Abstract
In the present study, we examined whether the effect of endogenously produced angiotensin II on proximal tubule transport in the male Sprague-Dawley rat is regulated by acute changes in extracellular volume. We measured the magnitude of endogenous angiotensin II-mediated stimulation of transport by sequentially perfusing proximal tubules in vivo, first with an ultrafiltrate-like solution, then by reperfusion of the same tubule with an ultrafiltrate-like solution containing 10−8 M losartan (angiotensin II receptor antagonist). During volume contraction, 10−8 M losartan decreased volume reabsorption from 4.20 ± 0.50 to 1.70 ± 0.30 nl · mm−1 · min−1 (P < 0.05), a decrease of 58.0 ± 7.0%. In contrast, after acute volume expansion, 10−8 M losartan decreased volume reabsorption from 1.84 ± 0.20 to 1.31 ± 0.20 nl · mm−1 · min−1 (P < 0.05), a decrease of 29.6 ± 9.0%. In hydropenic rats, addition of exogenous luminal angiotensin II had no effect on transport. However, in volume-expanded rats, addition of 10−8 M angiotensin II increased volume reabsorption from 2.10 ± 0.34 to 4.38 ± 0.59 nl · mm−1 · min−1 (P < 0.005). These data are consistent with endogenously produced angiotensin II augmenting proximal tubule transport to a greater degree during volume contraction than after volume expansion.
Keywords: autocrine, losartan, microperfusion, kidney
The renal proximal tubule can synthesize and secrete angiotensin II into the lumen (4, 5, 25, 27). Components of the renin-angiotensin system found within the proximal tubule include angiotensinogen and its mRNA, renin activity in lysates of proximal tubule cells in primary culture, and renin mRNA detected in proximal tubule cells in primary culture by reverse transcription and polymerase chain reaction (13, 20, 21, 24, 29). Angiotensin-converting enzyme activity has been localized to the luminal brush-border membrane, and receptors for angiotensin II have been found on both the basolateral and luminal membranes (6, 7, 9, 10, 29). Angiotensin II has been detected in native tubular fluid and in rat proximal tubules perfused with an artificial tubular fluid at concentrations ranging from 100- to 1,000-fold higher than in plasma (4, 5, 27).
Using in vivo microperfusion, we have recently shown that endogenously produced and luminally secreted angiotensin II modulates proximal tubule transport in the hydropenic rat (23). Luminal perfusion of 10−8 M losartan (angiotensin II receptor antagonist) or 10−4 M enalaprilat (angiotensin-converting enzyme inhibitor) decreases the rate of proximal tubule transport by 35–40% (23). The decrease in transport observed with enalaprilat was completely reversed with addition of exogenous 10−11 or 10−8 M luminal angiotensin II (23). These observations are consistent with endogenous angiotensin II stimulating proximal tubule transport in an autocrine or paracrine fashion.
The aim of this study was to examine whether the effect of endogenously produced and luminally secreted angiotensin II on proximal tubule volume reabsorption is regulated by acute changes in extracellular volume. The magnitude of the reduction in proximal tubule transport observed after addition of luminal losartan was used as a measure of the magnitude of the effect of angiotensin II on proximal tubule transport.
METHODS
Preparation of animals
Male Sprague-Dawley rats weighing between 180 and 240 g were used for this study. Rat preparation and the in vivo microperfusion procedure described below have been previously described in detail (16, 17, 23). Briefly, all animals were allowed free access to food and water before anesthesia with intraperitoneal Inactin (100 mg/kg). Rats were placed on a servo-controlled heated table set to maintain body temperature at 37°C. The jugular vein was cannulated for infusion of normal saline at 2.8 ml/h. A flank incision was used to expose the left kidney, which was then immobilized in a Lucite cup. The kidney was bathed with water-equilibrated light mineral oil heated to 37°C that was previously bubbled with 95% O2-5% CO2. The ureter was cannulated with polyethylene tubing to ensure free flow of urine. After microperfusion experiments during the hydropenic period were completed, the rat was volume expanded with normal saline and a normal saline solution containing 5% bovine serum albumin (1, 12). Normal saline was infused at a volume equal to 10% body weight over 1 h (~20 ml/h), followed by 3% body wt/h (~6 ml/h) (1). The 5% bovine serum albumin solution contained 140 mM NaCl and 4 mM KCl and was infused at 1 ml/100 g weight over 45 min (~2 ml/45 min), followed by 0.15 ml · 100 g wt−1 · h−1 (~0.3 ml/h) (12). Serial hematocrits were obtained at the time of vessel cannulation (prior to further surgery), 1 h after all surgery had been completed, and 1 h after volume expansion had been completed. Micropuncture surgery has previously been shown to reduce plasma volume and raise serial hematocrits (18, 23). As seen in Table 1, hematocrits and plasma angiotensin II levels rose with volume contraction and fell to presurgical levels following volume expansion (P < 0.001). Conversely, urine flow rate and urinary sodium excretion were low during volume contraction and rose significantly with volume expansion. Using a very similar volume expansion protocol, Braam et al. (5) demonstrated a similar fall in hematocrit and a rise in plasma angiotensin II levels, mean arterial pressure, urinary flow rate, and urinary sodium excretion, as well as fractional excretion of sodium.
