Abstract
Introduction
Computed tomography (CT) radiation exposure has come under increasing scrutiny due to dramatically increased utilization. Multiphase CT studies (repeated scanning before and after contrast injection) are potentially important, overlooked source of medically unnecessary radiation due to the dose-multiplier effect of extra phases. The purpose of this study is to determine the frequency of unindicated multiphase scanning and resultant excess radiation exposure in a sample referral population.
Methods
This study was IRB approved and HIPAA compliant. Abdomen/pelvis CT exams (n=500) performed at outside institutions submitted for tertiary interpretation were retrospectively reviewed for 1) appropriateness of each phase based on clinical indication and American College of Radiology (ACR) Appropriateness Criteria, and 2) per phase and total radiation effective dose.
Results
A total of 978 phases were performed in 500 patients, 52.8% (264/500) received phases that were not supported by ACR criteria. Overall, 35.8% (350/978) of phases were unindicated, most commonly being delayed imaging (272/350). The mean overall total radiation effective dose per patient was 25.8 mSv (95% CI 24.2, 27.5 mSv). Mean effective dose for unindicated phases was 13.1 (12.3, 14.0) mSv, resulting in a mean excess effective dose of 16.8 (15.5, 18.3) mSv per patient. Unindicated radiation comprised 33.3% of the total radiation effective dose in this population. Radiation effective doses exceeding 50 mSv were found in 21.2% (106/500) of patients.
Discussion
The results of this study suggest that a large proportion of patients undergoing abdominal/pelvic CT scanning receive unindicated additional phases that add substantial excess radiation dose with no associated clinical benefit.
INTRODUCTION
Computed tomography (CT) scanning has become ubiquitous in medicine. Recent technical advances including faster scan times, improved spatial resolution, and advanced multi-planar reconstruction techniques have increased the usefulness of CT for virtually every anatomic abnormality. Concomitantly, a rise in defensive medicine and ownership interest in CT centers by referring physicians have resulted in a dramatic increase in utilization.1,2,3 Approximately 3 million scans were performed annually in the United States in 1980, and by 2008 that number had grown to 67 million.4 Along with this increased number of scans, an increasing awareness of medical radiation has permeated the popular and scientific press. Over two-thirds of all medical radiation can now be attributed to CT, with the majority resulting from examinations of the chest, abdomen, and pelvis. 5,6,7
While there is no doubt that radiation exposure from CT has been increasing rapidly, the significance of this exposure remains unclear. High levels of ionizing radiation exposure are known to increase cancer risk8,9,10 but the data for lower doses of radiation is less clear and remains controversial.11,12,13 Therefore, in the absence of clarity on this topic, the American College of Radiology (ACR), Health Physics Society (HPS) and other interested organizations have adopted the principle of As Low As Reasonably Achievable (ALARA) in which physicians should minimize the amount of radiation exposure to only what is medically necessary.7,14,15
Most strategies to reduce radiation associated with CT have focused on vetting CT as the appropriate diagnostic test, limiting the examination to the anatomic area in question, and optimizing scanning parameters (particularly in pediatric patients).2,16,17,18 Applying optimized technical parameters alone can decrease radiation exposure by up to 65%.15,16 However, an important, but potentially overlooked source of medically unnecessary radiation is the use of multiphase examinations when a single or lesser number of phases would suffice.16 The different phases that are possible with state-of-the-art CT scanners are myriad and include scanning before and after contrast administration, delayed imaging, venous and arterial phases, and others. Considering the dose-multiplication effect of extra phases, it is possible that inappropriate multiphase CT could be an important source of excess radiation exposure. Recognizing the need for guidelines addressing multiphase examinations, the American College of Radiology (ACR) has developed evidence-based appropriateness criteria describing scanning protocols with specific phase selections for various clinical conditions.19
The purpose of this study is to determine the frequency with which the ACR Appropriateness Criteria for abdominal and pelvic CT are being followed, the frequency of unindicated phases, and the magnitude of excess radiation exposure for patients when unindicated phases are performed.
