA), and secondary antibody Streptavidin-APC (BD Biosciences). Specimens were then permeabilized with digitonin (Sigma-Aldrich) and incubated with JC-1 mitochondrial dye (Enzo Life Sciences, Farmingdale, NY, USA) and peptides (BIM 100 μM, BIM 0.1 μM, PUMA 100 μM, PUMA 10 μM, NOXA 100 μM, BAD 100 μM, BMF 100 μM, HRK 100 μM or PUMA2A 100 μM) or with dimethyl sulfoxide (DMSO (1%) or carbonyl cyanide m-chlorophenyl hydrazone (CCCP (10 μM)) at room temperature. Samples were run in duplicate except in cases where insufficient viable cells were available. Samples were analyzed on a FACS CantoII (BD Biosciences) using the BD FACS Diva software (BD Biosciences). The blast population was identified as CD45 dim, CD3 and CD20 negative. Intensely stained CD45 cells (mature lymphocytes) were excluded from analyses as described previously.20, 21 The quantifiable propensity of a pro-apoptotic peptide to induce mitochondrial depolarization relative to an uncoupling reagent control is referred to as percent priming. For the blast population, this was calculated using the median signal intensity of the PE channel normalized for DMSO as background (negative control), and CCCP served as 100% priming (positive control). For calculation of % priming, the following formula is utilized.
 |
Statistical Analysis
BH3-profiling biomarkers were analyzed by testing the association between the biomarker status (% priming) and responder or nonresponder classification. Univariate comparisons were made using the Mann–Whitney test; all reported P-values are two sided. The predictive ability of markers was assessed using the area under the curve (AUC) statistic. Analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA), R version 2.14.2 (R Core Team) and/or Graphpad Prism version 5.04 (Prism Software Corporation).
Results
Knockdown of antiapoptotic BCL-2 family proteins results in differential effects on viability and 5-Azacytidine sensitization
Preliminary RNAi drug modifier screens targeting genes capable of sensitizing AML-derived cell lines TF-1 and ML-2 to 5-Aza identified BCL-2 family members as potential targets. Validation studies confirmed that RNAi-mediated knockdown of BCL-XL resulted in three- to fourfold 5-Aza sensitization in TF-1 (data not shown). To further explore the role of BCL-2 family members as therapeutic targets in myeloid malignancies, we examined the role of three major antiapoptotic BCL-2 family proteins, BCL-2, MCL-1 and BCL-XL, and tested their impact on cell proliferation and sensitivity to 5-Aza. siRNA silencing of BCL-XL or MCL-1 effectively reduced viability in most AML cell lines examined, whereas BCL-2 siRNA had less of an effect (Table 1a). In regards to 5-Aza sensitization, silencing of BCL-XL potently sensitized erythroid cells TF-1 and HEL (Supplementary Figure 1A), while MCL-1 sensitized more broadly across the AML cells examined (Table 1b). Growth inhibitory effects of siRNA silencing alone generally paralleled the effects of siRNA sensitization to 5-Aza. Effective siRNA silencing was demonstrated for BCL-XL by reduction in protein levels in cells that were sensitized (TF-1) or were not sensitized (THP-1) (Supplementary Figures 1B and C), showing that differential 5-Aza sensitization was not due to differences in knockdown.
Table 1a. In vitro % viability reduction by siRNA alone.
| Cell line |
siRNA |
||
|---|---|---|---|
| BCL-2 | BCL-XL | MCL-1 | |
| SET-2 | 0–2 | 39–52 | 22–65 |
| TF-1 | 0–4 | 11–71 | 0–17 |
| HEL | 0–1 | 21–67 | 14–39 |
| THP-1 | 0–21 | 11–32 | 33–87 |
| OCI-AML3 | 0–20 | 66–71 | 49–99 |
| ML-2 | 0–4 | 11–38 | 18–35 |
Antiapoptotic BCL-2 family siRNA drug-dose-response assays with 5-Azacytidine in myeloid cells in vitro. Cell lines are listed in the left-most column. The BCL-2 family member silenced by siRNA is shown as the heading for each column. The range of % viability reduction due to the given siRNA alone (without 5-Aza) is shown (0=no affect and 100= maximal reduction in viability).
