Abstract
Exaggerated effects of word length upon reading-aloud performance define pure alexia, but have also been observed in semantic dementia. Some researchers have proposed a reading-specific account, whereby performance in these two disorders reflects the same cause: impaired orthographic processing. In contrast, according to the primary systems view of acquired reading disorders, pure alexia results from a basic visual processing deficit, whereas degraded semantic knowledge undermines reading performance in semantic dementia. To explore the source of reading deficits in these two disorders, we compared the reading performance of 10 pure alexic and 10 semantic dementia patients, matched in terms of overall severity of reading deficit. The results revealed comparable frequency effects on reading accuracy, but weaker effects of regularity in pure alexia than in semantic dementia. Analysis of error types revealed a higher rate of letter-based errors and a lower rate of regularization responses in pure alexia than in semantic dementia. Error responses were most often words in pure alexia but most often nonwords in semantic dementia. Although all patients made some letter substitution errors, these were characterized by visual similarity in pure alexia and phonological similarity in semantic dementia. Overall, the data indicate that the reading deficits in pure alexia and semantic dementia arise from impairments of visual processing and knowledge of word meaning, respectively. The locus and mechanisms of these impairments are placed within the context of current connectionist models of reading.
Keywords: Reading aloud, Pure alexia, Letter-by-letter reading, Semantic dementia, Surface dyslexia
Efficient activation and integration of orthographic knowledge is essential in fluent reading. Any disruption to this process as a consequence of brain damage will result in some form of reading deficit, or acquired dyslexia. One such disorder is pure alexia (PA), which is seen after damage to or disconnection of the left ventral occipito-temporal cortex (vOTC). Behaviourally, the traditional definition of PA is as a highly selective reading deficit, without associated problems in spoken language (aphasia), spelling (dysgraphia), or object recognition (agnosia) (Déjerine, 1892). PA patients experience difficulties in accurate and rapid parallel activation of the letters in words, which undermines their reading. This is evident in a very marked effect of the number of letters in a word on patients' reading speed (Behrmann & Plaut, 2013a; Roberts et al., 2013), which stands in contrast to the minimal effects of word length seen in normal individuals' reading aloud (Henderson, 1982; Weekes, 1997). This exaggerated length effect in PA is interpreted as reflecting sequential letter identification, or letter-by-letter (LBL) reading, and indeed some patients show this reading strategy overtly.
While the hallmark length effect that defines PA is well established and accepted, the cognitive cause of the reading deficit has been the matter of considerable debate. By one account, PA is a reading-specific disorder, and reports of patients who have shown normal visual processing and recognition of objects have been used to support such a view (e.g., Kay & Hanley, 1991; Miozzo & Caramazza, 1998) and vice versa (e.g., Yong, Warren, Warrington, & Crutch, 2013). Within this approach, a number of researchers have suggested that PA arises as a result of damage to an orthographic input lexicon or its incoming connections (e.g., Marshall & Newcombe, 1973; Noble, Glosser, & Grossman, 2000; Warrington & Langdon, 1994; Warrington & Shallice, 1979, 1980), which contains entries for all known word forms and has been associated with left vOTC (Cohen et al., 2002; Vinckier et al., 2007). As a result, these patients can no longer efficiently activate word forms, so the LBL reading strategy functions to boost activation of appropriate candidate lexical entries.
Reading-specific accounts that focus on damage to orthographic lexical representations should predict an increased incidence of nonlexical reading responses, which, in the case of irregular words, would take the form of regularization errors (e.g., sew read as “sue”). While PA patients do show some evidence of enhanced effects of regularity on reading aloud (Behrmann, Nelson, & Sekuler, 1998; Rapcsak & Beeson, 2004), regularization responses are relatively rare (Cumming, Patterson, Verfaellie, & Graham, 2006; Patterson & Kay, 1982). Hence a different form of a reading-specific account proposed that PA patients may in fact have difficulties with letter recognition, which would compromise input to both lexical and nonlexical processing (Arguin & Bub, 1993; Behrmann & Shallice, 1995; Bub, Black, & Howell, 1989; Hanley & Kay, 1996; Howard, 1991; Patterson & Kay, 1982; Perri, Bartolomeo, & Silveri, 1996; Reuter-Lorenz & Brunn, 1990; Rosazza, Appollonio, Isella, & Shallice, 2007). This account is consistent with the observation that PA patients often misidentify the component letters of words (Cumming et al., 2006).
A contrasting perspective on PA is that it arises from a particular kind of visual deficit that undermines the input to the reading system (Behrmann, Plaut, & Nelson, 1998; Farah & Wallace, 1991). This view falls within the primary systems account of acquired dyslexia, whereby reading disorders arise due to disruption of more basic visual, phonological, and semantic processing (Patterson & Lambon Ralph, 1999), which has been implemented in connectionist models of reading (Chang, Furber, & Welbourne, 2012a; Plaut & Behrmann, 2011; Welbourne, Woollams, Crisp, & Lambon Ralph, 2011; Woollams, Lambon Ralph, Plaut, & Patterson, 2007). Neuroimaging studies reveal that vOTC receives high-acuity foveal visual input (Hasson, Harel, Levy, & Malach, 2003; Hasson, Levy, Behrmann, Hendler, & Malach, 2002; Levy, Hasson, Avidan, Hendler, & Malach, 2001; Malach, Levy, & Hasson, 2002; Woodhead, Wise, Sereno, & Leech, 2011), which is particularly salient when dealing with complex and confusable visual stimuli like letter strings. In line with this view, pure alexia patients show reduced sensitivity to higher spatial frequency information (Roberts et al., 2013), although this is not universal (Starrfelt, Nielsen, Habekost, & Andersen, 2013). Also in keeping with a visual deficit account, the exaggerated length effect is accompanied by increased sensitivity to the visual confusability of letters (Arguin, Fiset, & Bub, 2002; Fiset, Arguin, Bub, Humphreys, & Riddoch, 2005; Harris, Olson, & Humphreys, 2013; Johnson & Rayner, 2007). Interestingly, when higher spatial frequencies are artificially removed, normal individuals show increased effects both of word length and letter confusability (Fiset, Arguin, & Fiset, 2006; Tadros, Fiset, Gosselin, & Arguin, 2009). Yet letter strings are by no means the only stimuli that rely on such information, with this same brain region activated in face and object recognition (Behrmann & Plaut, 2013b; Malach et al., 2002; Nestor, Behrmann, & Plaut, 2013; Price & Devlin, 2003, 2011; Vogel, Petersen, & Schlaggar, 2012; Woodhead et al., 2011). By this account then, patients with damage to left vOTC should show impairments in processing any visual stimuli that require medium- to high-spatial frequency information for effective recognition.
When it has been assessed, the accuracy of non-linguistic visual processing in PA has varied across cases, with some patients apparently showing normal performance (e.g., Kay & Hanley, 1991; Miozzo & Caramazza, 1998), while others have shown significant impairments (e.g., Cumming et al., 2006; Roberts et al., 2013). In studies that have also considered reaction times, which is of course the measure by which their reading deficit is defined, clear evidence of visual processing impairments has emerged, particularly for complex stimuli. Behrmann, Nelson, et al. (1998) reported five pure alexia patients to be slowed in naming pictures, but only those high in visual complexity. Similarly, a large case series of 21 PA patients revealed significantly impaired performance in matching chequerboard stimuli and unfamiliar logographic characters, most markedly for complex items in the presence of visually similar distracters (Roberts et al., 2013; see also Mycroft, Behrmann, & Kay, 2009). Moreover, performance for this condition was strongly related to the severity of the reading deficit, as measured by the size of the length effect.
Despite mounting evidence for a visual deficit in PA, this is unlikely to be the only possible cause of abnormal word length effects, as these have also been reported in other neuropsychological conditions, such as semantic dementia (SD; Cumming et al., 2006; Gold et al., 2005; Patterson & Hodges, 1992). SD is a selective and progressive disorder of conceptual knowledge associated with atrophy and hypometabolism of the anterior temporal lobes (ATL) (Adlam et al., 2006; Nestor, Fryer, & Hodges, 2006). Reading aloud in SD shows a near-universal pattern of surface dyslexia, where words with exceptional spelling–sound correspondences, particularly those low in frequency, are read aloud according to more typical correspondences (regularized). Moreover, accuracy for these exception items is strongly related to the extent of the patients' receptive and expressive semantic deficits (Graham, Patterson, & Hodges, 2000; Patterson et al., 2006; Woollams et al., 2007). The primary systems interpretation of these findings is that whole-word semantic knowledge supports the pronunciation of exception-word items (Patterson & Lambon Ralph, 1999; Patterson et al., 2006).