Table 1.
Comparison of data in presurgical, volume-contracted, and volume-expanded rats
| Presurgery | Volume Contraction |
Volume Expansion |
|
|---|---|---|---|
| Mean arterial pressure, mmHg | 96.7 ± 3.0 | 106.7 ± 2.1c | |
| Hematocrit, % | 45.1 ± 0.7 | 51.3 ± 0.8a | 43.0 ± 0.7a |
| Plasma ANG II, pM | 115.8 ± 34.5 | 198.4 ± 57.5b | 85.6 ± 23.1b |
| Urine flow, µl/min | 1.2 ± 0.2 | 19.7 ± 5.3d | |
| Urine Na excretion, nmol/min | 99.6 ± 36.2 | 2,994.5 ± 998.2e |
P < 0.001, volume contraction vs. presurgery and volume expansion vs. volume contraction;
P < 0.05, volume contraction vs. presurgery and volume expansion vs. volume contraction;
P < 0.05, compared with volume contraction;
P < 0.0005, compared with volume contraction;
P < 0.02, compared with volume contraction.
In vivo microperfusion
Proximal tubule segments on the surface of the kidney were initially mapped with an injection of a small droplet of oil, and early and late loops were identified. A wax block was inserted into the lumen of an early loop by a hydraulic Microdrive (Trent Wells, Coulterville, CA), which prevented any native glomerular ultrafiltrate from flowing into the tubule segments distal to the block. The wax block also prevented the native glomerular ultrafiltrate from escaping the tubule lumen and thus likely raised the hydrostatic pressure in Bowman’s space and stopped ongoing single-nephron glomerular filtration. Subsequently, a microperfusion pipette was inserted into the lumen immediately distal to the wax block, and an ultrafiltrate-like solution was perfused at 30 nl/min. Microperfusion was accomplished using a microperfusion pump system (K. Effenberger; Vestavia Scientific, Birmingham, AL). In a late proximal tubule loop distal to the perfusion pipette, a collection pipette was inserted, and the perfused ultrafiltrate-like solution was collected after an oil block was placed distally. Fluid collections were made over a 2- to 3-min period. The length of the tubule between the perfusion and collection sites averaged 2.6 ± 0.2 mm. The composition of the ultrafiltrate-like solution was (in mM) 120 NaCl, 25 NaHCO3, 5 KCl, 1 MgSO4, 1.8 CaCl2, 1 Na2PO4, 5 glucose, 5 alanine, 5 urea, and FD & C green dye no. 3. Exhaustively dialyzed [methoxy-3H]inulin was added as a volume marker.
To examine the effect of 10−8 M losartan (angiotensin II receptor antagonist; DuPont, Wilmington, DE) on proximal tubule transport, each proximal tubule was perfused twice, first with the control ultrafiltrate-like solution, followed by a second perfusion with the same ultrafiltrate-like solution containing 10−8 M losartan (30). Both initial and subsequent tubule perfusions and fluid collections were made from the same tubule puncture sites, thus allowing paired data to be obtained. The “double micropuncture” technique was performed in both volume-contracted and volume-expanded animals. Luminally perfused losartan acts primarily on the luminal brush border receptors. It is unknown whether losartan can traverse the proximal tubule cell and act on the basolateral angiotensin II receptors. However, luminal losartan has been shown to inhibit volume reabsorption and bicarbonate transport in rabbit proximal convoluted tubules perfused in vitro in the absence of bath angiotensin II (2).