MATERIALS AND METHODS
Selection and Description of Participants
This study was approved by the human subjects committee of our institutional review board with a waiver of informed consent. The patient group consisted of 708 consecutive abdomen/pelvis CT scans performed at outside institutions during a 4 month period (2/26/2008–6/6/2008) and submitted to our institution for an official “over-read”. Exclusion criteria included: non-digitized images; pelvis-only examinations; specialty examinations including CT colonography, CT-guided biopsies and vascular studies; and studies where the clinical indication was unknown. The final cohort was comprised of 500 patients with a median age of 60 years (range: 9 months–90 years). There were 263 females (53%) and 237 males (47%) with 18 patients 18 years of age and younger. The studies were primarily from referring institutions in Wisconsin and Illinois, with a smaller number coming from Michigan, Minnesota, Iowa, Ohio, Florida, Missouri, and Alaska.
Appropriateness Criteria
CT examinations were reviewed by one of two experienced abdominal radiologists (Fred T. Lee or J. Louis Hinshaw) to determine which phases were indicated for the given clinical indication. American College of Radiology (ACR) appropriateness criteria19 was used as the gold standard. A CT phase was considered to be appropriate (indicated) if the ACR appropriateness criteria was ≥ 4 (scale 1–9, 4–6 “may be appropriate”, 7–9 “usually appropriate”), and unindicated if the score was <4. Each examination that had an “unindicated” phase(s) was reviewed to determine if there was an incidental finding on the scan that could justify additional scanning for further characterization (e.g. incidental liver mass necessitating delayed imaging). If so, these phases were categorized as “unindicated but justified.”
Technical Information
Radiation Effective Dose Calculations
The clinical history, indication, phases performed, scanning parameters (including CT scanner make and model, tube current, kilovoltage, slice thickness, collimation, rotation time, and pitch) and body part were all recorded. CT scanner models from GE, Siemens, Toshiba, and Phillips were represented. The collected parameters were used to calculate effective dose for each phase using the ImPACT CT Patient Dosimetry Calculator (Version 0.99x 20/01/06) and the effective dose in millisieverts (mSv) was recorded.20 For patients with more than one phase, doses were added together to obtain a total dose per patient. The patients with unindicated but justified phases were analyzed with the unindicated group. These patients were initially identified to determine which patients had incidental findings that if noted on the CT scanner, could warrant additional phases. However, as it was impossible to determine if these findings were identified before or after the patient left the CT scanner (with the latter thought to be more likely), we analyzed these patients as part of the unindicated group.
Rotation time and pitch was unavailable in 56 of 500 subjects. For these patients, the rotation time and pitch were estimated using the mean values obtained from all other scans that used the same CT scanner model.
Statistical Analysis
The distribution of total effective dose, indicated effective dose and excess effective dose were all skewed so that a log transformation was necessary to obtain approximate Gaussian distributions. Differences between effective doses in various groups were assessed using 2 sample T-tests after transformation to the log scale. All reported means and 95% confidence intervals were calculated in the log scale and transformed back to the original units. Comparisons of proportions were done using Chi-squared tests. P-values < 0.05 were considered statistically significant. Mean radiation effective doses (95% confidence intervals) are reported in milliSieverts (mSv).
RESULTS
Scan Phases
The majority of patients (307/500, 61.4%) had a multiphase CT examination with 264/307 (86.0%) of these patients having at least one phase that was not indicated (Table 1). Overall, 264/500 (52.8%) of the total patient population had at least one unindicated phase and 350/978 (35.8%) of all phases were unindicated. The majority of the unindicated phases were delayed phase imaging (272/350, 78%), with the remainder being a combination of arterial phase (37/350, 11%) and non-contrast imaging (41/350, 12%).
Table 1.