Table 1b. In vitro 5-Azacytidine EC50 fold-shift enhancement by siRNA.
| Cell line |
siRNA |
||
|---|---|---|---|
| BCL-2 | BCL-XL | MCL-1 | |
| SET-2 | 1.0±0.0 (P=0.20) | 1.4±0.2 (P=0.052) | 1.1±0.1 (P=0.14) |
| TF-1 | 1.2±0.1 (P=0.011) | 3.4±0.7 (P=3.5e–5) | 1.6±0.1 (P=0.061) |
| HEL | 1.2±0.1 (P=0.38) | 4.4±1.8 (P=0.031) | 3.0±0.4 (P= 0.086) |
| THP-1 | 1.0±0.1 (P=0.82) | 1.1±0.2 (P=0.079) | 1.2±0.0 (P=0.076) |
| OCI-AML3 | 1.2±0.5 (P=0.61) | −1.1±0.2 (P=0.016) | 2.2±0.8 (P=0.056) |
| ML-2 | 1.0±0.0 (P=0.35) | 1.1±0.1 (P=0.026) | 1.4±0.0 (P=0.024) |
Anti-apoptotic BCL-2 family siRNA drug-dose-response assays with 5-Azacytidine in myeloid cells in vitro. Cell lines are listed in the left-most column. The BCL-2 family member silenced by siRNA is shown as the heading for each column. 5-Aza EC50 fold-shifts are listed with P values associated with EC50 fold-shift measurements averaged for the different siRNA sequences against each BCL-2 family member. ‘−' denotes antagonistic fold-shift.
ABT-737 synergizes with 5-Azacytidine more potently than ABT-199 in myeloid cell lines
Two therapeutic agents, ABT-263 and ABT-199, directly targeting antiapoptotic BCL-2 family members by acting as BH3-domain mimetics are currently undergoing clinical testing. Thus far, ABT-263 and ABT-199 have been tested primarily for the treatment of solid tumors and lymphoid malignancies, and their efficacy in myeloid malignancies remains to be determined.22, 23, 24 ABT-263 (navitoclax), an orally available analog and the clinical grade compound of the experimental tool compound ABT-737 with a nearly identical binding profile, inhibits BCL-XL, BCL-2 and BCL-w with Ki values <1 nM.25, 26 Because of the on-target effects of ABT-263 on BCL-XL, a megakaryocytic lineage gene, ABT-263 induces thrombocytopenia.27 Recently ABT-199, a more selective inhibitor of BCL-2 that does not inhibit BCL-XL at low-to-moderate concentrations, has shown promising clinical responses in lymphoid malignancies, without some of the clinical toxicities of ABT-263, particularly thrombocytopenia.28, 29
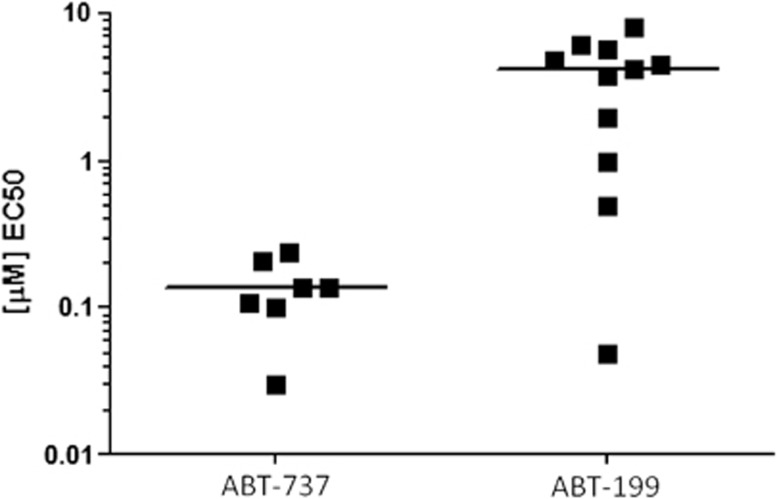
To determine which agent is more potent in myeloid malignancies, we assessed single-agent activity and 5-Aza sensitization with ABT-737 (the tool compound of ABT-263) versus ABT-199 across a spectrum of genomically heterogeneous AML cell lines. ABT-737 exhibited lower single-agent EC50 values (median 0.14 μM for ABT-737 versus 4.3 μM for ABT-199) (Figure 1), and resulted in greater 5-Aza sensitization, as determined by EC50 fold-enhancement and Combination Index synergy with CalcuSyn (Figures 2a–c and Supplementary Figures 2A and B). Generally, higher concentrations of ABT-199, than ABT-737, were required to enhance 5-Aza activity (Figure 2b). Moreover, ABT-737 exhibited dose-dependent sensitization in all AML cells, whereas ABT-199 sensitization was not dose-dependent except in MDS-L (Supplementary Figure 2A). ABT-737 also resulted in greater synergy than ABT-199 in most cell lines, as determined by lower combination index values and/or greater Fractional Effect by CalcuSyn analysis, which again were seen at lower doses of ABT-737 than ABT-199 (low nM versus low μM concentrations, respectively) (Supplementary Figure 2B). As expected, ABT-737 sensitization to 5-Aza was mediated by enhancing apoptosis (Supplementary Figures 2C and D). We conclude that, although BCL-2 inhibition with ABT-199 is effective in myeloid malignancies, in most cell lines simultaneous targeting of BCL-XL, BCL-2 and Bcl-w with ABT-737 is more potent than targeting BCL-2 alone, and in combination with 5-Aza.
Figure 1.