We next examined the effect of luminal angiotensin II on proximal tubule transport in the volume-expanded animals with luminal perfusion of exogenous angiotensin II at 10−6, 10−8, and 10−10 M ([Asn1,Val5]angiotensin II; Sigma Chemical, St. Louis, MO). In vivo microperfusion of the volume-expanded animals began 1 h after volume expansion had been completed.
After all collections were performed, the entire tubule was injected with liquid Microfil (Flow-Tech, Carver, MA) and allowed to harden overnight in the refrigerator. The kidney was later placed in 6 N HCl at 37°C for 1 h. The Microfil tubule casts were dissected and photographed, and the tubular length between the perfusion and collection sites was measured.
Plasma angiotensin II assay
Plasma samples were collected 1 h after completion of animal surgery, during volume contraction, and 1 h after volume expansion was completed. Plasma samples were extracted on a phenyl-bonded sodium phosphate-EDTA column (Bond-Elut; Analytichem, Harbor City, CA). Prior to sample application, the column was prewashed with 3 ml of 90% methanol in water and 6 ml of distilled deionized water. After the sample was applied, the column was then washed with 3 ml of distilled deionized water, followed by 1.5 ml hexane, and finally 1.5 ml chloroform. The angiotensin peptides retained on the column were then eluted with 2 ml of 90% methanol in water, dried under vacuum in a Speed Vac, and subsequently stored at −80°C until assay.
The angiotensin II assay was performed with an enzyme immunoassay kit (Peninsula Laboratories, Belmont, CA). Briefly, samples and standards are added to microtiter wells containing antibody to angiotensin II bound to the walls of the well. Next, biotinylated “tracer angiotensin II” is added to the well, which competes with angiotensin II in the sample or standard for binding to the angiotensin II antibody. The amount of biotinylated angiotensin II bound to the angiotensin II antibody is inversely proportional to the amount of angiotensin II in the sample. After incubation, avidinhorseradish peroxidase conjugate is added, which binds only to biotinylated angiotensin II. In the last step, a colorimetric agent (3,3, 5,5 -tetramethyl benzidine dihydrochloride) is added to each well and allowed to react with bound horseradish peroxidase. The intensity of color depends on the amount of horseradish peroxidase bound to biotinylated angiotensin II. All microtiter wells are read in a colorimetric microtiter plate reader (Titertek Multiscan, McLean, VA), a standard curve is constructed from the optical density readings, and sample angiotensin II concentrations are read from this curve. A standard curve is constructed each time the assay was performed.
Analysis
All collected tubular fluid was transferred to constant-bore capillary tubing for measurement of volume with a micrometer (Mitutoyo, City of Industry, CA) and then mixed with scintillation fluid for radioactivity counting. The rate of fluid reabsorption was calculated as the difference between perfused and collected volumes divided by the time of collection divided by the tubule length. One tubule per rat was used for micropuncture. Thus, n represents the number of tubules and rats. Analysis of variance and Student’s t-tests (paired and unpaired) were used to determine statistical significance. The post hoc test used following analysis of variance included the Student-Newman-Keuls multiple comparisons test. All data are expressed as means ± SE.