Yet there have been a few reports of SD patients with accuracy of low-frequency exception-word reading falling within the normal range despite an appreciable semantic deficit (Blazely, Coltheart, & Casey, 2005; Cipolotti & Warrington, 1995). This has led some researchers to propose that exception-word reading in SD is undermined not by semantic deficits associated with ATL damage, but rather the posterior spread of atrophy into the left vOTC region (Coltheart, Tree, & Saunders, 2010). This account predicts that there should be clear similarities in the reading-aloud performance of SD and PA patients. The observation of abnormally strong length effects in SD (Cumming et al., 2006; Gold et al., 2005), combined with reports of SD cases who have adopted an explicit LBL reading strategy (Noble et al., 2000), have been considered evidence for this view. An alternative perspective, however, is that it is these length effects arising as a consequence of reduced support from whole-word semantic knowledge that would usually bind the letters of a word together, offsetting costs associated with processing more letters.
In a direct comparison of the visual processing and reading performance of three PA patients with three SD patients (Cumming et al., 2006), performance on nonverbal visual processing tasks for both familiar and unfamiliar objects was normal in SD, but impaired in PA. Letter matching was normal for SD at longer durations, whereas in PA it was universally impaired. Length effects were seen in both types of disorder, but these were significantly smaller for the SD than PA patients (although it should be kept in mind that accuracy was higher in SD than in PA). Interestingly, error responses were usually words for the PA patients, but nonwords for the SD patients. This is consistent with work showing enhanced influences of whole-word variables in PA (e.g., Roberts, Lambon Ralph, & Woollams, 2010). The notion of a bottom-up visual and a top-down semantic impairment both increasing length effects was reinforced by the finding that PA patients showed smaller length effects for words than for nonwords, while SD patients showed equivalent effects. Taken together, these results speak to a visual origin of length effects in PA and a semantic cause in SD.
The goal of the present research was to illuminate the source of reading deficits in PA and SD by comparing patients matched on overall severity. Previous work has already compared the effects of length and lexicality in PA and SD (Cumming et al., 2006), so here we explored the impacts of frequency and regularity using the Surface List (Patterson & Hodges, 1992) and considered not only overall accuracy but also the nature of the patients' reading errors. If the deficits in both PA and SD arise from damage to reading-specific orthographic processing, we would expect to see similar reading performance across the two groups. If, in contrast, the two reading deficits arise from underlying visual and semantic causes, respectively, then we would expect (a) weaker effects of regularity for PA than for SD, (b) a higher proportion of nonword and regularization responses in SD than in PA, and (c) a higher proportion of incorrect word responses and letter-based errors in PA than in SD.
METHOD
Participants
Pure alexia
For this study we operationally characterized pure alexia in terms of a combination of damage to the left occipito-temporal cortex combined with slowed reading and an abnormally large word length effect. Ten PA patients with overt LBL reading of varying degrees participated. All were native speakers of English who had suffered from acute brain injury more than two years prior to the time of testing. These patients were recruited from local NHS speech and language therapy services on the basis of marked increases in word-reading latency as a function of letter length. On our reading list of 180 words (Roberts et al., 2010), overt LBL responses were produced by every patient.
As can be seen in Table 1, all patients had damage in the occipito-temporal region, as judged by a neurologist, as a consequence of stroke or tumour resection. Scans for eight of the 10 patients are provided in the Appendix. Scans for two other patients (P.M. and K.W.) were not available, hence the determination of damage was made on the basis of the neurologist's written report. Overall, neuropsychological background assessment indicated that the patients had preserved working memory (digit span; Wechsler, 1987) and phonological processing, with only one patient slightly impaired on the more demanding tests of phonological segmentation (E.W.). Deficits in visual processing on at least one subtest of the Visual Object and Space Perception Battery (VOSP; Warrington & James, 1991) were apparent in all patients.
Table 1.
Demographic and background neuropsychological data for the 10 pure alexic patients included in the current study, ordered from least to most impaired according to high-frequency regular-word reading accuracy
| Variable | Max. | P.M. | J.W. | J.M. | T.S. | K.W. | S.C. | J.W.F. | M.S. | A.T. | E.W. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| Age (years) | — | 64 | 59 | 67 | 57 | 44 | 81 | 54 | 70 | 73 | 74 |
| Years of education Lesion information | — | 10 | 11 | 10 | 10 | 10 | 11 | 10 | 10 | 10 | 10 |
| Neuroimaging summary | — | Occipitotemporal | Occipitotemporal | Occipitotemporal | Occipitotemporal | Occipitotemporal | Occipitotemporal | Occipitotemporal | Occipitotemporal | Occipitoparietal | Occipitotemporoparietal |
| Aetiology | — | PCA stroke | PCA stroke | PCA tumour resection | PCA tumour resection | MCA stroke | PCA stroke | Post aneurism PCA infarct | PCA stroke | PCA stroke | MCA stroke |
| Working memory Digit span (scaled score) | 18 | NT | 9 | 15 | 8 | 14 | 7 | 10 | 14 | 10 | 7 |
| Visual processing Right visual field impairmenta | – | Upper quadrant | Hemianopia | Upper quadrant | Hemianopia | Hemianopia | Hemianopia | Hemianopia | Hemianopia | Upper quadrant | Hemianopia |
| VOSP | |||||||||||
| Incomplete letters | 20 | NT | 19 | 20 | 19 | 20 | 18 | 17 | 16 | 16 | 19 |
| Silhouettes | 30 | NT | 25 | 18 | 22 | 19 | 3 | 24 | 19 | 13 | 12 |
| Object decision | 20 | NT | 17 | 17 | 18 | 20 | 14 | 19 | 16 | 17 | 17 |
| Progressive silhouettes | 20 | NT | 8 | 11 | 5 | 16 | NT | NT | 9 | 9 | 12 |
| Dot counting | 10 | NT | 10 | 10 | 10 | 9 | 10 | 10 | 9 | 10 | 10 |
| Position discrimination | 20 | NT | 20 | 20 | 18 | 20 | 17 | 16 | 19 | 20 | 20 |
| Number location | 10 | NT | 10 | 10 | 10 | 10 | 10 | 8 | 10 | 9 | 10 |
| Cube analysis | 10 | NT | 9 | 10 | 10 | 4 | 9 | 10 | 7 | 10 | 7 |
| Semantic processing | |||||||||||
| Namingb | 64 | 64 | 59 | 60 | 40 | 56 | 59 | 54 | 45 | 54 | 45 |
| Spoken Word to | 64 | NT | 64 | 63 | 63 | 63 | 62 | NT | 62 | 63 | 57 |
| Picture Matchingb Camel and Cactus (pictures)b | 64 | 64 | 52 | 61 | 24 | NT | NT | 61 | 47 | NT | 45 |
| 96 Synonyms (%)c | 96 | NT | 93 | 93 | 83 | 74 | 71 | 94 | 81 | NT | 76 |
| Phonological processing PALPA 2: | 72 | NT | 71 | 72 | 68 | 71 | NT | 72 | 71 | NT | 65 |
| Phonological judgement PALPA IS: Rhyme judgement | 60 | NT | 57 | 56 | 56 | 59 | NT | 58 | 53 | NT | 56 |
| Phonological segmentationd | 96 | NT | 96 | 94 | 87 | 97 | NT | 96 | 91 | NT | 69 |
| Spelling | |||||||||||
| PALPA 39 | |||||||||||
| Written | |||||||||||
| Short | 100 | NT | 92 | 100 | 100 | NT | NT | NT | 100 | NT | 75 |
| Long | 100 | NT | 50 | 75 | 100 | NT | NT | NT | 75 | NT | 17 |
| Reading aloud | |||||||||||
| 180-item list: | — | ||||||||||
| Mean RT (ms) | — | 1013 | 7530 | 5432 | 5158 | 5903 | 7910 | 6484 | 12,667 | 15,683 | 7010 |
| Mean accuracy (%) | — | 100 | 91 | 96 | 95 | 94 | 83 | 53 | 75 | 57 | 58 |
| Length effect (ms per letter) | — | 170 | 1299 | 911 | 1060 | 651 | 1843 | 1369 | 1650 | 523 | 2248 |
| Length effect (% per letter)e Surface List: | — | 0 | 0.75 | −1.25 | −1.25 | 0 | −3.75 | 1.5 | −8.75 | −4 | −3.25 |
| High-frequency regular | 42 | 41 | 41 | 41 | 40 | 39 | 36 | 34 | 32 | 30 | 29 |
| Low-frequency regular | 42 | 40 | 38 | 37 | 39 | 36 | 26 | 39 | 24 | 22 | 15 |
| High-frequency exception | 42 | 39 | 40 | 39 | 37 | 41 | 31 | 37 | 30 | 29 | 25 |
| Low-frequency exception | 42 | 37 | 28 | 33 | 34 | 32 | 16 | 34 | 20 | 27 | 26 |
Note: Values in italics denote abnormal performance represented by scores falling beyond two standard deviations below control performance where normative data available; for Digit Span, abnormal scores are two standard deviations below age-appropriate means (Ivnik et al., 1992). VOSP = Visual Object and Space Perception Battery (Warrington & James, 1991); PALPA = Psycholinguistic Assessments of Language Processing in Aphasia (Kay, Lesser, & Coltheart, 1992a); MCA = middle cerebral artery; PCA = posterior cerebral artery; RT = reaction time; NT = not tested; NA = not available.