Single-agent BCL-2 family inhibitor activity in AML cell lines. Seven AML-derived cells lines (TF-1, HEL, THP-1, U937, ML-2, HL-60 and MDS-L) are plotted for ABT-737, whereas 11 are plotted for ABT-199 activity (UKE-1, SET-2, M07e, TF-1, HEL, THP-1, ML-2, OCI-AML3, OCI-AML2, HL-60 and MDS-L). Six cell lines were tested with both compounds (TF-1, HEL, THP-1, ML-2, HL-60 and MDS-L).
Figure 2.
ABT-737 compared with ABT-199 in vitro synergy with 5-Azacytidine. (a) Maximal 5-Aza EC50 fold-shifts for ABT-737 and ABT-199 are shown side-by-side for each cell line. These EC50 fold-shifts are a ‘one-sided' measurement of 5-Aza enhancement. (b) Corresponding ABT-737 or ABT-199 doses at which maximal 5-Aza EC50 fold-shifts occurred are shown side-by-side. (c) *CalcuSyn Combination Index (CI) values corresponding to the greatest synergy (thus doses shown in b) nearest to the 5-Aza EC50 dose are listed. It is important to note that CI values are a ‘two-sided' measurement of drug synergy for two specific doses, thus a single CI value is not a universal characteristic of the interaction between two drugs because interactions can be dose dependent. See Supplementary Figure 2B for an extensive data set of CI values across multiple dose combinations plotted against the corresponding Fractional Effect, where 0=no effect and 1.0=maximal effect. CI values <0.8 indicate synergy, whereas CI values >1.1 indicate antagonism.
RNAi silencing of antiapoptotic BCL-2 family proteins in combination with ABT-199 or ABT-737
Next we aimed to explore the individual contribution of inhibiting BCL-XL or MCL-1 in combination with BCL-2. Thus, BCL-XL or MCL-1 was silenced by siRNA and ABT-199 activity subsequently assessed. BCL-XL or MCL-1 silencing strongly potentiated ABT-199 as shown by average ABT-199 EC50 fold-shifts (Table 2). MCL-1 has been shown to confer resistance to ABT-737.30 Conversely, inhibition of MCL-1 enhances ABT-737 sensitivity of several tumor types;31 however, this has not been shown for myeloid cells. Indeed, siRNA silencing of MCL-1 strongly sensitized to ABT-737 (Supplementary Figure 3). These data confirm BCL-XL and MCL-1 as critical targets in myeloid malignancies, and inhibiting either enhances effects of BCL-2 inhibition.
Table 2. In vitro ABT-199 EC50 fold-shift enhancement by siRNA.
| Cell line |
siRNA |
|
|---|---|---|
| BCL-XL | MCL-1 | |
| SET-2 | 1.2±0.1 (P=0.032) | 1.5±0.3 (P=0.049) |
| TF-1 | 1.6±0.2 (P=0.0073) | 2.3±1.0 (P=0.076) |
| THP-1 | 4.2±2.8 (P=0.11) | 8.5±2.4 (P=0.0079) |
| OCI-AML3 | 2.4±1.0 (P=0.067) | 6.9±0.9 (P=0.0084) |
BCL-XL and MCL-1 siRNA knockdown combined with ABT-199 drug-dose-response in myeloid cells in vitro. Cell lines are listed in the left-most column. The BCL-2 family member silenced by siRNA before assessing ABT-199 drug-dose-response is shown as the heading for each column. ABT-199 EC50 fold-shifts, determined relative to non-silencing siRNA, are listed with P-values associated with EC50 fold-shift measurements averaged for four different siRNA sequences against each BCL-2 family member.
Protein and mRNA expression of antiapoptotic BCL-2 family members in primary AML specimens
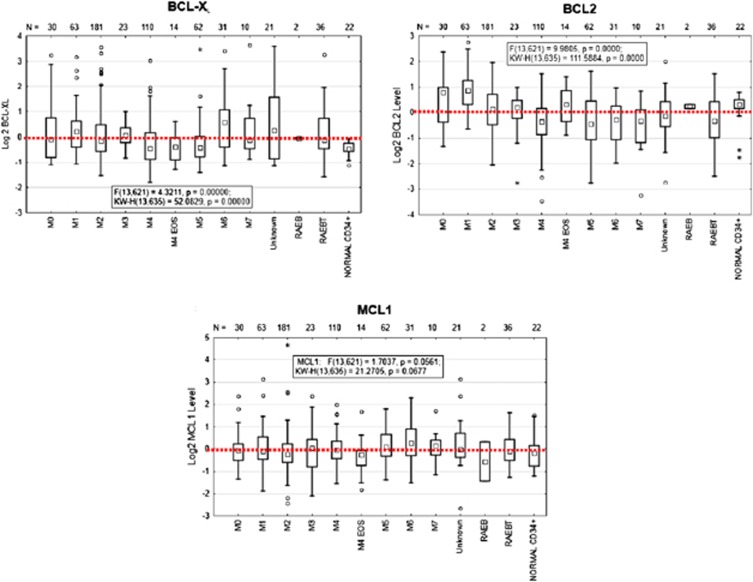
To assess putative correlations between BCL-2 family expression levels and pharmacological/functional siRNA data, we measured the expression of BCL-XL, BCL-2 and MCL-1 in 577 primary AML samples using a reverse phase protein array (RPPA) proteomic approach. There was no strong association between BCL-XL, BCL-2 or MCL-1 expression and cytogenetic-molecular features (data not shown), consistent with our previous report.17 However, when analyzed by AML FAB classification, BCL-XL was highest in M6, whereas BCL-2 was highest in M1 and M0 (Figure 3). Median BCL-2 levels were higher in normal CD34+-selected hematopoietic progenitor cells as compared with all FAB classifications except M0, M1 and M4 EOS. In contrast, median BCL-XL and MCL-1 levels were lower in normal CD34+ progenitor cells as compared with the majority of FAB classifications. MCL-1 expression was more uniformly distributed across FAB classifications than BCL-XL or BCL-2. There were often strong differences in expression levels of BCL-XL, BCL-2 and MCL-1 within a given FAB subgroup, indicating significant patient-to-patient variability. Similar trends were confirmed by mRNA expression in AML data sets reanalyzed and in public databases (Supplementary Figures 4A–C).