RESULTS
Effect of luminal losartan on proximal tubule transport during volume contraction and volume expansion
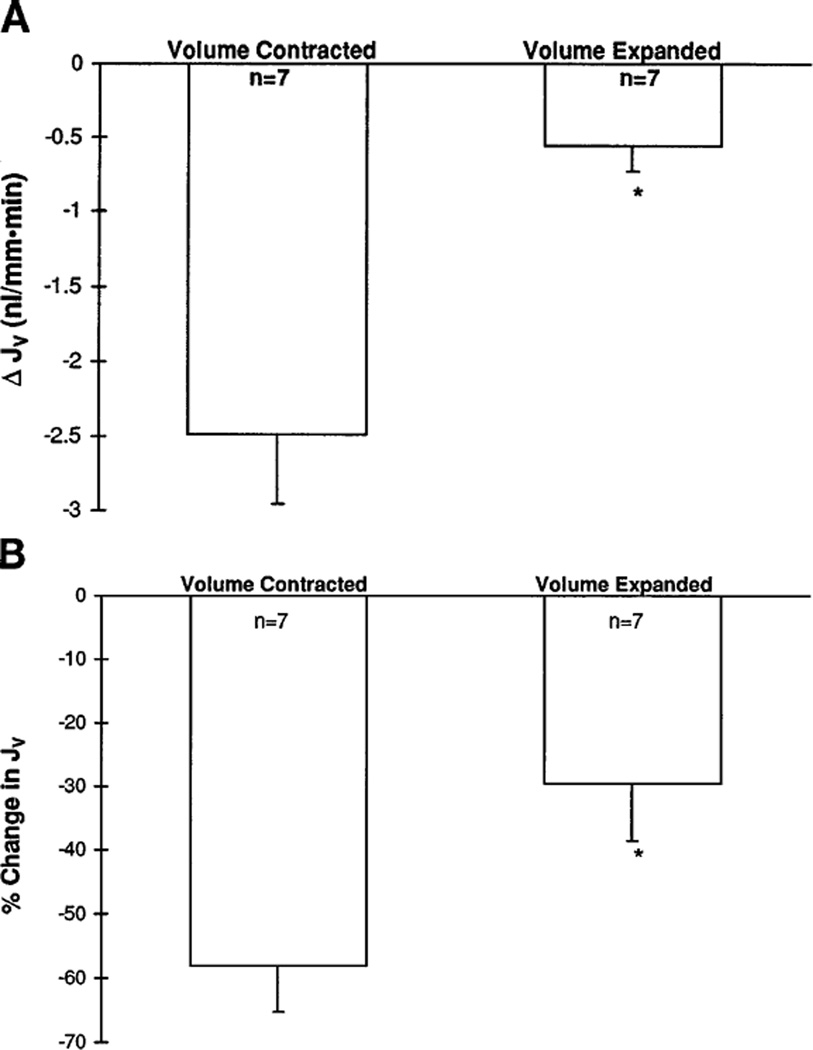
These studies were performed to investigate the effect of losartan, an angiotensin II receptor antagonist, on the proximal tubule volume reabsorptive rate during volume contraction and volume expansion. As seen in Fig. 1, administration of luminal 10−8 M losartan significantly inhibited the rate of volume reabsorption by the proximal tubule during volume contraction (4.2 ± 0.50 vs. 1.70 ± 0.30 nl · mm−1 · min−1, P < 0.05). In contrast, after acute volume expansion, administration of luminal 10−8 M losartan decreased volume reabsorption to a far lesser degree (1.84 ± 0.20 vs. 1.31 ± 0.20 nl · mm−1 · min−1, P < 0.05). Time controls performed in the volume-expanded rats demonstrated no change in volume reabsorption rates between the first and second perfusions (2.22 ± 0.78 vs. 2.13 ± 0.81 nl · mm−1 · min−1). As seen in Fig. 2, the absolute and percent decrement in proximal tubule transport resulting from the administration of luminal 10−8 M losartan was significantly greater during volume contraction (58 ± 7.0%) than after volume expansion (29.6 ± 9.0%, P < 0.05).
Fig. 1.
Effect of 10−8 M luminal losartan on proximal tubule volume reabsorption (Jv) during volume contraction and volume expansion. Luminal losartan decreased volume reabsorption both during volume contraction and volume expansion (*P < 0.005 and ‡P < 0.05, respectively). Magnitude of the decrement in volume reabsorption is much greater during volume contraction than after volume expansion. †P < 0.05, compared with volume-contracted control.
Fig. 2.
A: absolute change in proximal tubule volume reabsorption (ΔJv) observed with luminal perfusion of losartan in volume-contracted and volume-expanded rats. Absolute decrease in volume reabsorption during volume contraction (2.49 ± 0.46 nl · mm−1 · min−1) was significantly greater than that observed after acute volume expansion (0.56 ± 0.16 nl · mm−1 · min−1, *P < 0.01). B: percent change in proximal tubule volume reabsorption observed with luminal perfusion of losartan in volume-contracted and volume-expanded rats. Percent decrease during volume contraction (58.0 ± 7.0%) was significantly greater than that observed after volume expansion (29.6 ± 9.0%, *P < 0.05).