Assessed using LernReha from Kasten, Strasburger, and Sabel (1997).
Tests from Bozeat, Gregory, Lambon Ralph, and Hodges (2000).
Tests from Patterson and Marcel (1992).
Represents decrease in accuracy for each additional letter in string.
Performance on the Cambridge Picture Naming test (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, 2000) revealed impaired performance in all bar one case (P.M.). Receptive semantic processing tests included the Cambridge Spoken Word to Picture Matching test (Bozeat, Lambon Ralph, et al., 2000), where a spoken word was matched to a target picture amongst nine semantically related alternatives; the Camel and Cactus Pictures test (Bozeat, Lambon Ralph, et al., 2000), where a target picture was matched to a picture of an associated item in the context of three semantically similar items; and the 96 Synonyms test (Jefferies et al., 2009), where a written target word was matched to a synonym in the context of two other related words of similar frequency and imageability (options were also read to the patient by the experimenter). Six patients (T.S., K.W., S.C., E.W., M.S., & A.T.) showed mild but measureable impairments on at least one of these receptive semantic tests.
While the prevalence of deficits on these semantic tests could be interpreted as indicating deficits in conceptual knowledge, it is worth noting that all of the tests involved either pictures or written words. Poor performance on these tests is therefore consistent with optic aphasia, if conceptualized as a disconnection of semantics from visual input (Plaut & Shallice, 1993). Yet in light of the demonstrated visual impairments on the VOSP, it seems plausible that impaired performance on the semantic tests in this patient group may have arisen as a consequence of problems in visual processing. We hypothesize that reduced sensitivity to higher spatial frequencies could impair performance on (a) the more demanding subtests of the VOSP such as progressive silhouettes; (b) semantic tests that involve picture identification; and (c) semantic tests that also involve reading written words. Such an account would of course be consistent with the primary systems view and previous reports of object processing deficits in this population (e.g., Behrmann, Nelson, et al., 1998; Mycroft et al., 2009; Roberts et al., 2013).
Data for spelling words of different lengths from the Psycholinguistic Assessments of Language Processing in Aphasia (PALPA) 39 subtest (Kay, Lesser, & Coltheart, 1992b) were available for only five cases. Although this test does not have published norms, according to Medler and Binder (2005), the mean frequency of items is 108 per million, and the control range on the PALPA 40 is between 30 and 100% for items with a mean frequency 105 per million. Hence, although spelling performance was not perfect in all cases, it would seem there was good performance for shorter words of three or four letters, and performance for longer words was also good in most cases, with the only clearly impaired case being E.W. Given that E.W. also showed deficits in tests of phonological and semantic processing, it is possible that aphasic deficits may have contributed to her impaired orthographic processing.
All patients showed elevated mean reading speeds on the 180-item list from Roberts et al. (2010; reaction times, RTs, were derived using a voice recorder and manual analysis of reaction time data using WavePad software). All patients showed an appreciable influence of word length upon their reading speed, although the strength of the effect varied across different patients. This variability is also reflected in accuracy of Surface List reading and demonstrates that any comparisons across patient types must take into account overall severity of the reading disorder.
Semantic dementia
Ten SD patients with reading accuracy comparable to that of the PA patients on high-frequency regular words were selected from the cohort presented in Woollams et al. (2007). All patients had received a diagnosis of semantic dementia according to the Neary et al. (1998) consensus criteria, which include atrophy of the ATL. Their selective semantic impairment is apparent in Table 2.
Table 2.
Demographic and background neuropsychological data for the 10 semantic dementia patients included in the current study, ordered from least to most impaired according to high-frequency regular-word reading accuracy
| Variable | Max. | GC6 | LS3 | MB1 | DC1 | DA1 | AM4 | NS2 | MA6 | FM8 | AT6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| Age (years) | — | 60 | 62 | 65 | 77 | 75 | 65 | 69 | 73 | 57 | 68 |
| Years of education | — | 12 | 13 | 11 | 8 | 16 | 16 | 9 | 13 | 10 | 19 |
| Cognitive status | |||||||||||
| MMSE | — | NT | 24 | 22 | 18 | 9 | 8 | 25 | 5 | 22 | 15 |
| Raven's coloured | — | NT | NT | 17 | 33 | NT | 30 | 36 | NT | 25 | 34 |
| Working memory | |||||||||||
| Digit span (scaled score) | 18 | 5 | 7 | 4 | 7 | 3 | 6 | 6 | NT | 4 | 9 |
| Visual processing | |||||||||||
| Rey Immediate Copy | 36 | 34 | 29 | 26 | 32 | 34 | 35 | 36 | NT | 32 | 36 |
| VOSP | |||||||||||
| Incomplete letters | 20 | 20 | 19 | 19 | 18 | 17 | NT | 19 | NT | NT | 18 |
| Silhouettes | 30 | 5 | NT | 9 | 1 | NT | NT | NT | NT | NT | NT |
| Object decision | 20 | 14 | NT | 13 | 17 | 18 | NT | NT | NT | NT | NT |
| Progressive silhouettes | 20 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| Dot counting | 10 | 10 | 10 | 9 | 10 | 9 | NT | 10 | NT | NT | 10 |
| Position discrimination | 20 | 20 | 20 | 20 | 20 | 19 | NT | 20 | NT | NT | 20 |
| Number location | 10 | 8 | 10 | 7 | 10 | 10 | NT | 9 | NT | NT | 8 |
| Cube analysis | 10 | 10 | 9 | 8 | 10 | 8 | NT | 10 | NT | NT | 10 |
| Semantic processing | |||||||||||
| Naminga | 64 | 13 | 34 | 44 | 20 | 12 | 0 | 8 | 10 | 0 | 5 |
| Spoken Word to Picture Matchinga | 64 | 35 | 60 | 58 | 44 | 50 | 17 | 42 | 43 | 59 | 29 |
| Pyramids and Palm Trees (pictures) | 52 | NT | 30 | 39 | 40 | 39 | 35 | 39 | 26 | 46 | 38 |
| Pyramids and Palm Trees (words) | 52 | NT | NT | NT | 30 | 41 | 30 | 33 | 26 | NT | 27 |
| Category (8 categories) | — | NT | NT | 25 | 13 | 4 | 1 | 19 | 0 | 0 | NT |
| Reading aloud | |||||||||||
| Surface List: | |||||||||||
| High-frequency regular | 42 | 41 | 41 | 40 | 39 | 39 | 36 | 36 | 33 | 32 | 29 |
| Low-frequency regular | 42 | 40 | 35 | 41 | 39 | 37 | 34 | 15 | 25 | 29 | 29 |
| High-frequency exception | 42 | 38 | 40 | 40 | 33 | 35 | 33 | 31 | 37 | 21 | 21 |
| Low-frequency exception | 42 | 20 | 26 | 32 | 20 | 28 | 21 | 7 | 26 | 15 | 10 |
Note: Values in italics denote abnormal performance represented by scores falling beyond two standard deviations below performance of a group of between 100 and 24 (depending on the test) control participants comparable in terms of age and education; for Digit Span, abnormal scores are two standard deviations below age-appropriate means (Ivnik et al., 1992); for the Raven's, abnormal performance is that below the 50th percentile for older controls in norms. NT = not tested; MMSE = Mini-Mental State Examination; VOSP = Visual Object and Space Perception battery (Warrington & James, 1991); PALPA = Psycholinguistic Assessment of Language Processing in Aphasia (Kay et al., 1992b).
Tests from Bozeat, Lambon Ralph, et al. (2000).
Mini-Mental State Examination (MMSE) scores (Folstein, Folstein, & McHugh, 1975) were below the control range for all patients, as would be expected given that this test assesses some aspects of verbal ability. Working memory performance as assessed by digit span (Wechsler, 1987) was within the normal range in all but one case (DA1). Visuoperceptual processing was reasonably intact, as indicated by scores within the normal range for all patients on the Rey Immediate Copy Test (Lezak, 1976). Where available, data from the VOSP showed preserved performance except for the Silhouettes subtest and in one case (MB1) on the Object Decision subtest, which is understandable given this draws on knowledge of object identity.
Marked impairments were apparent on tests tapping semantic memory. Performance was outside the control range for all patients on both the Cambridge Picture Naming and Spoken Word to Picture Matching (WPM) tests (Bozeat, Lambon Ralph, et al., 2000; Hodges, Patterson, Oxbury, & Funnell, 1992) and on the Pyramids and Palm Trees Test (Howard & Patterson, 1992), reflecting the progressive anomia and declining comprehension that are key features of SD. Deficits in semantically generated output are apparent on the Category Fluency Test (Hodges, Salmon, & Butters, 1992), in which patients are asked to generate as many examples as they can in one minute each for eight semantic categories, arguing against a visual contribution to the decreased performance seen on the semantic tests. Performance on the Surface List shows a consistent pattern of surface dyslexia, with all patients showing poor performance for low-frequency exception words.