Figure 3.
BCL-XL, MCL-1 and BCL-2 protein expression in primary specimens determined by Reverse Phase Protein Array. 577 primary AML patient samples are shown grouped by AML FAB classification.
5-Azacytidine and ABT-737 exhibit synergy ex vivo across a spectrum of myeloid malignancies: AML, MDS and MPNs
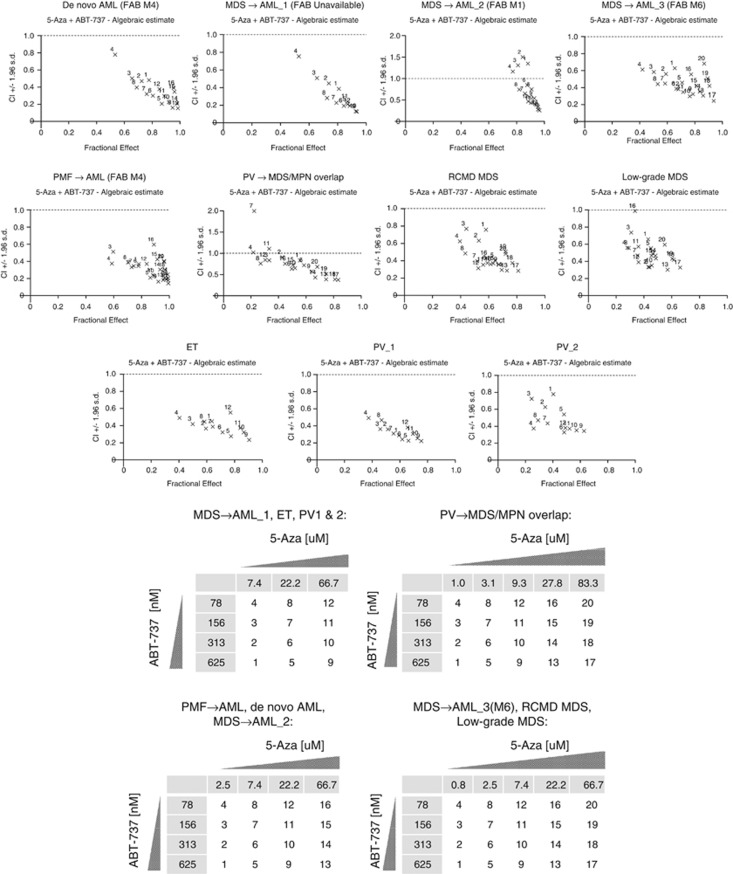
To demonstrate that inhibition of antiapoptotic BCL-2 family proteins is a potent 5-Aza sensitization strategy for AML and myeloid malignancies including MDS and MPNs, we evaluated the combination of ABT-737 with 5-Aza in short-term ex vivo cultures. ABT-737 sensitizes primary specimens from de novo AML and secondary AML (prior MDS or PMF) (Figure 4 and Table 3). We reasoned that the combination would be particularly effective in MPNs, given evidence for BCL-XL as a lineage-specific proto-oncogene, and its importance in erythroid progenitors.32, 33 Indeed, primary samples from MDS and MPN patients were sensitive to the combination, exhibiting strong synergy (Figure 4 and Table 3).
Figure 4.
5-Azacytidine and ABT-737 synergistically combine in primary myeloid malignancy specimens (N=11). Combination Index (CI) versus Fractional Effect (FE) plots were calculated using CalcuSyn Software Version 2.1. The numbers in the tables (Figure 4 key) correspond to the dose combinations shown on the CI versus FE plots for the indicated primary specimens. CI values <0.8 indicate synergy, whereas CI values >1.1 indicate antagonism.