Number of phases per patient
| Number of phases per study | Number of patients (N=500) | Total number of phases | Number of Unindicated Phases | % of total phases that are unindicated |
|---|---|---|---|---|
| 1 | 192 | 192 | 0 | 0% |
| 2 | 176 | 350 | 152 | 43.4% |
| 3 | 101 | 303 | 146 | 48.2% |
| 4 | 23 | 92 | 36 | 39.1% |
| 5 | 8 | 40 | 16 | 40% |
Radiation Effective Dose
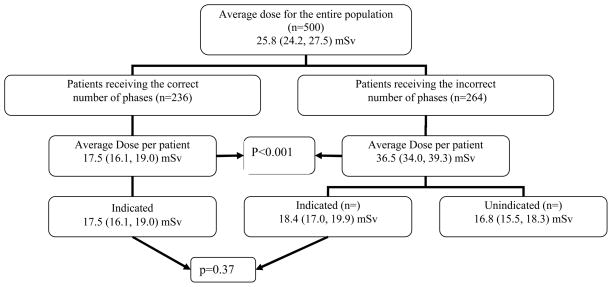
The mean effective dose per patient for the entire patient population was 25.8 (24.2, 27.5) mSv (range: 3.5–144 mSv) with a mean effective dose per CT phase of 14.1 (13.6, 14.7) mSv (range: 2.1–71.0 mSv). In patients who received the correct number of phases (n=236), the mean effective dose per patient was 17.5 (16.1, 19.0) mSv. When unindicated phases were performed (n=264) the total mean effective dose per phase was 13.1 (12.3, 14.0) and the mean effective dose per patient was substantially higher at 36.5 (34.0, 39.3), with 18.4 (17.0, 19.9) mSv being indicated and 16.8 (15.5, 18.3) mSv being unindicated (figure 1). The total excess effective dose over all patients is 5,484.2 mSv and the total effective dose over all patients is 16,449.1. Thus 33.3% of the total radiation effective dose to the patient population was due to unindicated phases.
Figure 1.
Mean total radiation dose for the entire population was 25.8 mSv. The mean dose for patients who received unindicated phases was substantially higher than patients that only received indicated phases (P < 0.001). The indicated dose for both groups was not significantly different (p=0.37)
Patients who had unindicated phases had a mean effective dose that was significantly higher than for the patients who had an appropriate imaging protocol (36.5 [34.0, 39.2] mSv vs. 17.5 [16.1, 19.0] mSv, p < 0.001). For patients who had an imaging protocol not supported by ACR criteria, a mean of 46.1% (44.4%, 47.9%) of their radiation effective dose was attributable to unindicated phases. Notably 106/500 (21.2%) patients received a radiation effective dose in excess of 50 mSv, and 7/500 (1.4%) patients received a radiation effective dose in excess of 100 mSv for a single examination, levels that are not appropriate in any setting. Patients were more likely to have a greater overall radiation effective dose if unindicated phases were performed (add doses, p < 0.001). No significant difference in the total indicated radiation effective dose was seen between the patients who had unindicated phases and those that received only indicated phases (add doses, p = 0.37).
Radiation Effective Dose by Age
Patients less than 50 years of age were less likely to receive excess radiation (57/128, 44.5%), versus those ages 50 and above (207/372, 55.6%, p = 0.044) (Table 2). However, excess radiation effective dose was seen in patients in all age groups, and patients 30–39 years old had the highest mean excess radiation. The patients aged 30–39 that had unindicated phases had a total mean radiation effective dose of 41.7 (32.5, 53.4) mSv, of which 23.2 (27.6, 30.5) mSv was not indicated (Table 2).
Table 2.
