Abstract
Goals of work
Ginger has been used to treat numerous types of nausea and vomiting. Ginger has also been studied for its efficacy for acute chemotherapy-induced nausea and vomiting (CINV). However, its efficacy for delayed CINV in a diverse oncology population is unknown.
Materials and methods
We performed a randomized, double-blind, placebo-controlled trial in 162 patients with cancer who were receiving chemotherapy and had experienced CINV during at least one previous round of chemotherapy. All participants were receiving a 5-HT3 receptor antagonists and/or aprepitant. Participants were randomized to receive either 1.0 g ginger, 2.0 g ginger daily, or matching placebo for 3 days. The primary outcome was change in the prevalence of delayed CINV. Secondary outcomes included acute prevalence of CINV, acute and delayed severity of CINV, and assessment of blinding.
Main results
There were no differences between groups in the prevalence of delayed nausea or vomiting, prevalence of acute CINV, or severity of delayed vomiting or acute nausea and vomiting. Participants who took both ginger and aprepitant had more severe acute nausea than participants who took only aprepitant. Participants were able to accurately guess which treatment they had received. Ginger appeared well tolerated, with no difference in all adverse events (AEs) and significantly less fatigue and miscellaneous AEs in the ginger group.
Conclusions
Ginger provides no additional benefit for reduction of the prevalence or severity of acute or delayed CINV when given with 5-HT3 receptor antagonists and/or aprepitant.
Keywords: Ginger, Apripetant, Chemotherapy-induced nausea and vomiting
Introduction
Chemotherapy-induced nausea, retching, and vomiting (CINV) has historically had significant negative impacts on the quality of life (QoL) and daily functioning of patients receiving chemotherapy [5]. CINV that occurs within the first 24 h of chemotherapy treatment is considered acute. Delayed CINV occurs at greater than 24 h post-treatment and can persist for several days. The negative impacts of CINV on QoL persist despite the introduction of newer treatments for nausea and vomiting, such as serotonin (5-HT3) antagonists for acute CINV and aprepitant [neurokinin-1 (NK-1) antagonist] [10] for delayed CINV.
Several recent surveys have found that the prevalence of CINV after receiving conventional anti-emetic therapy ranged from 48% to 67% [3, 6, 12, 16]. In one survey conducted in ten community oncology clinics, only 33% of the patients did not have either delayed or acute CINV, and the majority of patients who developed CINV experienced both delayed and acute CINV [6]. In a US national survey, only fatigue was a more prevalent side effect of chemotherapy treatment compared to CINV [16]. In particular, patients consistently reported significantly more delayed nausea and vomiting compared to acute CINV, indicating that delayed CINV continues to be difficult to control despite the introduction of anti-emetic agents targeted at this timeframe [6, 13]. Patients experiencing CINV reported lower satisfaction with their care [12], missed work days [16], and negative effects on QoL [3, 6, 12, 16]. Agents that could safely further decrease the rates of CINV, and especially delayed CINV, are needed.
Ginger root (Zingiber officinale Roscoe, Zingiberaceae) was first cultivated in Asia and has been used as a medicinal herb for at least 2,000 years [27]. In Chinese, Indian, Middle Eastern, and western herbal medicine, ginger is used primarily as a remedy for digestive disorders including dyspepsia, nausea, vomiting, and diarrhea [17, 25].
Ginger root contains approximately 1.5% to 2.0% of a number of pungent compounds [4]. Gingerols are the most abundant pungent compounds in fresh roots, and several gingerols of various chain lengths (n6 to n10) are present, with the most plentiful being 6-gingerol. Shogaols, the dehydrated form of gingerols, are mainly found in the dried and thermally treated roots, with 6-shogaol being the most abundant [20]. Gingerols and shogaols appear to be the compounds that confer most of the medicinal properties to ginger root.