Table 3. Ex vivo 5-Azacytidine EC50 fold-shift enhancement by ABT-737.
| Primary sample type | Maximal 5-Aza EC50 fold-shift; (ABT-737 dose) | Cytogenetics, mutations |
|---|---|---|
| De novo AML | 3.3 (1.3 μM) | Inv 16, FLT3 neg., NPM1 neg. |
| MDS → AML_1 | 2.7 (630 nM) | Restricted patient info. |
| MDS → AML_2 | −6.8 (2.5 μM) | +8 (1 of 20), FISH normal, FLT3 neg., NPM1 pos. |
| MDS → AML_3 | 1.1 (80 nM) | −7 (20 of 20, 90% by FISH), NPM1 neg. |
| PMF → AML | 2.1 (630 nM) | 46XY, JAK2 neg. |
| PV → MDS/MPN | 2.0 (1.3 μM) | 46XY, JAK2V617F pos. |
| RCMD MDS | 1.1 (160 nM) | Del 20 (8 of 20, 41% by FISH), JAK2 neg. |
| Low-grade MDS | 1.5 (1.3 μM) | 46XX, MDS FISH neg. |
| ET | 2.3 (1.3 μM) | 46XY, JAK2 neg., MPL neg. |
| PV_1 | 1.4 (310 nM) | 46XY, JAK2 pos. |
| PV_2 | 1.8 (1.3 μM) | JAK2 pos. |
Ex vivo 5-Azacytidine fold-shift enhancement by ABT-737. 5-Aza enhancement as determined by maximal 5-Aza EC50 shifts for the same 11 primary specimens shown in Figure 5 are shown with the dose of ABT-737 at which maximal EC50 enhancement occurred (shown in brackets), aside clinical cytogenetics and mutations. Although only doses corresponding to maximal enhancement are shown, significant enhancement often occurred at lower doses of ABT-737. Similarly, greater synergy by CI was frequently observed with increasing doses of 5-Aza beyond the EC50 dose. For example, see the MDS-to-AML_2 sample in Table 3 in comparison with this same sample in Figure 4, noting the antagonistic CI values near the lower, 2.5 μM 5-Aza EC50 dose (points 1–4), yet strong synergistic CI and FE at the higher 7.4 μM concentration of 5-Aza (points 5–8). ‘−' denotes antagonistic fold-shift.
BH3 profiling discriminates clinical response to 5-Azacytdine-based therapies
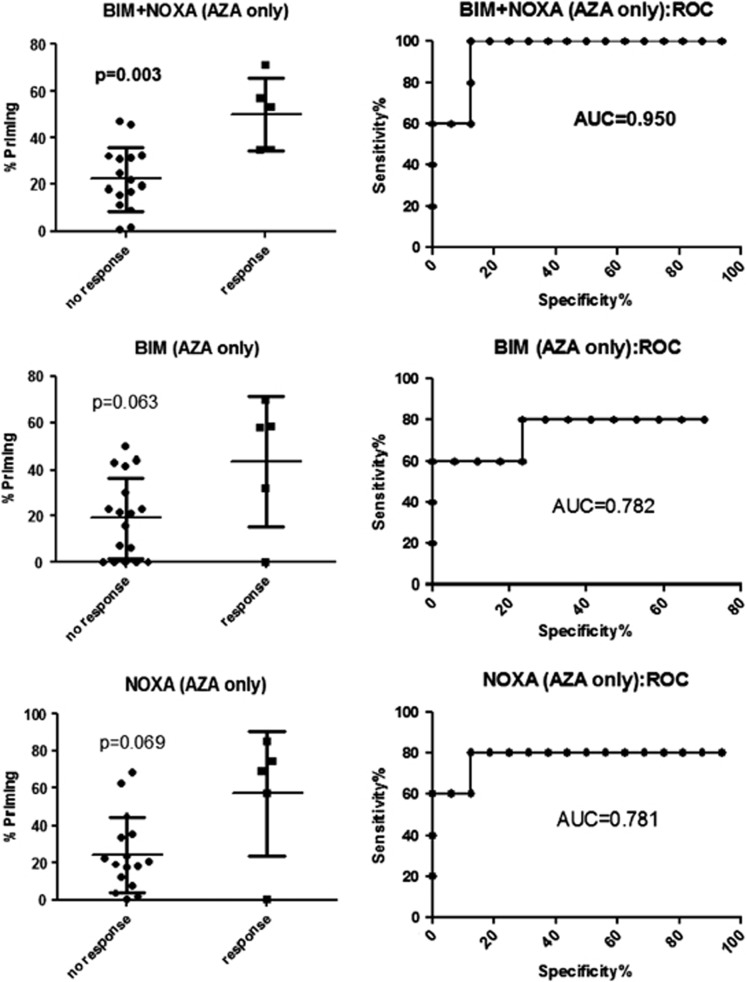
Although there are protein expression differences for BCL-XL, BCL-2 and MCL-1 by RPPA, within and between AML FAB subtypes, we did not find a strong correlation with clinical response to 5-Aza-based therapies (data not shown). This may be expected considering possible mechanistic distinctions34 and functional redundancies amongst the different antiapoptotic BCL-2 family members35 exhibiting overlapping expression patterns, as well as the distinct, yet overlapping binding affinities between anti- and proapoptotic proteins.36, 37 Thus, we aimed to functionally interrogate the overall balance of pro- and antiapoptotic BCL-2 family proteins, a cellular-molecular characteristic described as ‘primedness',38 by BH3 profiling. This assay uses peptides derived from proapoptotic BH3-only proteins and determines the degree to which these peptides induce apoptosis. As proof-of-concept in vitro, several BH3-profiling metrics significantly correlated with 5-Aza sensitivity (Supplementary Figure 5). To correlate clinical responses to 5-Aza with BH3 profiling, specimens from 22 AML, MDS and MDS/MPN overlap patients treated with 5-Aza-based regimens, and for whom clinical outcome was available, were assayed (Supplementary Table 1). Clinically, the best BH3 metric comprised combined values from NOXA and BIM peptides, which discriminated clinical responses, defined as achieving a response (sensitive) versus a patient being resistance/refractory, with statistical significance (Mann–Whitney two-tailed P=0.001) and an area under the receiver operating characteristic curve of 0.950 (Figure 5).