Age distribution of indicated and unindicated phases and dose
| Age | Number of phases per patient | Mean dose per patient | ||||
|---|---|---|---|---|---|---|
| indicated | unindicated | % Indicated phases | Total | Indicated | Excess* | |
| 0–9 | 15 | 5 | 68.4 | 8.5 | 6.6 | 6.4 |
| 10–19 | 11 | 2 | 63.2 | 11.8 | 10.3 | 7.3 |
| 20–29 | 14 | 9 | 60.9 | 18.3 | 12.4 | 14.6 |
| 30–39 | 34 | 24 | 67.8 | 25.5 | 16.6 | 23.2 |
| 40–49 | 81 | 39 | 71.8 | 27.3 | 19.6 | 20.8 |
| 50–59 | 149 | 82 | 64.9 | 27.4 | 19.0 | 17.3 |
| 60–69 | 184 | 108 | 62.4 | 27.3 | 18.6 | 15.6 |
| 70–79 | 89 | 50 | 60.1 | 26.3 | 18.8 | 16.1 |
| 80–89 | 50 | 31 | 62.4 | 27.5 | 18.7 | 18.3 |
| 90+ | 1 | 0 | 100.0 | 26.0 | 26.0 | 0.0 |
| All patients | 628 | 350 | 64.2 | 25.8 | 17.5 | 16.8 |
Mean excess dose is calculated using patients with excess dose only.
Radiation Effective Dose For Benign and Malignant Indications
Patients being evaluated for an underlying malignancy (i.e. known malignancy, palpable abdominal mass, suspicious lesion/carcinomatosis identified on another imaging modality, or painless jaundice) comprised 238/500 (47.6%) of the patient population. The remaining 262 patients were being evaluated for likely benign indications. Overall, individuals being evaluated for malignancy were significantly more likely to receive excess effective dose than those being evaluated for benign indications (148/262, 56.5% vs. 88/238, 37.0%, p < 0.001).
Unindicated, but Justified Phases
Additional scanning for further characterization could be justified on the basis of scan findings in 20/307 (6.5%) of patients. It is not known whether these phases were obtained in response to the finding, or if they were performed as standard practice at the referring institution. The radiation effective dose from these phases represented 3.9% of the total effective dose seen. The incidental findings identified included: renal lesions (n=12), an unsuspected liver lesion (n=3), and hydronephrosis (n=5).
DISCUSSION
The motivation for this study was the anecdotal observation that a large proportion of CT studies submitted to our tertiary center for re-interpretation were performed with multiphase scan protocols that were not appropriate for the clinical indication. Since extra phases effectively multiply radiation dose, we hypothesized that unindicated multiphase examinations were an important source of excess radiation, particularly if extrapolated across larger populations. The results of this study confirm the hypothesis: More than 50% of patients were exposed to at least one unindicated phase, resulting in a mean excess dose of 16.8 mSv of medically unnecessary radiation. The overall mean radiation dose in our population was 25.8 mSv which far exceeds21 the national benchmark of 10–15 mSv per CT examination,20,22,23 and the majority of this difference was due to unindicated phases. If patients had received only phases indicated by ACR Appropriateness Criteria, the mean radiation dose would have been 17.9 mSv. This suggests that the high radiation doses seen in this population (and potentially throughout the country) are correctable with simple changes in practice. Conforming to ACR guidelines in this patient population would have reduced the radiation exposure by 46.1% for patients that received inappropriate multiphase examinations, or 33.3% for the entire population. Thus, it appears that inappropriate multiphase scanning could be an even more important source of medically unnecessary radiation than non-optimized technical scanner settings. Prior studies of scanner settings have suggested that a potential decrease in radiation dose of 20–65%15,16 is possible with parameter optimization,2,,16,17,18 but given recent attention in the medical and lay press, much of this improvement has likely already been realized.