Effect of luminal angiotensin II on proximal tubule transport during volume expansion
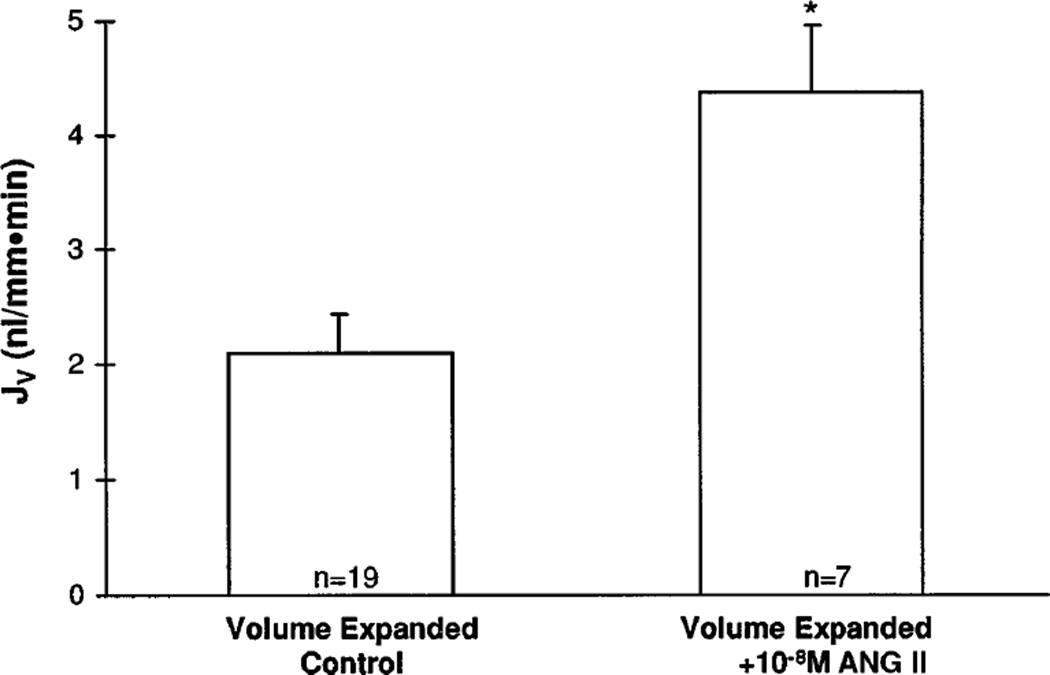
These studies were conducted to investigate the effect of 10−6, 10−8, and 10−10 M luminal angiotensin II on proximal tubule volume reabsorption in the acutely volume-expanded rat. We and others have previously shown that administration of 10−6, 10−8, and 10−11 M luminal angiotensin II in the volume-contracted rat had little or no effect on proximal tubule transport (11, 17, 23). As shown in Fig. 3, 10−8 M luminal angiotensin II increased proximal tubule volume reabsorption in the volume-expanded rat from 2.10 ± 0.34 to 4.38 ± 0.59 nl · mm−1 · min−1 (P < 0.005). This rate of proximal tubule volume absorption was comparable to that observed in volume-contracted rats. In contrast, the rates of proximal tubule volume reabsorption observed with 10−6 and 10−10 M luminal angiotensin II (2.22 ± 0.29 and 1.97 ± 0.20 nl · mm−1 · min−1, respectively) were unchanged, compared with that observed in the volume-expanded control (2.10 ± 0.34 nl · mm−1 · min−1).
Fig. 3.
Effect of luminal 10−8 M angiotensin II on proximal tubule volume reabsorption after acute volume expansion. Volume reabsorptive rate rose from 2.10 ± 0.34 to 4.38 ± 0.49 nl · mm−1 · min−1, a reabsorptive rate observed in volume-contracted rats (*P < 0.005).
DISCUSSION
Angiotensin II has long been known to directly affect proximal tubule transport independent of changes in the glomerular filtration rate (3, 11, 14, 16, 17, 26). Physiological doses of angiotensin II stimulate proximal tubule transport in the absence of changes in glomerular hemodynamics or blood pressure (3, 11, 14, 16, 17). Using in vitro and in vivo microperfusion, physiological doses of peritubular or systemic angiotensin II stimulate proximal tubule transport (15–17). Systemic administration of the angiotensin II antagonist saralasin in whole animal studies decreased proximal tubule fractional and absolute volume reabsorption (22, 28).