Figure 5.
BH3-profiling metrics correlate with clinical 5-Azacytidine-based response. Clinical response to 5-Aza-based therapy (N=22) is plotted against % priming by BH3 profiling for the indicated BH3 peptide(s). Clinicopathologic variables are shown in Supplementary Table 1.
Discussion
Therapeutic progress in the treatment of myeloid malignancies has been slow. Despite increased molecular knowledge, the underlying mechanisms of many therapeutic agents, including the hypomethylating agent 5-Aza, are still poorly understood.39, 40 This lack of mechanistic understanding has impeded development of novel rational combinations. We aimed to circumvent these challenges by employing a previously successful RNAi sensitizer screening approach as a mechanistically unbiased strategy to discover genes that modulate sensitivity to 5-Aza.15 Primary and secondary RNAi screens indicated that inhibition of antiapoptotic BCL-2 family proteins constitutes an important concept for sensitizing myeloid cells to 5-Aza.
Direct comparison of BCL-XL, MCL-1 and BCL-2 inhibition by siRNA and pharmacologically suggests that, although inhibition of individual antiapoptotic BCL-2 proteins (that is, BCL-2 with ABT-199) can be effective alone or in combination with 5-Aza, inhibiting more than one BCL-2 member simultaneously (that is, BCL-XL, BCL-2 and BCL-w with ABT-737, or siRNA against MCL-1 or BCL-XL combined with ABT-199) has more potent antileukemic activity. Importantly, dual or multiple BCL-2 family targeting sensitizes to 5-Aza across a broader range of AML cell lines. Proteomic data from 577 primary AML samples showed overlapping expression of BCL-XL, MCL-1 and BCL-2 across samples for the FAB subtypes. This overlap of expression, together with siRNA and pharmacological data, suggests functional redundancy between BCL-XL, BCL-2 and MCL-1 in myeloid malignancies. Thus, we conclude that two or more antiapoptotic BCL-2 family members determine the apoptotic threshold and response to 5-Aza in most AML cases. Consequently, dual or multiple simultaneous antiapoptotic BCL-2 family protein targeting may have greater antileukemic activity alone or with 5-Aza. Also supporting this concept, we show that knockdown of BCL-XL or BCL-2 alone with siRNA did not significantly sensitize THP-1 or ML-2 to 5-Aza; however, simultaneous inhibition of BCL-XL, BCL-2 and BCL-w by ABT-737 resulted in potent 5-Aza sensitization in these cells. In addition, siRNA knockdown of BCL-XL or MCL-1 strongly sensitized AML cell lines to BCL-2 inhibition with ABT-199, demonstrating that BCL-XL and MCL-1 can cooperate with BCL-2 in governing apoptotic response. Furthermore, ABT-737 generally resulted in broader and more potent sensitization than ABT-199, which occurred at lower doses of ABT-737 than ABT-199 (7- to 200-fold lower ABT-737 doses in five of seven AML cell lines). Single-agent activity of BCL-2 family inhibitors in AML cell lines confirms this trend, as the median ABT-737 EC50 dose was found to be >30-fold lower than that of ABT-199.
Although the aforementioned collection of data suggests partial functional redundancy of BCL-XL, BCL-2 and MCL-1 in myeloid malignancies, other interesting distinctions were observed in the proteomic data set. Median levels of BCL-2 were greater in normal CD34+ progenitor cells as compared with most FAB groups. In contrast, median BCL-XL and MCL-1 levels were lower in normal CD34+ progenitor cells. This raises speculation that compounds more selectively targeting BCL-XL and MCL-1, without inhibiting BCL-2, could widen a therapeutic index in some cases of AML. These preclinical observations can now be validated clinically with the availability of an increasing number of BCL-2 family member-targeting drugs. In fact, the first clinical trials combining 5-Aza with BCL-2-targeting compounds are in development (Tibes, personal communication).