Stimuli
The reading performance of all PA and SD patients was assessed using the Surface List (Patterson & Hodges, 1992; see Woollams et al., 2007, Appendix A). The Surface List consists of a factorial manipulation of frequency and regularity, with 42 items per cell. Within each level of frequency, the regular and exception items are matched on initial phoneme and do not differ according to Kučera and Francis (1967) written frequency [high-frequency regular (HFR) = 811.43, high-frequency exception (HFE) = 798.83, t(1, 80) < 1; low-frequency regular (LFR) = 5.78, low-frequency exception (LFE) = 5.41, t(1,78) < 1 or orthographic length [HFR = 4.14, HFE = 4.24, t(1, 82) < 1; LFR = 4.83, LFE = 4.81, t(1, 82) < 1].
Procedure
For the PA patients, after an initial series of 12 practice items, patients viewed each item of the Surface List one at a time in the centre of a laptop screen. Items were displayed using E-Prime software (Schneider, Eschman, & Zuccolotto, 2002) with an input of Arial 18 point that translated to the equivalent of 34 point once displayed on the screen (ascender/descender height = 0.9 cm). Responses were digitally recorded for later coding. For the SD patients, practice and test items were presented one at a time on cards in Geneva 26-point font (ascender/descender height = 0.7 cm), and responses were coded in written form by the experimenter. Note that although presentation format differed over patient group, the two are near-identical proportional fonts (e.g., pint vs. pint), and while the font size was larger for the PA patients than for the SD patients, this in fact works against our hypothesis of more visual errors for PA than SD patients. Moreover, letter identification has been shown to be relatively independent of such variations in size (Pelli, Burns, Farell, & Moore-Page, 2006). For both groups, test items were presented in a fixed pseudorandom order that ensured a representative distribution of items from each condition over four blocks.
RESULTS
Accuracy
Reading accuracy for the PA and SD patients is presented in Figure 1. Data were analysed using a 2 (patient: PA/SD) by 2 (frequency: high/low) by 2 (regularity: regular/exception) analysis of variance (ANOVA), with repeated measures on the second and third factors. The results revealed no main effect of patient type, F(1, 18) = 0.74, p = .401, indicating that the severity matching had been successful. There were significant main effects of both frequency, F(1, 18) = 41.25, p < .0005, and regularity, F(1, 18) = 28.55, p < .0005, and their interaction, F(1, 18) = 12.49, p = .002. The two patient types showed comparable effects of frequency, F(1, 18) = 1.85, p = .191, but the impact of regularity was significantly stronger in SD than in PA, F(1, 18) = 8.95, p = .008. The significant three-way interaction, F(1, 18) = 6.27, p = .022, was driven by the SD patients' significantly worse performance specifically on the low-frequency exception words, t(18) = 2.49, p = .011, one-tailed. Repeated measures ANOVAs on the PA patients alone showed significant main effects of frequency, F(1, 9) = 19.10, p = .002, a marginal effect of regularity, F(1, 9) = 4.51, p = .063, and no interaction between them, F(1, 9) = 0.38, p = .551. A parallel analysis on the SD patients alone showed significant main effects of frequency, F(1, 9) = 22.79, p = .001, regularity, F(1, 9) = 25.05, p = .001, and an interaction between them, F(1, 9) = 29.72, p < .0005.
Figure 1.
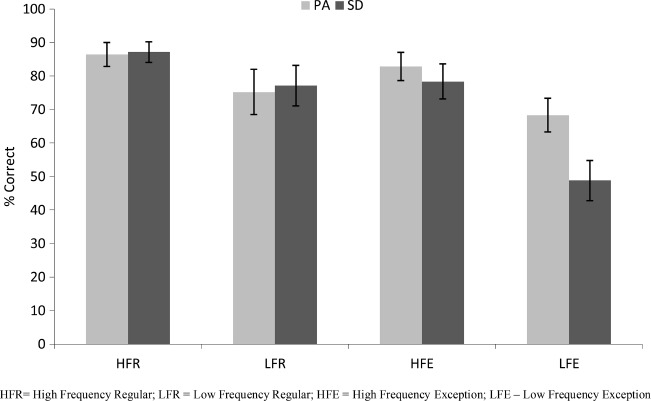
Reading-aloud accuracy for 10 pure alexic (PA) and 10 semantic dementia (SD) patients according to frequency and regularity. Error bars represent ± standard error.
Error types
All errors were transcribed in order to maximize orthographic similarity to the target. A variety of error types were observed amongst both PA and SD patients, and a summary of these is provided in Table 3. We classified each error into one of the following mutually exclusive categories: (a) omissions (which were rare in both groups); (b) legitimate alternative reading of components (LARC), in which the patient pronounced the word in line with spelling–sound correspondences of one or more other known words (e.g., sew → “sue”, as in few and stew); (c) visual errors, in which the response had at least 1 letter (out of 3 or 4) or 2 letters (out of 5 or 6) in common with target (e.g., saw → “save”; cough → “coach”); (d) letter omissions, where all letters of the response were found in the target, but the response was one letter shorter than the target (e.g., learn → “lean”); (e) letter additions, where all letters of the response were found in the target, but the response was one letter longer than the target (e.g., per → “pear”); (f) letter transpositions, where the response was the same length and contained all the letters of the target, but two adjacent letters had switched order (e.g., trial → “trail”); and (g) letter substitutions, where the response was the same length as the target but one letter had been replaced (e.g., food → “fool”). As can be seen in Table 3, omission errors were very rare in the PA patients, but as they were nonexistent in the SD patients, this group difference was significant, t(18) = 1.97, p = .032, one-tailed). As expected, LARC errors were the most prevalent type of error for the SD patients, and, while some LARC errors were made by the PA patients, these were significantly less common, t(18) = 3.21, p = .002, one-tailed. Visual errors were marginally more common for the PA than for SD patients, t(18) = 1.47, p = .079, one-tailed. Neither letter omissions nor additions differed significantly between PA and SD patients [t(18) = 1.25, p = .115, one-tailed; t(18) = 0.89, p = .194, one-tailed, respectively]. Letter transpositions, although rare overall, were significantly more common in PA than in SD patients, t(18) = 2.16, p = .022, one-tailed. The most prevalent error type for the PA patients was letter substitutions, and while such errors were also seen in the SD patients, they were significantly less common, t(18) = 2.42, p = .013, one-tailed. No difference between PA and SD patients on other error types was apparent, t(18) = 0.35, p = .364, one-tailed. To summarize, LARC errors were significantly more common for the SD than for the PA patients, whereas at least some types of letter-based errors (visually related responses, transpositions, and substitutions) were significantly more common in the PA than in the SD patients. This pattern is displayed in Figure 2 and is consistent with reading performance disrupted by a semantic deficit in SD and by a visual deficit in PA.
Table 3.
Proportion of different error types for the 10 PA and 10 SD cases
| Error type | Patient type | HFRa | LFRa | HFEa | LFEa | Totalb |
|---|---|---|---|---|---|---|
| Omission errors | ||||||
| PA | 0 (0) | .017 (.017) | 0 (0) | .038 (.017) | .021 (.034)* | |
| SD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | |
| LARC errors | ||||||
| PA | .017 (.017) | .096 (.052) | .248 (.092) | .268 (.042) | .198 (.111)* | |
| SD | .31 (.098) | .142 (.056) | .36 (.107) | .547 (.054) | .434 (.203)* | |
| Visual errors | ||||||
| PA | .213 (.066) | .365 (.081) | .191 (.051) | .288 (.048) | .279 (.152)** | |
| SD | .139 (.074) | .193 (.07) | .289 (.081) | .17 (.033) | .187 (.128)** | |
| Letter omissions | ||||||
| PA | .052 (.034) | .161 (.057) | .185 (.074) | .097 (.018) | .122 (.067) | |
| SD | .028 (.021) | .137 (.069) | .16 (.068) | .074 (.018) | .086 (.065) | |
| Letter additions | ||||||
| PA | .035 (.019) | .066 (.035) | .008 (.008) | 0 (0) | .021 (.021) | |
| SD | .089 (.05) | .006 (.006) | .022 (.015) | .014 (.007) | .029 (.019) | |
| Letter transpositions | ||||||
| PA | .112 (.054) | .013 (.009) | 0 (0) | .004 (.004) | .024 (.022)* | |
| SD | .061 (.036) | 0 (0) | 0 (0) | 0 (0) | .007 (.012)* | |
| Letter substitutions | ||||||
| PA | .332 (.098) | .258 (.076) | .34 (.092) | .166 (.044) | .242 (.07)* | |
| SD | .177 (.075) | .446 (.075) | .125 (.04) | .117 (.016) | .175 (.051)* | |
| Other errors | ||||||
| PA | .24 (.129) | .024 (.01) | .029 (.015) | .139 (.034) | .093 (.069) | |
| SD | .197 (.1) | .077 (.032) | .044 (.021) | .078 (.015) | .083 (.054) |
Note: Standard deviations are provided in parentheses. PA = pure alexia; SD = semantic dementia; LARC = legitimate alternative reading of components; HFR = high-frequency regular, HFE = high-frequency exception, LFR = low-frequency regular, and LFE = low-frequency exception.