Recently, the proximal tubule has been found to synthesize and luminally secrete angiotensin II at concentrations ranging from 100- to 1,000-fold higher than that in plasma (4, 5, 27). This robust endogenous production of angiotensin II within the proximal tubule is consistent with an autocrine/paracrine role for endogenous angiotensin II to regulate proximal tubule transport independent of systemic angiotensin II. We recently demonstrated that blockade of the production or action of endogenous angiotensin II in in vivo microperfused proximal tubules by administration of luminal 10−8 M losartan or 10−4 M enalaprilat inhibited the proximal tubule volume reabsorptive rate by 35–40% (23).
Our current data is consistent with an autocrine/paracrine role of angiotensin II in the regulation of proximal tubule transport by demonstration that the effect of luminal angiotensin II is regulated by acute changes in extracellular volume. The decrease in proximal tubule transport caused by luminal losartan is a measure of angiotensin II’s contribution to regulation of proximal tubule transport. The proportionately greater decrease in transport observed with luminal losartan during volume contraction (58.0 ± 7.0 vs. 29.6 ± 9.0%, P < 0.05) indicates greater stimulation of net proximal tubule transport by luminal angiotensin II levels during acute volume contraction than during acute volume expansion.
The proposed autocrine or paracrine role for angiotensin II in the regulation of proximal tubule transport does not preclude the role of other systemic and peritubular physical factors that also concomitantly affect proximal tubule transport. Alterations in extracellular volume affect the systemic renin-angiotensin system, the permeability properties of the paracellular pathway, peritubular physical forces, renal nerve activity, and systemic catecholamine levels, which were not examined in this study (19). The net change in proximal tubule transport occurring with alterations in extracellular volume likely result from the combined effect of these systemic and peritubular factors, as well as endogenous proximal tubule angiotensin II.
Previous studies have demonstrated little or no effect of exogenous luminal angiotensin II on proximal tubule transport in the hydropenic rat (16, 23). To examine whether this was the result of elevated endogenous production and luminal levels of angiotensin II during volume contraction, we perfused angiotensin II into the lumen of proximal tubules after acute volume expansion. The twofold increase in volume reabsorption with addition of 10−8 M luminal angiotensin II during volume expansion is consistent with endogenous physiological concentrations of angiotensin II having a greater stimulatory effect on volume absorption during volume contraction. Pharmacological or subphysiological concentrations of exogenous luminal angiotensin II (10−6 and 10−10 M) had neither an inhibitory nor stimulatory effect on proximal tubule volume reabsorption.
The above data (Figs. 2 and 3) underscore the important and central role played by luminal angiotensin II levels. These data are supported by the results of two other recent studies that have examined the effect of changes in extracellular volume on luminal angiotensin II levels (4, 5). Braam et al. (5) found that, although not statistically significant, the luminal angiotensin II concentration in native tubular fluid fell from 14 nM during volume contraction to 8 nM after acute volume expansion. Conversely, Boer et al. (4) found that proximal tubule angiotensin II rose 3.5-fold when renal perfusion pressure was decreased 20%. This rise in angiotensin II with renal hypoperfusion and potential fall in angiotensin II with volume expansion are consistent with regulation of endogenously produced and luminally secreted angiotensin II by acute changes in extracellular volume. The absence of a statistically significant change in luminal angiotensin II levels induced by acute changes in extracellular volume in both studies might result from the lack of adequate sensitivity and precision in detecting small differences of luminal angiotensin II during different volume states. The small tubular fluid sample volumes along with sample dilution during the assay all contribute to variation in obtaining the final angiotensin II level. Such “background variation or noise” could potentially obscure any differences in luminal angiotensin II levels occurring between different volume states.
Alternatively, the decrease in luminal angiotensin II levels might represent dilution by the greater volume of tubular fluid rather than an actual decrease in production and secretion of angiotensin II. Regulation of the luminal concentration of angiotensin II may ultimately not be the modus operandi of endogenous proximal tubule angiotensin II. An alternate autocrine role for angiotensin II might involve regulation of transport by intracellular angiotensin II. Angiotensin II may be synthesized intracellularly and act within the proximal tubule cell to regulate transport independent of “extracellular” luminal angiotensin II levels. As an autocrine hormone, intracellular or tissue angiotensin II, rather than luminal angiotensin II, may fall with volume expansion and rise with volume contraction to regulate proximal tubule transport. In support of this notion, Boer et al. (4) also found that volume expansion markedly decreased the whole kidney tissue angiotensin II content, whereas reduced renal pefusion pressure raised whole kidney tissue angiotensin II.