As we previously reported,41, 42 and recently confirmed by another group,43 we demonstrate formal synergy of ABT-737 and 5-Aza in AML. Herein, we also show for the first time that ABT-737 and 5-Aza synergize in secondary AML arising from MDS and MPNs, and in primary MDS, PV and ET samples ex vivo, providing a preclinical rationale to include MDS and MPN patients in future trials of 5-Aza and BCL-2 family-targeting agents.
Expression of BCL-2 alone is not sufficient for predicting clinical response to navitoclax,23, 44 although expression signatures of combined antiapoptotic BCL-2 family members may predict response in CLL.45 However, analyzing expression of antiapoptotic BCL-2 family proteins may prove futile in some cells deficient in effector BCL-2 proteins BAX and BAK function.46 Together these observations suggest a functional assay such as BH3 profiling may have an advantage in capturing the BCL-2 family-regulated apoptotic threshold of malignant myeloid cells. Consequently, we provide the first evidence that BH3 profiling can distinguish clinical response to 5-Aza. A NOXA plus BIM-profiling metric exhibited the most significant correlation with clinical response to 5-Aza-based therapy. We show that BIM (broad complementary binding affinity to several BCL-2 members) and NOXA (specific affinity for MCL-1) metrics are critical determinants of 5-Aza response, in agreement with data showing 5-Aza upregulation of NOXA and reduction of MCL-1.43, 47 The compiled data, including BH3 profiling, further support a model, whereby the overall balance of pro- and antiapoptotic BCL-2 family proteins, and thus a specific apoptotic threshold, is a critical determinant of response to 5-Aza. The complementary results of BH3 profiling to RNAi, pharmacological and gene expression data, support an underlying biological relevance and putative role for BH3 profiling to identify patients more likely to respond to 5-Aza, a crucially important unmet clinical need, even if important mechanistic questions remain unanswered. Accordingly, patients with an unfavorable BH3 profile for single-agent 5-Aza response could be considered for a trial combining 5-Aza with antiapoptotic BCL-2 family-targeting agents.
In summary, we provide functional genomic and pharmacologic evidence to (i) further refine and define optimal inhibitory spectra for the development of novel BCL-2 family inhibitory compounds for application in myeloid malignancies, (ii) propose a clinical trial with 5-Aza and antiapoptotic BCL-2 family-targeting agents in myeloid malignancies including MDS and MPN patients and (iii) propose to prospectively test BH3 profiling as a predictor of 5-Aza response.
Acknowledgments
This work was supported in part by American Cancer Society Postdoctoral Fellowship Grant 119364-PF-10-123-01 awarded to JMB, in part by a grant from the IBIS Foundation for Individualized Medicine awarded to RT, in part by a Mayo Clinic Career Development award to RT, and in part by NCI-SBIR #HHSN261201200039C and #HHSN261201299985C contracts to Eutropics Pharmaceuticals. Institutional support was provided by TGen and Mayo Clinic.
Author contributions
JMB performed research, designed and executed experiments, analyzed and interpreted data, and wrote the manuscript; SK performed research, RPPA experiments and analysis; WEP, RL, and MC performed BH3-profiling assays and analysis; DC assisted RNAi assay development and experiments; C-XS assisted with experiments; JM and GA isolated primary patient samples; IG assisted RNAi screening experiments; AC and RT performed RNAi screen analysis; RV, JC, VF, and RAM provided primary patient samples and clinical information; YQ and KRC performed RPPA experiments and analysis; EB performed research and analysis; HHY directed assay development and provided essential infrastructure; DOA designed and analyzed experiments, and provided essential infrastructure; AKS provided essential infrastructure; and RT conceived and directed the project, performed research, designed experiments, analyzed and interpreted data, and wrote the manuscript.