Data from experimental models suggests that a carcinogenic risk secondary to high dose ionizing radiation is real.24,25 However, the risk at low doses of radiation typical in medical imaging, and/or the threshold required for carcinogenesis remains unclear.10,11 The linear-no-threshold (LNT) model championed by some authors suggests proportionality between dose and cancer risk (even at very low doses) and represents the worst possible scenario for low dose exposure.26,27 There is serious doubt about the validity of the LNT theory,10,28 but it is often quoted as a means to estimate cancer risk for patients exposed to low dose radiation.29 There are several studies that argue against the LNT model, and no clear evidence for increased cancer risk with low dose radiation exposure has been identified to date.30,31,32 However, in light of the current uncertainty and the unknown “true” risk of low dose radiation, the concept of ALARA should be followed. Notably, 21.2% of the patients in this study received ≥ 50 mSv, and 1.4% received ≥100 mSv for a single examination, levels that meet or exceed thresholds for increased cancer risk proposed by various studies and certainly higher than is acceptable by any current published standard for diagnostic CT.8,9,10,11 Interestingly, the patients with doses >100mSv, all had at least one unindicated phase.
Although younger patients being evaluated for benign indications were less likely to receive excess phases, these patients still received large amounts of medically unnecessary radiation. Overall, the greatest excess radiation dose was in the 30–39 year age group. This is important because excess radiation in younger patients has a higher potential for adverse outcomes than in older adults33 due to more radiosensitive tissues,8 and a longer lifespan with more opportunities for any radiation-induced genetic mutations to be expressed as neoplasia.
Although the retrospective nature of this study did not allow us to investigate the reasons behind the performance of inappropriate multiphase exams, we suspect that a lack of focus on performing protocols tailored for the individual patient and clinical indication is the predominant factor, with most patients being prospectively scanned with extra phases to reduce call backs. Based on the results of this study, the risk of call backs appears to be negligible with a paucity of cases where inappropriate extra phases were justified. In addition, it is likely that if given the choice, most patients would accept the inconvenience of a call back for additional scanning rather than routine exposure to unindicated series. As professional societies, accrediting bodies, insurers, and health care institutions increasingly use radiation exposure as a measure of health care quality, the routine use of multiphase examinations will become increasingly difficult to defend. Already, radiologists are being asked to dictate radiation exposure into reports as a pay-for-performance measure.34 However, currently we are unaware of payments for inappropriate multiphase examinations being systematically denied due to excess radiation exposure (excluding contrast/non-contrast phases where an inappropriate increase in charges could be an issue). The results of this study make it clear that there is substantial room for improvement and specialty societies can aid in this effort by providing parameters and protocols that set diagnostic dose references, radiation safety education beginning in medical school, and other tools to support optimization efforts. 26
This study had several limitations. The small sample size and limited geographical area make it difficult to be certain that our results can be extrapolated across the entire US population. Because of the small number of pediatric patients in the study, strong conclusions about excess radiation exposure in children caused by multiphase CT are not possible. Perhaps this issue could be better addressed with a similar study based at a large children’s hospital. An additional limitation of our study is a possible selection bias towards highly ill patients based on the referral to a tertiary care center. Based on a non-quantitative evaluation of the patient population, this does not appear to be the case. The most frequent indications for scanning in our study were relatively routine: abdominal pain (50/500), flank pain (41/500), follow up prostate cancer (24/500), and follow up colon cancer (19/500). An additional potential limitation is that this study used the ACR Appropriateness Criteria as the sole adjudication method to determine the appropriateness of each CT phase. While it is our belief that these criteria are the most widely accepted guidelines available for this purpose, they do not cover every clinical situation and they are not being continuously updated. In terms of radiation dose calculators, an exhaustive description of the various methods to calculate radiation dose is beyond the scope of this study. However, the methods used in this study have been previously validated, have been used in many peer-reviewed publications, and are widely accepted.35 A different calculator may have resulted in different overall radiation exposures, but the relative impact of medically inappropriate scanning would likely not be changed.
Conclusion
In summary, our study suggests that a large proportion of patients who undergo abdominal/pelvic CT scanning receive medically unnecessary multiphase examinations, resulting in substantial excessive radiation exposure. This source of excess radiation appears almost entirely correctable by widespread adoption of individual scan protocoling tailored specifically for the patient’s clinical condition and guided by the ACR Appropriateness Criteria or other evidence-based criteria. Lastly, the routine use of “one size fits all” multiphase protocols for abdomen and pelvis CT should cease immediately.