Proportion = error type/errors per condition.
Proportion = error type/total errors.
Significant group difference at p < .05, one-tailed.
Marginally significant group difference at p < .08, one-tailed.
Figure 2.
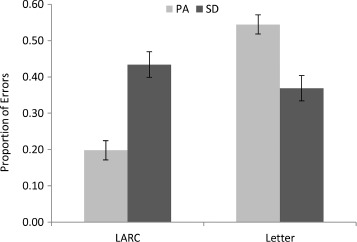
Proportion of legitimate alternative reading of components (LARC) and letter (visual + transposition + substitution) errors for the 10 pure alexic (PA) and 10 semantic dementia (SD) patients. Error bars represent ± standard error.
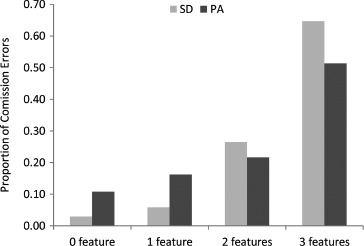
For all errors of commission, we also coded whether the responses corresponded to another known word, and these proportions are displayed in Table 4. There was a highly significant difference between the PA and SD patients on this measure, t(18) = 6.87, p < .000005, one-tailed. As can be seen in Figure 3, the vast majority of errors of commission produced by the PA patients were words. The SD patients, on the other hand, were somewhat more likely to produce nonword than word errors. This striking difference is consistent with the idea that reading aloud in SD is characterized by a reduction of semantic activation, such that there is insufficient top-down information to prevent nonword responses. In contrast, the high proportion of word errors in the PA patients suggests that reading responses in the face of compromised bottom-up visual input are typically constrained by top-down information.
Table 4.
Proportion of errors of commission that were phonologically identical to another known word for the 10 PA and 10 SD cases
| Patient type | HFRa | LFRa | HFEa | LFEa | Totalb |
|---|---|---|---|---|---|
| PA | .823 (.1) | .903 (.04) | .826 (.062) | .649 (.057) | .777 (.084)* |
| SD | .665 (.1) | .556 (.063) | .43 (.077) | .411 (.039) | .46 (.111)* |
Note: Standard deviations are provided in parentheses. PA = pure alexia; SD = semantic dementia; HFR = high-frequency regular, HFE = high-frequency exception, LFR = low-frequency regular, and LFE = low-frequency exception.
Proportion = word errors/commission errors per condition.
Proportion = word errors/total commission errors.
Significant group difference at p < .000005, one-tailed.
Figure 3.
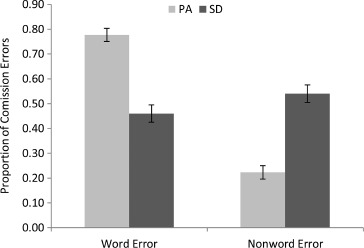
Proportion of word and nonword errors for the 10 pure alexic (PA) and 10 semantic dementia (SD) patients. Error bars represent ± standard error.
Letter substitutions
The analysis of error types demonstrated that PA patients were significantly more likely than SD patients to substitute one of the component letters of a word. If these substitutions result from a visual processing deficit in PA, then we would also expect that the form of these errors will be driven more by visual similarity than in SD. To assess this hypothesis, we coded the letter presented and letter “reported” (as reflected in the whole response) according to the letter confusability matrix in Patterson and Kay (1982), derived from the errors made by normal participants in identifying letters presented briefly in peripheral vision (Bouma, 1971). We selected this confusability matrix because: (a) it was based on lower-case letters, as used in our reading list; (b) it was derived from peripheral vision, resulting in perception with reduced medium- to high-spatial frequency information, akin to deficits suggested in PA patients (Roberts et al., 2013); and (c) it has been used before with reference to letter substitutions in cases of PA (Patterson & Kay, 1982). The results for each group can be seen in Figure 4, where the values represent the proportion of all substitutions. The cells closest to the diagonal represent maximum visual similarity, and the substitutions of the PA patients fall closer to the diagonal than do those of the SD patients, as hypothesized. In order to quantify this difference, we computed the Euclidean distance between the presented and reported letters within the matrix for each error in the following way: We created a matrix where each letter was assigned a number from 1 to 26 (e.g., a = 2, o = 3), and the absolute difference between the presented and reported letter yielded a distance for that confusion for a given patient (e.g., cat read as “cot” had a distance of 1). The average distance for PA patients across all letter substitution errors was 5.1, while that for SD patients was 7.0, which was significantly lower, t(165) = 2.38, p = .009, one-tailed. This result is again consistent with a visual deficit undermining reading in PA.
Figure 4.
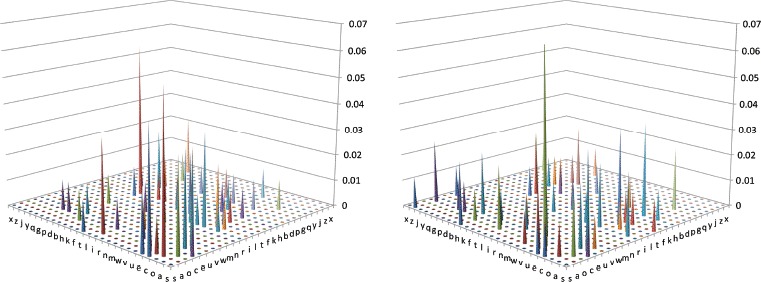
Visual similarity of letter substitution errors for the 10 pure alexic (PA; left) and 10 semantic dementia (SD; right) patients. Values representation proportion of all substitution errors. [To view this figure in colour, please see the online version of this journal].
The preceding analysis indicates a key role for visual similarity in the specific letter substitution errors of the PA patients. What might be the relevant relationship between stimulus and response words in SD reading errors? One possibility is that semantic impairment exerts its effects on reading aloud through mild perturbation of phonological/phonetic processing. To assess this hypothesis, we used the Bailey and Hahn (2005) coding scheme to capture the sound similarity—in terms of number of shared features (place, manner, voice, sonorance)—corresponding to the phonemes involved in letter substitution errors. The results can be seen in Figure 5, where the values represent the proportion of all consonant–consonant substitutions. This reveals that the SD patients' letter substitutions were more likely to equate to phonemes sharing two or three features with the target phoneme, whereas for PA patients such substitutions typically shared either no, or just a single, phonetic feature. A comparison of the average number of shared phonetic features demonstrated greater phonemic similarity of substitutions amongst the SD (2.53) than amongst the PA patients (2.14), t(165) = 1.69, p = .038, one-tailed.
Figure 5.
Proportion of consonant substitution errors according to number of phonetic features (place, manner, voice, sonorance) shared between presented and reported phonemes for the 10 pure alexic (PA) and 10 semantic dementia (SD) patients.
DISCUSSION
This study investigated the extent to which the reading deficits seen in PA and SD arise from similar or different causes. The fact that increased word length effects have been seen in reading performance in both these disorders has led some researchers to propose that they share a common cause in terms of disruption to reading-specific orthographic processing (e.g., Coltheart et al., 2010; Noble et al., 2000). In contrast, the primary systems view attributes all characteristics of these two reading disorders, including length effects, to a deficit in general visual processing in PA and to a deficit in central semantic processing in SD (Patterson & Lambon Ralph, 1999; Roberts et al., 2013; Woollams et al., 2007). These two accounts therefore diverge in the extent to which they predict resemblances between reading performance in the two disorders. Here, we explored this issue by directly comparing the impact of frequency and regularity on reading accuracy, and the nature of error types, in 10 cases of PA and 10 cases of SD who were matched on their accuracy in reading single words aloud.
In terms of reading accuracy, the PA and SD patients were similar in that they showed comparable effects of frequency, which concurs with results previously reported in the literature (e.g., Behrmann, Plaut, et al., 1998; Graham et al., 2000). While this result could be consistent with a shared locus of impairment in orthographic processing, it could also arise from different sources. In PA, the perception of high-frequency words may be less disrupted due to feedback from intact higher order linguistic/semantic representations (Roberts et al., 2010), whereas in SD, the production of low-frequency words may be more disrupted because semantic representations of these items are most vulnerable to damage (Lambon Ralph, Graham, Ellis, & Hodges, 1998; Rogers et al., 2004; Woollams, Cooper-Pye, Hodges, & Patterson, 2008). This notion that the influence of top-down activation is increased in PA but reduced in SD is consistent with the striking finding reported here and previously (Cumming et al., 2006) that PA patients are much more likely to produce errors that are nevertheless known words, while SD patients are in fact more likely to produce errors that are nonwords.