Another equally plausible hypothesis to explain regulation of proximal tubule transport by angiotensin II involves modulation of angiotensin II receptor density or modulation of the sensitivity of the signal transduction mechanism for angiotensin II. After changes in extracellular volume, the effect of luminal angiotensin II would “translate” into greater or lesser stimulation of proximal tubule transport via alterations in receptor density or sensitivity of signal transduction. Cheng et al. (8) recently found that angiotensin II upregulates expression of angiotensin II receptors in the proximal tubule. Thus the stimulation of proximal tubule transport by angiotensin II could be significantly amplified via upregulation of its own receptor. Our observations regarding heightened stimulation of proximal tubule transport by endogenous angiotensin II during volume contraction may, in part, result from an increase in angiotensin II receptor expression caused by angiotensin II itself. Alternatively, changes in extracellular volume might also alter the affinity of the angiotensin II receptor for angiotensin II. Extracellular volume expansion may decrease the angiotensin II receptor affinity, whereas extracellular volume contraction may increase the angiotensin II receptor affinity. This notion would reconcile the currently observed stimulation of proximal tubule transport with 10−8 M angiotensin II in volume-expanded but not volume-contracted rats with the previous observation by Harris and Young (11) of stimulation of proximal tubule transport with 10−9 M angiotensin II in volume-contracted rats (11, 23). Although speculative, these possibilities provide an intriguing alternative to the mechanism of action of endogenous angiotensin II.
Acknowledgments
We are grateful for the able secretarial assistance of Patricia Wilson and Janell McQuinn and the technical assistance of Lisa K. Worrall.
This work was supported by a grant from the American Heart Association, Texas Affiliate (to A. Quan), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41612 (to M. Baum), and by grants from the National Kidney Foundation of Texas (to A. Quan and M. Baum).
REFERENCES
- 1.Alpern RJ, Cogan MG, Rector FC. Effects of extracellular fluid volume and plasma bicarbonate concentration on proximal acidification in the rat. J. Clin. Invest. 1983;71:736–746. doi: 10.1172/JCI110821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum M, Quigley R, Quan A. Effect of luminal angiotensin II on rabbit proximal convoluted tubule bicarbonate absorption. Am. J. Physiol. 1997;273:F595–F600. doi: 10.1152/ajprenal.1997.273.4.F595. (Renal Physiol. 42) [DOI] [PubMed] [Google Scholar]
- 3.Barraclough MA, Jones NF, Marsden CD. Effect of angiotensin on renal function in the rat. Am. J. Physiol. 1967;212:1153–1157. doi: 10.1152/ajplegacy.1967.212.5.1153. [DOI] [PubMed] [Google Scholar]
- 4.Boer WH, Braam B, Fransen R, Boer R, Koomans HA. Effects of reduced renal perfusion pressure and acute volume expansion on proximal tubule and whole kidney angiotensin II content in the rat. Kidney Int. 1997;51:44–49. doi: 10.1038/ki.1997.6. [DOI] [PubMed] [Google Scholar]
- 5.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am. J. Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. (Renal Fluid Electrolyte Physiol. 33) [DOI] [PubMed] [Google Scholar]
- 6.Brown GP, Douglas JG. Angiotensin II binding sites on isolated rat renal brush border membranes. Endocrinology. 1982;111:1830–1836. doi: 10.1210/endo-111-6-1830. [DOI] [PubMed] [Google Scholar]
- 7.Brown GP, Douglas JG. Angiotensin II binding sites in rat and primate isolated renal tubular basolateral membranes. Endocrinology. 1983;112:2007–2014. doi: 10.1210/endo-112-6-2007. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-l angiotensin II receptors in renal proximal tubule. J. Clin. Invest. 1995;95:2012–2019. doi: 10.1172/JCI117886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox HM, Munday KA, Poat JA. The binding of [125I]-angiotensin to rat renal epithelial cell membranes. Br. J. Pharmacol. 1983;79:63–70. doi: 10.1111/j.1476-5381.1983.tb10496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox HM, Munday KA, Poat JA. Localization of [125I]-angiotensin II receptors on rat kidney cortex epithelial cells. Br. J. Pharmacol. 1984;82:891–895. doi: 10.1111/j.1476-5381.1984.tb16487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflügers Arch. 1977;367:295–297. doi: 10.1007/BF00581370. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa I, Maddox DA, Cogan MG, Brenner BM. Dynamics of glomerular ultrafiltration in euvolemic Munich-Wistar rats. Renal Physiol. 1978;1:121–131. [Google Scholar]