Acknowledgments
Funding: Mary J Lindstrom’s contribution to this manuscript was supported by a NIH grant 1UL1RR025011.
Footnotes
The authors of this manuscript have no conflict of interest or financial disclosures to report.
References
- 1.Gazelle Scott G, Halpern, et al. Utilization of Diagnostic Medical Imaging: Comparison of Radiologist Referral versus Same-Specialty Referral. Radiology. 2007;245(2):517–522. doi: 10.1148/radiol.2452070193. [DOI] [PubMed] [Google Scholar]
- 2.Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Ownership or Leasing of CT Scanners by Nonradiologist Physicians: A Rapidly Growing Trend That Raises Concern About Self-Referral. Journal of the American College of Radiology. 2008;5(12):1206–1209. doi: 10.1016/j.jacr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609–17. doi: 10.1001/jama.293.21.2609. [DOI] [PubMed] [Google Scholar]
- 4.IMV 2006 CT Market Summary Report. Des Plains, IL: IMV Medical Information Division; 2006. [Google Scholar]
- 5.Brenner DJ, Hall EJ. Computed Tomography – An Increasing Source of Radiation Exposure. NEJM. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 6.Mettler FA, Jr, Wiest PW, Locken JA, Kelsey CA. CT Scanning : Pattern of Use and Dose. Journal of Radiological Protection. 2000;204:353–359. doi: 10.1088/0952-4746/20/4/301. [DOI] [PubMed] [Google Scholar]
- 7.Brix G, Nissen-Meyer S, Lechel U, et al. Radiation exposures of Cancer Patients from Medical X-rays: How Relevant are they for Individual Patients and Population Exposure? European Journal of Radiology. 2009;72(2):342–347. doi: 10.1016/j.ejrad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Pierce DA, Preston DL. Radiation-Related Cancer Risks at Low Doses Among Atomic Bomb Survivors. Radiat Res. 2000;154:178–186. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the Mortality of Atomic Bomb Survivors. Report 12, Part I. Cancer- 1950–1990. Radiation Res. 1996;146:1–27. [PubMed] [Google Scholar]
- 10.Muirhead CR. Studies on the Hiroshima and Nagasaki Survivors, and Their Use in Estimating Radiation Risks. Radiation Protection Dosimetry. 2003;104:331–335. doi: 10.1093/oxfordjournals.rpd.a006196. [DOI] [PubMed] [Google Scholar]
- 11.Mezerich R. Are CT Scans Carcinogenic? American College of Radiology. 2008:691–693. doi: 10.1016/j.jacr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Cardis E, Vrijheid M, Blettner M, et al. Risk of Cancer After Low Doses of Ionizing Radiation: Retrospective Cohort Study in 15 Countries. BMJ. 2005:331–377. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little MP, Wakeford R, Tawn JE, Bouffler SD, Berrington de Gonzalez A. Risks Associated with Low Doses and Low Dose rates of Ionizing radiation: Why Linearity May be (Almost) the Best We Can Do. Radiology. 2009;251(1):6–12. doi: 10.1148/radiol.2511081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee on the Biological Effects of Ionizing Radiation. Health Effects of Exposure to Low Levels of Ionizing Radiation. Washington, DC: National academy Press; 1990. [Google Scholar]
- 15.ICRP. ICRP Publication no 60. Oxford, UK: Pergamon; 1991. 1990 Recommendations of the International Commission on Radiological Protection. [PubMed] [Google Scholar]
- 16.Greess H, Nomayr A, Wolf H, Baum U, et al. Dose Reduction in CT Examinations of Children by an attenuation-based on-line modulation of tube current(CARE dose) European Radiology. 2002;12:1571–1576. doi: 10.1007/s00330-001-1255-4. [DOI] [PubMed] [Google Scholar]
- 17.Tack D, De Maertelaer V, Gevenois PA. Dose Reduction in Multidetector CT Using Attenuation-Based Online Tube Current Modulation. American Journal of Roentgenology. 2003;181:331–334. doi: 10.2214/ajr.181.2.1810331. [DOI] [PubMed] [Google Scholar]
- 18.Paterson A, Frush D, Donnelly LF. Helical CT of the Body : Are Settings Adjusted for Pediatric Patients? American Journal of Radiology. 2001;176:297–301. doi: 10.2214/ajr.176.2.1760297. [DOI] [PubMed] [Google Scholar]
- 19.ACR Appropriateness Criteria. 2008 Available at: http://acr.org/ac.