The impact of regularity on reading accuracy was significantly weaker in PA than in SD, and the incidence of LARC errors was also significantly lower. Consistent with previous work, there was a marginally significant effect of regularity on PA reading accuracy (Behrmann, Nelson, et al., 1998; Rapcsak & Beeson, 2004), but LARC errors were the least common error type for the PA patients (Cumming et al., 2006; Patterson & Kay, 1982). This contrasts with the very strong impact of regularity on reading accuracy for the SD patients and the fact that LARC errors were the most common error type in SD, as has been previously seen in larger samples (Graham et al., 2000; Woollams et al., 2007). The prevalence of LARC errors in SD speaks to intact processing along a direct pathway between orthography and phonology in the face of compromised whole-word knowledge due to damage to the semantic system.
Consideration of the nature of reading errors also highlighted a higher incidence of certain letter-based errors in PA than SD—specifically those where the stimulus and response shared most of their letters (visual errors, see also Rapcsak & Beeson, 2004), where letters in the response reordered those in the stimulus (transpositions: see also Pflugshaupt et al., 2011), and where a single letter in the stimulus was replaced by another in the response (substitutions, see Patterson & Kay, 1982). Indeed, it was letter substitutions that were the most common type of error for the PA patients, but some substitution errors were also produced by the SD patients. To understand the source of the substitution errors in the two patient types, we first considered the extent to which the presented and reported letters were visually similar, as measured by their degree of confusability by normal participants when letters are presented in peripheral vision (Bouma, 1971; Patterson & Kay, 1982), a technique that may simulate the lower spatial frequency information available to PA patients with unlimited duration central presentation (Roberts et al., 2013). The visual similarity of the presented and reported letters was significantly higher in PA than in SD, consistent with a visual processing impairment as the cause of the reading deficit in PA.
We then further explored the source of letter substitution errors in SD by considering the extent to which they were driven by phonological similarity, as measured by overlap in terms of the phonetic features of the presented and reported consonant phonemes (Bailey & Hahn, 2005). The motivation behind this analysis was the possibility that semantic damage could exert its effects on reading aloud through disruption of phonological processing. This notion is supported by a body of literature demonstrating poorer repetition by SD patients of short sequences of words whose meanings they no longer know than of words with meanings that are still known (Knott, Patterson, & Hodges, 1997, 2000; Patterson, Graham, & Hodges, 1994). This poorer performance is characterized by phoneme migration errors (e.g., mint, rug will be reproduced as rint, mug), suggesting that semantic activation helps to bind together phonological elements. Consistent with this view, the phonological similarity of the letter substitutions of SD patients was significantly higher than that for PA patients.
The phonological similarity of letter substitution errors in SD does suggest that semantic impairment exerts effects on reading aloud through disruption of phonological processing, but there are multiple mechanisms by which this could occur. SD patients' poor performance in repetition of lists of words with degraded meaning has been viewed as reflecting dramatically reduced semantic activation of phonology, consistent with the prevalence of omission errors in SD patients' picture naming (Woollams et al., 2008) and the ineffectiveness of phonological cueing for their anomia (Graham, Patterson, & Hodges, 1995; Jefferies, Patterson, & Lambon Ralph, 2008). It is possible that degraded knowledge not only reduces phonological activation but also adds noise to it, consistent with the occurrence of errors of commission in SD picture naming (Woollams et al., 2008). This noisy activation would be inherited by phonological representations during reading, and indeed this is the approach taken by Woollams et al. (2007) in their simulations of reading aloud in SD within the connectionist triangle model of Plaut, McClelland, Seidenberg, and Patterson (1996). To the extent that phonological representations are organized according to phonetic features (e.g., Harm & Seidenberg, 2004), then this noisy activation would result in the substitution of similar phonemes during reading aloud, as observed in the present study.
Overall then, a consideration of the reading-aloud performance in PA and SD patients matched for accuracy of reading aloud has shown that the two groups perform very differently. The prevalence of visual errors and the visual similarity of letter substitutions in PA indicate a general visual processing deficit, whereas the prevalence of LARC errors and the phonetic similarity of phoneme substitutions in SD are consistent with a semantic impairment, in line with a primary systems account of reading disorders. This account of PA and SD reading is represented schematically in Figure 6 within the connectionist triangle framework. The assumption of a general visual processing deficit in PA is supported not only by the present data, but also previous work showing visual processing deficits to varying degrees in these patients (Behrmann, Nelson, et al., 1998; Behrmann & Plaut, 2013a; Behrmann & Shallice, 1995; Farah & McClelland, 1991; Friedman & Alexander, 1984; Mycroft et al., 2009; Roberts et al., 2013; Starrfelt & Behrmann, 2011; Starrfelt, Habekost, & Gerlach, 2010; Starrfelt, Habekost, & Leff, 2009) and recent neuroimaging work implicating the vOTC in the processing of high-spatial-frequency foveal visual information (Hasson et al., 2003, 2002; Levy et al., 2001; Malach et al., 2002; Vogel et al., 2012; Woodhead et al., 2011). The assumption of disruption specifically to semantics is similarly supported by patient neuroimaging data: SD patients have structural and functional abnormality of the ATL but not vOTC (Acosta-Cabronero et al., 2011; Nestor et al., 2006; Woollams, Lambon Ralph, Plaut, & Patterson, 2010), and the extent of ATL damage has been directly linked to level of success on nonreading semantic tasks (Adlam et al., 2006; Mion et al., 2010).
Figure 6.
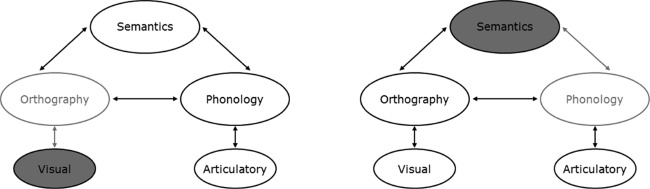
Schematic representation of the loci of deficits undermining reading in pure alexic (PA; left) versus semantic dementia (SD; right) within a triangle model of reading. Filled ovals represent damaged components; grey ovals represent subsequently disrupted processing.
Within the primary systems account, the visual deficit in PA undermines input to orthographic processing, producing the patients' visual errors, letter transpositions, and visually similar letter substitutions. This can, however, be offset to some extent by top-down activation from intact semantic and phonological information feeding back to orthography, producing the effects of frequency (and possibly regularity) observed here, combined with the prevalence of real-word error responses. In contrast, the semantic impairment in SD reduces and disrupts activation of phonology during reading, increasing the incidence of nonword error responses. Effects of frequency arise because semantic representations of low-frequency words are less robust to damage, while regularity effects arise as reading of words with atypical spelling–sound mappings come to rely more upon semantic activation of phonology over the course of learning (Plaut et al., 1996). The intact mappings directly between orthography and phonology produce LARC errors in the case of words with atypical mappings, particularly those low in frequency. In some cases, the direct activation of phonology can be disrupted by the noise from degraded semantic activations, and the result is the substitution of a phonetically similar phoneme.
Our account requires further exploration within implemented connectionist computational models of reading aloud. Some of these models incorporate phonological representations in the form of phonetic features (e.g., Harm & Seidenberg, 2004), allowing exploration of SD patients' errors. More recently, connectionist models have been extended to accept raw visual input (Chang et al., 2012a; Chang, Furber, & Welbourne, 2012b) and could therefore potentially simulate PA patients' reading behaviour. This investigation has provided target data for such simulations and has demonstrated that despite surface similarities in the reading impairments of PA and SD patients, a deeper consideration indicates that these arise due to distinct impairments of visual processing versus semantic representation.
APPENDIX
Structural scans for eight PA patients
Patients are ordered from least to most impaired according to high-frequency regular-word reading accuracy.