- 13.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. J. Clin. Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson MD, Malvin RL. Stimulation of renal sodium reabsorption by angiotensin II. Am. J. Physiol. 1977;232:F298–F306. doi: 10.1152/ajprenal.1977.232.4.F298. (Renal Fluid Electrolyte Physiol. 1) [DOI] [PubMed] [Google Scholar]
- 15.Li L, Wang YP, Capparelli AW, Jo OD, Yanagawa N. Effect of luminal angiotensin II on proximal tubule fluid transport: role of apical phospholipase A2. Am. J. Physiol. 1994;266:F202–F209. doi: 10.1152/ajprenal.1994.266.2.F202. (Renal Fluid Electrolyte Physiol. 35) [DOI] [PubMed] [Google Scholar]
- 16.Liu FY, Cogan MG. Angiotensin II: a potent regulator of acidification in the rat early proximal convoluted tubule. J. Clin. Invest. 1987;80:272–275. doi: 10.1172/JCI113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu FY, Cogan MG. Angiotensin II stimulation of hydrogen ion secretion in the rat early proximal tubule. J. Clin. Invest. 1988;82:601–607. doi: 10.1172/JCI113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddox DA, Price DC, Rector FC. Effects of surgery on plasma volume and salt and water excretion in rats. Am. J. Physiol. 1977;233:F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. (Renal Fluid Electrolyte Physiol. 2) [DOI] [PubMed] [Google Scholar]
- 19.Moe GW, Legault L, Skorecki KL. Extracellular fluid volume and pathophysiology of edema. In: Brenner BM, Rector FC, editors. The Kidney. 4th ed. I. 1991. pp. 628–643. [Google Scholar]
- 20.Moe OW, Alpern RJ, Henrich WL. The renal proximal tubule renin-angiotensin system. Semin. Nephrol. 1993;13:552–557. [PubMed] [Google Scholar]
- 21.Moe OW, Kazutomo U, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J. Clin. Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelayo JC, Blantz RC. Analysis of renal denervation in the hydropenic rat: interactions with angiotensin II. Am. J. Physiol. 1984;246:F87–F95. doi: 10.1152/ajprenal.1984.246.1.F87. (Renal Fluid Electrolyte Physiol. 15) [DOI] [PubMed] [Google Scholar]
- 23.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J. Clin. Invest. 1996;97:2878–2882. doi: 10.1172/JCI118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richoux JP, Cordonnier JL, Bouhnik J, Clausen E, Corvol P, Menard J, Grignon G. Immunocytochemical localization of angiotensinogen in the rat liver and kidney. Cell Tissue Res. 1983;233:439–451. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 25.Rosivall L, Narkates AJ, Oparil S, Navar LG. De novo intrarenal formation of angiotensin II during control and enhanced renin secretion. Am. J. Physiol. 1987;252:F1118–F1123. doi: 10.1152/ajprenal.1987.252.6.F1118. (Renal Fluid Electrolyte Physiol. 21) [DOI] [PubMed] [Google Scholar]
- 26.Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J. Clin. Invest. 1984;73:507–515. doi: 10.1172/JCI111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seikaly MG, Arant BS, Seney FD. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J. Clin. Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner RW, Tucker BJ, Blantz RC. Glomerular hemodynamics in rats with chronic sodium depletion: effect of saralasin. J. Clin. Invest. 1979;64:503–512. doi: 10.1172/JCI109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taugner R, Hackenthal E, Rix E, Nobling R, Poulsen K. Immunocytochemistry of the renin-angiotensin system: renin, angiotensinogen, angiotensin I, and angiotensin II and converting enzyme in the kidneys of mice, rats, and tree shrews. Kidney Int. 1986;22(Suppl.):S33–S43. [PubMed] [Google Scholar]
- 30.Wong PC, Price WA, Chiu AT, Duncia JV, Carini DJ, Wexler RR, Johnson AL, Timmermans PBMWM. Nonpeptide angiotensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by losartan, an orally active antihypetensive agent. J. Pharmacol. Exp. Ther. 1990;252:719–725. [PubMed] [Google Scholar]