- 20.McNitt-Gray MF. AAMP/RSNA Physics Tutorial for Residents: Topics in CT. Radiographics. 2002;22(6):1541–1553. doi: 10.1148/rg.226025128. [DOI] [PubMed] [Google Scholar]
- 21.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation Dose Associated With Common Computed Tomography Examinations and the Associated Lifetime Attributable Risk of Cancer. Archives of Internal Medicine. 2009;169(22):2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nationwide Evaluation of X-ray Trends :2000 Computed Tomography. Conference of Radiation Control Program Directors; Department of Health and Human Services; 2006. [Google Scholar]
- 23.Nafziger B. Rad Groups Recommend Keeping a Closer Eye on CT Scan Dosage Protocols After Cedars-Sinai Fiasco. 2009 Nov 4; Available at: http://www.dotmed.com/news/story/10650.
- 24.Dikomey E, Brammer I. Relationship between Cellular Radiosensitivity and Non-Repaired Double Strand Breaks Studied for Different Growth States, Dose Rates, and Plating conditions in a normal Fibroblast line. Int Journal Radiation Biology. 2000;76(6):773–781. doi: 10.1080/09553000050028922. [DOI] [PubMed] [Google Scholar]
- 25.Boucher D, Hindo J, Averbeck D. Increase Repair of Gamma-Induced DNA Doubls Strand Breaks at Lower Dose-Rate in CHO Cells. Canada Journal Physio Pharmacol. 2004;82(2):125–132. doi: 10.1139/y04-006. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Bindman Rebecca. Is Computed Tomgraphy safe? NEJM. 2010;363(1):1–4. doi: 10.1056/NEJMp1002530. [DOI] [PubMed] [Google Scholar]
- 27.Thrall JH. Utilization of Imaging: Challenges and Opportunities. American College of Radiology. 2009;4(1):287–288. doi: 10.1016/j.jacr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Tubiana Maurice, Feinendegen Ludiwg E, Yang Chichuan, Kaminski Joseph. The Linear NoThreshold Relation is Inconsistent with Radiation Biologic and Experimental Data. Radiology. 2009;251(1):13–21. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research Council. BEIR VII: Health risks from Exposure to Low levels of Ionizing Radiation. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 30.Gerber TC, Carr JJ, Arai AE, et al. Ionizing Radiation in Cardiac Imaging. Circulation. 2009;119:1056–1965. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 31.Fazel Reza, Krumholz Harlan M, Wang Yongfei, et al. Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures. NEJM. 2009;361(9):849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall BF, Kendall GM, Edwards AA, Bouffler S, Muirhead CR, Meara JR. What are the risks from medical X-rays and other low dose radiation? The British Journal of Radiology. 2006;79:285–294. doi: 10.1259/bjr/55733882. [DOI] [PubMed] [Google Scholar]
- 33.Brenner David J. One Size Does not Fit All: Reducing Risks from Pediatric CT. ACR Bulletin. 2001;57(2):20–23. [Google Scholar]
- 34.Radiology Physician Performance Measure Set. American Medical Association and National Committee for Quality Assurance. 2008:1–43. [Google Scholar]
- 35.Lewis MA, Edyvean S, Sassi SA, Kiremidjian H, Keat N, Britten AJ. Estimating patient dose on current CT scanners: Results of the ImPACT CT dose survey. London: ImPACT; 1999. ( www.impactscan.org/dosesurveysummary.htm) [Google Scholar]