REFERENCES
- Acosta-Cabronero J., Patterson K., Fryer T. D., Hodges J. R., Pengas G., Williams G. B., Nestor P. J. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2011;134:2025–2035. doi: 10.1093/brain/awr119. [DOI] [PubMed] [Google Scholar]
- Adlam A. L. R., Patterson K., Rogers T. T., Nestor P. J., Salmond C. H., Acosta-Cabronero J., Hodges J. R. Semantic dementia and fluent primary progressive aphasia: Two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Arguin M., Bub D. N. Single-character processing in a case of pure alexia. Neuropsychologia. 1993;31:435–458. doi: 10.1016/0028-3932(93)90059-9. [DOI] [PubMed] [Google Scholar]
- Arguin M., Fiset S., Bub D. Sequential and parallel letter processing in letter-by-letter dyslexia. Cognitive Neuropsychology. 2002;19:535–555. doi: 10.1080/02643290244000040. [DOI] [PubMed] [Google Scholar]
- Bailey T. M., Hahn U. Phoneme similarity and confusability. Journal of Memory and Language. 2005;52:347–370. [Google Scholar]
- Behrmann M., Nelson J., Sekuler E. B. Visual complexity in letter-by-letter reading: ’Pure’ alexia is not pure. Neuropsychologia. 1998;36:1115–1132. doi: 10.1016/s0028-3932(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Plaut D. C. Cereb Cortex. Advance online publication; 2013a. Bilateral hemispheric processing of words and faces: Evidence from word impairments in prosopagnosia and face impairments in pure alexia. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Plaut D. C. Distributed circuits, not circumscribed centers, mediate visual recognition. Trends in Cognitive Sciences. 2013b;17:210–219. doi: 10.1016/j.tics.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Plaut D. C., Nelson J. A literature review and new data supporting an interactive account of letter-by-letter reading. Cognitive Neuropsychology. 1998;15:7–51. doi: 10.1080/026432998381212. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Shallice T. Pure alexia – a nonspatial visual disorder affecting letter activation. Cognitive Neuropsychology. 1995;12:409–454. [Google Scholar]
- Blazely A. M., Coltheart M., Casey B. J. Semantic impairment with and without surface dyslexia: Implications for models of reading. Cognitive Neuropsychology. 2005;22:695–717. doi: 10.1080/02643290442000257. [DOI] [PubMed] [Google Scholar]
- Bouma H. Visual recognition of isolated lower-case letters. Vision Research. 1971;11:459–474. doi: 10.1016/0042-6989(71)90087-3. [DOI] [PubMed] [Google Scholar]
- Bozeat S., Gregory C. A., Lambon Ralph M. A., Hodges J. R. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of Neurology Neurosurgery and Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S., Lambon Ralph M. A., Patterson K., Garrard P., Hodges J. R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Bub D. N., Black S., Howell J. Word recognition and orthographic context effects in a letter-by-letter reader. Brain and Language. 1989;36:357–376. doi: 10.1016/0093-934x(89)90073-4. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Furber S., Welbourne S. Modelling normal and impaired letter recognition: Implications for understanding pure alexic reading. Neuropsychologia. 2012a;50:2773–2788. doi: 10.1016/j.neuropsychologia.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. N., Furber S., Welbourne S. “Serial” effects in parallel models of reading. Cognitive Psychology. 2012b;64:267–291. doi: 10.1016/j.cogpsych.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L., Warrington E. K. Semantic memory and reading abilities: A case report. Journal of the International Neuropsychological Society: JINS. 1995;1:104–110. doi: 10.1017/s1355617700000163. [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M., Tree J. J., Saunders S. J. Computational modeling of reading in semantic dementia: Comment on Woollams, Lambon Ralph, Plaut, and Patterson (2007) Psychological Review. 2010;117:256–271. doi: 10.1037/a0015948. [DOI] [PubMed] [Google Scholar]
- Cumming T. B., Patterson K., Verfaellie M. M., Graham K. S. One bird with two stones: Abnormal word length effects in pure alexia and semantic dementia. Cognitive Neuropsychology. 2006;23:1130–1161. doi: 10.1080/02643290600674143. [DOI] [PubMed] [Google Scholar]
- Déjerine J. Contribution a l’étude anatomo-pathologique et clinique des differentes variétés de cécité-verbale. Mémoires Société Biologique. 1892;4:61–90. [Google Scholar]
- Farah M. J., McClelland J. L. A computational model of semantic memory impairment: Modality specificity and emergent category specificity. Journal of Experimental Psychology: General. 1991;120:339–357. [PubMed] [Google Scholar]
- Farah M. J., Wallace M. Pure alexia as a visual impairment: A reconsideration. Cognitive Neuropsychology. 1991;8:313–334. [Google Scholar]
- Fiset D., Arguin M., Bub D., Humphreys G. W., Riddoch M. J. How to make the word-length effect disappear in letter-by-letter dyslexia: Implications for an account of the disorder. Psychological Science. 2005;16:535–541. doi: 10.1111/j.0956-7976.2005.01571.x. [DOI] [PubMed] [Google Scholar]
- Fiset S., Arguin M., Fiset D. An attempt to simulate letter-by-letter dyslexia in normal readers. Brain and Language. 2006;98:251–263. doi: 10.1016/j.bandl.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman R. B., Alexander M. P. Pictures, images, and pure alexia – A case-study. Cognitive Neuropsychology. 1984;1:9–23. doi: 10.1080/02643298408252014. [DOI] [Google Scholar]
- Gold B. T., Balota D. A., Cortese M. J., Sergent-Marshall S. D., Snyder A. Z., Salat D. H., Buckner R. L. Differing neuropsychological and neuroanatomical correlates of abnormal reading in early-stage semantic dementia and dementia of the Alzheimer type. Neuropsychologia. 2005;43:833–846. doi: 10.1016/j.neuropsychologia.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Graham K. S., Patterson K., Hodges J. R. Progressive pure anomia: Insufficient activation of phonology by meaning. Neurocase. 1995;1:25–38. [Google Scholar]
- Graham N. L., Patterson K., Hodges J. R. The impact of semantic memory impairment on spelling: Evidence from semantic dementia. Neuropsychologia. 2000;38:143–163. doi: 10.1016/s0028-3932(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Hanley J. R., Kay J. Reading speed in pure alexia. Neuropsychologia. 1996;34:1165–1174. doi: 10.1016/0028-3932(96)00039-5. [DOI] [PubMed] [Google Scholar]
- Harm M. W., Seidenberg M. S. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Harris L., Olson A., Humphreys G. Overcoming the effect of letter confusability in letter-by-letter reading: a rehabilitation study. Neuropsychological Rehabilitation. 2013;23:429–462. doi: 10.1080/09602011.2013.776500. [DOI] [PubMed] [Google Scholar]
- Hasson U., Harel M., Levy I., Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Hasson U., Levy I., Behrmann M., Hendler T., Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/S0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Henderson L. Orthography and word recognition in reading. London: Academic Press; 1982. [Google Scholar]
- Hodges J. R., Patterson K., Oxbury S., Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hodges J. R., Salmon D. P., Butters N. Semantic memory impairment in Alzheimer's disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Howard D. Letter-by-letter readers: Evidence for parallel processing. In: Besner D., Humphreys G. W., editors. Basic processes in reading: Visual word recognition. Hove: Lawrence Erlbaum; 1991. pp. 34–76. [Google Scholar]
- Howard D., Patterson K. Pyramids and palm-trees: A test of semantic access from pictures and words. Bury St. Edmunds: Thames Valley Test Company; 1992. [Google Scholar]
- Ivnik R. J., Malec J. F., Smith G. E., Tangalos E. G., Petersen R. C., Kokmen E., Kurland L. T. Mayo's older Americans normative studies: WAIS-R norms for ages 56 to 97. Clinical Neuropsychologist. 1992;6(SUPPL.):1–30. [Google Scholar]
- Jefferies E., Patterson K., Jones R. W., Lambon Ralph M. A. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23:492–499. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E., Patterson K., Lambon Ralph M. A. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. L., Rayner K. Top-down and bottom-up effects in pure alexia: Evidence from eye movements. Neuropsychologia. 2007;45:2246–2257. doi: 10.1016/j.neuropsychologia.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Kasten E., Strasburger H., Sabel B. A. Programs for diagnosis and therapy of visual field deficits in vision rehabilitation. Spatial Vision. 1997;10:499–503. doi: 10.1163/156856897x00456. [DOI] [PubMed] [Google Scholar]
- Kay J., Hanley R. Simultaneous form perception and serial letter recognition in a case of letter-by-letter reading. Cognitive Neuropsychology. 1991;8:249–273. [Google Scholar]
- Kay J., Lesser R., Coltheart M. Psycholinguistic assessments of language processing in aphasia (PALPA) Hove: Erlbaum; 1992a. [Google Scholar]
- Kay J., Lesser R., Coltheart M. Psycholinguistic assessments of language processing in Aphasia (PALPA): An introduction. Aphasiology. 1992b;10:159–180. [Google Scholar]
- Knott R., Patterson K., Hodges J. R. Lexical and semantic binding effects in short-term memory: Evidence from semantic dementia. Cognitve Neuropsychology. 1997;14:1165–1216. [Google Scholar]
- Knott R., Patterson K., Hodges J. R. The role of speech production in auditory-verbal short-term memory: Evidence from progressive fluent aphasia. Neuropsychologia. 2000;38:125–142. doi: 10.1016/s0028-3932(99)00069-x. [DOI] [PubMed] [Google Scholar]
- Kučera H., Francis W. N. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Lambon Ralph M. A. L., Graham K. S., Ellis A. W., Hodges J. R. Naming in semantic dementia – What matters? Neuropsychologia. 1998;36:775–784. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Levy I., Hasson U., Avidan G., Hendler T., Malach R. Center-periphery organization of human object areas. Nature Neuroscience. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1976. [Google Scholar]
- Malach R., Levy I., Hasson U. The topography of high-order human object areas. Trends in Cognitive Sciences. 2002;6:176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Marshall J. C., Newcombe F. Patterns of paralexia – Psycholinguistic approach. Journal of Psycholinguistic Research. 1973;2:175–199. doi: 10.1007/BF01067101. [DOI] [PubMed] [Google Scholar]
- Medler D. A., Binder J. R. MCWord: An on-line orthographic database of the English language. 2005. Retrieved from http://www.neuro.mcw.edu/mcword/ [Google Scholar]
- Mion M., Patterson K., Acosta-Cabronero J., Pengas G., Izquierdo-Garcia D., Hong Y. T., Nestor P. J. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Miozzo M., Caramazza A. Varieties of pure alexia: The case of failure to access graphemic representations. Cognitive Neuropsychology. 1998;15:203–238. doi: 10.1080/026432998381267. [DOI] [PubMed] [Google Scholar]
- Mycroft R. H., Behrmann M., Kay J. Visuoperceptual deficits in letter-by-letter reading? Neuropsychologia. 2009;47:1733–1744. doi: 10.1016/j.neuropsychologia.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J. S., Gustafson L., Passant U., Stuss D., Black S., Benson D. F. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor A., Behrmann M., Plaut D. C. The neural basis of visual word form processing: A multivariate investigation. Cereb Cortex. 2013;23:1673–1684. doi: 10.1093/cercor/bhs158. [DOI] [PubMed] [Google Scholar]
- Nestor P. J., Fryer T. D., Hodges J. R. Declarative memory impairments in Alzheimer's disease and semantic dementia. NeuroImage. 2006;30:1010–1020. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Noble K., Glosser G., Grossman M. Oral reading in dementia. Brain and Language. 2000;74:48–69. doi: 10.1006/brln.2000.2330. [DOI] [PubMed] [Google Scholar]
- Patterson K., Graham N., Hodges J. R. The impact of semantic memory loss on phonological representations. Journal of Cognitive Neuroscience. 1994;6:57–69. doi: 10.1162/jocn.1994.6.1.57. [DOI] [PubMed] [Google Scholar]
- Patterson K., Hodges J. R. Deterioration of word meaning: Implications for reading. Neuropsychologia. 1992;30:1025–1040. doi: 10.1016/0028-3932(92)90096-5. [DOI] [PubMed] [Google Scholar]
- Patterson K., Kay J. Letter-by-letter reading: Psychological descriptions of a neurological syndrome. Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 1982;34:411–441. doi: 10.1080/14640748208400852. [DOI] [PubMed] [Google Scholar]
- Patterson K., Lambon Ralph M. A. Selective disorders of reading? Current Opinion in Neurobiology. 1999;9:235–239. doi: 10.1016/s0959-4388(99)80033-6. [DOI] [PubMed] [Google Scholar]
- Patterson K., Marcel A. J. Phonological ALEXIA or PHONOLOGICAL alexia? 1992.