William Pierceall, Ryan Lena and Michael Cardone are employees and equity owners of Eutropics Pharmaceuticals. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Mesa RA, Tibes R. MPN blast phase: clinical challenge and assessing response. Leuk Res. 2012;36:1496–1497. doi: 10.1016/j.leukres.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Cherington C, Slack JL, Leis J, Adams RH, Reeder CB, Mikhael JR, et al. Allogeneic stem cell transplantation for myeloproliferative neoplasm in blast phase. Leuk Res. 2012;36:1147–1151. doi: 10.1016/j.leukres.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbert M, Suciu S, Baila L, Ruter BH, Platzbecker U, Giagounidis A, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- Thepot S, Itzykson R, Seegers V, Raffoux E, Quesnel B, Chait Y, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM) Blood. 2010;116:3735–3742. doi: 10.1182/blood-2010-03-274811. [DOI] [PubMed] [Google Scholar]
- Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenterphase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa RA, Verstovsek S, Rivera C, Pardanani A, Hussein K, Lasho T, et al. 5-Azacitidine has limited therapeutic activity in myelofibrosis. Leukemia. 2009;23:180–182. doi: 10.1038/leu.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013;27:1028–1036. doi: 10.1038/leu.2012.312. [DOI] [PubMed] [Google Scholar]
- Cluzeau T, Robert G, Mounier N, Karsenti JM, Dufies M, Puissant A, et al. BCL2L10 is a predictive factor for resistance to azacitidine in MDS and AML patients. Oncotarget. 2012;3:490–501. doi: 10.18632/oncotarget.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25:1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- Ettou S, Audureau E, Humbrecht C, Benet B, Jammes H, Clozel T, et al. Fas expression at diagnosis as a biomarker of azacitidine activity in high-risk MDS and secondary AML. Leukemia. 2012;26:2297–2299. doi: 10.1038/leu.2012.152. [DOI] [PubMed] [Google Scholar]
- Tohyama K, Tsutani H, Ueda T, Nakamura T, Yoshida Y. Establishment and characterization of a novel myeloid cell line from the bone marrow of a patient with the myelodysplastic syndrome. Br J Haematol. 1994;87:235–242. doi: 10.1111/j.1365-2141.1994.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Tibes R, Bogenberger JM, Chaudhuri L, Hagelstrom RT, Chow D, Buechel ME, et al. RNAi screening of the kinome with cytarabine in leukemias. Blood. 2012;119:2863–2872. doi: 10.1182/blood-2011-07-367557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med. 2005;110:173–183. doi: 10.1385/1-59259-869-2:173. [DOI] [PubMed] [Google Scholar]
- Kornblau SM, Tibes R, Qiu YH, Chen W, Kantarjian HM, Andreeff M, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–164. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Qiu Y, Huang X, Diao L, Zhang N, Coombes KR, et al. Survivin is highly expressed in CD34(+)38(-) leukemic stem/progenitor cells and predicts poor clinical outcomes in AML. Blood. 2012;120:173–180. doi: 10.1182/blood-2012-02-409888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CM, Bruncko M, Adickes J, Bauch J, Ding H, Kunzer A, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–6915. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Vandenberg CJ, ABT-199 CoryS. a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121:2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernandez-Luna JL. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338:564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- Zeuner A, Pedini F, Francescangeli F, Signore M, Girelli G, Tafuri A, et al. Activity of the BH3 mimetic ABT-737 on polycythemia vera erythroid precursor cells. Blood. 2009;113:1522–1525. doi: 10.1182/blood-2008-03-143321. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, El-Sabban M, Awaraji C, Abboushi N, D4baibo GS. Distinct sites of action of Bcl-2 and Bcl-xL in the ceramide pathway of apoptosis. Biochem J. 1998;336 (Pt 3:735–741. doi: 10.1042/bj3360735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Aimiuwu J, Wang H, Chen P, Xie Z, Wang J, Liu S, et al. RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood. 2012;119:5229–5238. doi: 10.1182/blood-2011-11-382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komashko VM, Farnham PJ. 5-azacytidine treatment reorganizes genomic histone modification patterns. Epigenetics. 2010;5:229–240. doi: 10.4161/epi.5.3.11409. [DOI] [PubMed] [Google Scholar]
- Bogenberger JM, Shi C-X, Gonzales I, Tiedemann R, Noel P, Slack J, et al. RNAi screening identifies BCL-XL as an erythroid lineage-specific 5-Azacytidine sensitizer while the BCL-2/BCL-XL/BCL-2 inhibitor ABT-737 results in more universal sensitization in leukemia cells Blood (ASH Annual Meeting Abstracts) 2011118Abstract 3513. [Google Scholar]
- Bogenberger JM, Shi C-X, Hagelstrom RT, Gonzales I, Choudhary A, Tiedemann R, et al. Synthetic lethal RNAi screening identifies inhibition of Bcl-2 family members as sensitizing to 5-Azacytidine in myeloid cells AACR (Annual Meeting Abstracts) 201070Abstract LB128 [Google Scholar]
- Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91:1861–1870. doi: 10.1007/s00277-012-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harbi S, Hill BT, Mazumder S, Singh K, Devecchio J, Choudhary G, et al. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood. 2011;118:3579–3590. doi: 10.1182/blood-2011-03-340364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Duvvuri S, Ruvolo V, Samaniego F, Younes A, Andreeff M. Decreased sensitivity of 17p-deleted chronic lymphocytic leukemia cells to a small molecule BCL-2 antagonist ABT-737. Cancer. 2012;118:1023–1031. doi: 10.1002/cncr.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiziltepe T, Hideshima T, Catley L, Raje N, Yasui H, Shiraishi N, et al. 5-Azacytidine, a DNA methyltransferase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol Cancer Ther. 2007;6:1718–1727. doi: 10.1158/1535-7163.MCT-07-0010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.