- Patterson K., Ralph M. A. L., Jefferies E., Woollams A., Jones R., Hodges J. R., Rogers T. T. “Presemantic” cognition in semantic dementia: Six deficits in search of an explanation. Journal of Cognitive Neuroscience. 2006;18:169–183. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Pelli D. G., Burns C. W., Farell B., Moore-Page D. C. Feature detection and letter identification. Vision Research. 2006;46:4646–4674. doi: 10.1016/j.visres.2006.04.023. http://dx.doi.org/10.1016/j.visres.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Perri R., Bartolomeo P., Silveri M. C. Letter dyslexia in a letter-by-letter reader. Brain and Language. 1996;53:390–407. doi: 10.1006/brln.1996.0055. [DOI] [PubMed] [Google Scholar]
- Pflugshaupt T., Suchan J., Mandler M. A., Sokolov A. N., Trauzettel-Klosinski S., Karnath H. O. Do patients with pure alexia suffer from a specific word form processing deficit? Evidence from 'wrods with trasnpsoed letetrs'. Neuropsychologia. 2011;49:1294–1301. doi: 10.1016/j.neuropsychologia.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Plaut D. C., Behrmann M. Complementary neural representations for faces and words: A computational exploration. Cognitive Neuropsychology. 2011;28:251–275. doi: 10.1080/02643294.2011.609812. [DOI] [PubMed] [Google Scholar]
- Plaut D. C., McClelland J. L., Seidenberg M. S., Patterson K. Understanding normal and impaired word reading: Computational principles in Quasi-Regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Plaut D. C., Shallice T. Perseverative and semantic influences on visual object naming errors in optic aphasia: A connectionist account. Journal of Cognitive Neuroscience. 1993;5:89–117. doi: 10.1162/jocn.1993.5.1.89. [DOI] [PubMed] [Google Scholar]
- Price C. J., Devlin J. T. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price C. J., Devlin J. T. The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak S. Z., Beeson P. M. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62:2221–2229. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Brunn J. L. A prelexical basis for letter-by-letter reading – A case-study. Cognitive Neuropsychology. 1990;7:1–20. [Google Scholar]
- Roberts D. J., Lambon Ralph M. A., Woollams A. M. When does less yield more? The impact of severity upon implicit recognition in pure alexia. Neuropsychologia. 2010;48:2437–2446. doi: 10.1016/j.neuropsychologia.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Woollams A. M., Kim E., Beeson P. M., Rapcsak S. Z., Lambon Ralph M. A. Efficient visual object and word recognition relies on high spatial frequency coding in the left posterior fusiform gyrus: Evidence from a case-series of patients with ventral occipito-temporal cortex damage. Cerebral Cortex. 2013;23:2568–2580. doi: 10.1093/cercor/bhs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. T., Lambon Ralph M. A., Garrard P., Bozeat S., McClelland J. L., Hodges J. R., Patterson K. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rosazza C., Appollonio I., Isella V., Shallice T. Qualitatively different forms of pure alexia. Cognitive Neuropsychology. 2007;24:393–418. doi: 10.1080/02643290701377877. [DOI] [PubMed] [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A. E-Prime user's guide. 2002.
- Starrfelt R., Behrmann M. Number reading in pure alexia–a review. Neuropsychologia. 2011;49:2283–2298. doi: 10.1016/j.neuropsychologia.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Starrfelt R., Habekost T., Gerlach C. Visual processing in pure alexia: A case study. [Case Reports] Cortex. 2010;46:242–255. doi: 10.1016/j.cortex.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Starrfelt R., Habekost T., Leff A. P. Too little, too late: Reduced visual span and speed characterize pure alexia. Cereb Cortex. 2009;19:2880–2890. doi: 10.1093/cercor/bhp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrfelt R., Nielsen S., Habekost T., Andersen T. S. How low can you go: Spatial frequency sensitivity in a patient with pure alexia. Brain and Language. 2013;126:188–192. doi: 10.1016/j.bandl.2013.05.006. http://dx.doi.org/10.1016/j.bandl.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Tadros K., Fiset D., Gosselin F., Arguin M. A medium spatial frequency trough causes letter-by-letter dyslexia in normal readers. Journal of Vision. 2009;9:822. doi: 10.1167/9.8.822. [DOI] [Google Scholar]
- Vinckier F., Dehaene S., Jobert A., Dubus J. P., Sigman M., Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Vogel A. C., Petersen S. E., Schlaggar B. L. The left occipitotemporal cortex does not show preferential activity for words. Cereb Cortex. 2012;22:2715–2732. doi: 10.1093/cercor/bhr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E. K., James M. The visual object and space perception battery. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1991. [Google Scholar]
- Warrington E. K., Langdon D. Spelling dyslexia – A deficit of the visual word-form. Journal of Neurology Neurosurgery and Psychiatry. 1994;57:211–216. doi: 10.1136/jnnp.57.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E. K., Shallice T. Semantic access dyslexia. Brain. 1979;102:43–63. doi: 10.1093/brain/102.1.43. [DOI] [PubMed] [Google Scholar]
- Warrington E. K., Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Wechsler D. A. Wechsler memory scale – Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- Weekes B. S. Differential effects of number of letters on word and nonword naming latency. Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 1997;50:439–456. [Google Scholar]
- Welbourne S. R., Woollams A. M., Crisp J., Lambon Ralph M. A. The role of plasticity-related functional reorganization in the explanation of central dyslexias. Cognitive Neuropsychology. 2011;28:65–108. doi: 10.1080/02643294.2011.621937. [DOI] [PubMed] [Google Scholar]
- Woodhead Z. V. J., Wise R. J. S., Sereno M., Leech R. Dissociation of sensitivity to spatial frequency in word and face preferential areas of the fusiform gyrus. Cerebral Cortex. 2011;21:2307–2312. doi: 10.1093/cercor/bhr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams A. M., Cooper-Pye E., Hodges J. R., Patterson K. Anomia: A doubly typical signature of semantic dementia. Neuropsychologia. 2008;46:2503–2514. doi: 10.1016/j.neuropsychologia.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Woollams A. M., Lambon Ralph M. A., Plaut D. C., Patterson K. SD-squared: On the association between semantic dementia and surface dyslexia. Psychological Review. 2007;114:316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- Woollams A. M., Lambon Ralph M. A., Plaut D. C., Patterson K. SD-Squared revisited: Reply to coltheart, tree, and Saunders (2010) Psychological Review. 2010;117:273–281. doi: 10.1037/a0017641. [DOI] [PubMed] [Google Scholar]
- Yong K. X., Warren J. D., Warrington E. K., Crutch S. J. Intact reading in patients with profound early visual dysfunction. Cortex. 2013;49:2294–2306. doi: 10.1016/j.cortex.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