Ginger demonstrates numerous properties that may be beneficial in treating CINV, including reversing the inhibitory effect of cisplatin on gastric emptying in rats [15, 31], as a 5-HT3 receptor antagonist [18, 32, 35], and as an antioxidant [1, 22, 34].
Previous clinical trials have examined the effect of ginger on CINV with mixed results, with one study showing no effect [24], another with mixed results [29], and two others with positive outcomes [28, 33] of ginger compared to placebo or metoclopramide. Apart from their mixed outcomes, it is difficult to assess these studies as they are limited by their small sample sizes, no clear identification or quality control of the ginger product used, unclear or limited patient population, and no examination of appropriate ginger dose. Further, only one study [24] investigated delayed nausea and vomiting. Using a well-defined and broad patient sample, a well-characterized ginger product at several doses, adequate sample size, and investigation into both acute and delayed CINV, we conducted a randomized, placebo-controlled, double-blind clinical trial to determine the efficacy of a ginger extract for the treatment of CINV in adults with a histologically confirmed diagnosis of cancer that were currently being treated with chemotherapy.
Materials and methods
Participants
The study protocol and all procedures were approved by the University of Michigan (UM) Medical School Institutional Review Board and all participating clinical sites review boards. All participants provided written informed consent. The study took place between June 2003 and May 2006. Individuals 18 years and older who had a histologically confirmed diagnosis of cancer currently being treated with chemotherapy (for adjuvant, neoadjuvant, curative, or palliative means) were eligible. Patients must also have received at least one previous chemotherapy treatment with the same chemotherapeutic agent and have experienced nausea or vomiting of any severity as a result of that treatment. Patients were recruited from the UM Health System oncology clinics and from ten other sites that were part of the National Cancer Institute’s (NCI’s) Community Clinic Oncology Program (CCOP). Patients were ineligible if they: (a) were receiving multiple-day chemotherapy; (b) were receiving concurrent radiotherapy that was classified as high or intermediate risk of causing emesis (i.e., total body irradiation, hemi-body, upper abdomen, abdominal–pelvic mantle, cranium, or craniospinal irradiation); (c) were taking therapeutic doses of coumadin (individuals on low-dose coumadin to maintain peripheral or central venous catheters were allowed), aspirin (individuals taking low-dose 81 mg aspirin were allowed), or heparin; (d) had a history of a bleeding disorder(s) and those experiencing clinically significant thrombocytopenia; (e) had an allergy to ginger or had taken ginger in the last week; or (f) were nursing mothers, pregnant women, or planning a pregnancy during the study period. Patients were eligible to participate if they were scheduled to have a single-day chemotherapy regime and to receive a 5-HT3 receptor antagonist antiemetic and/or the antiemetic aprepitant.
All potentially eligible participants were approached by a research assistant or nurse after their visit at various oncology clinics, and interested patients were scheduled for their screening visit. The screening visit was within 28 days of when the study medication was administered and could be replaced with a pre-chemotherapy visit if all study procedures were conducted. Written and verbal informed consent were obtained at the beginning of the screening visit. Patients then had a physical exam, medical history, a list of concomitant medications collected, a complete blood count (with platelets and differentials), a comprehensive chemistry screen, and a prothrombin time/international ratio test. Participants were also given a questionnaire to assess the amount of ginger in their typical diet. Those participants who had acceptable physical exams, laboratory values, and were not eating a significant amount of ginger were deemed eligible and were scheduled to receive their study medication during their next round of chemotherapy.
Intervention
Eligible participants were randomly assigned to receive a ginger extract, manufactured by Pure Encapsulations® (Sudbury, MA, USA), 1.0 g (four capsules ginger and four capsules placebo daily), 2.0 g (eight capsules daily), or a matching placebo (eight capsules daily). These doses were chosen based on the manufacturer’s recommendations and on doses used in previous clinical studies using this extract. Each capsule contained 250 mg dry extract of ginger root [10:1 (v/v) extraction solvent (ethanol 50%)/root] standardized to 15 mg (5%) of total gingerols. The University of Michigan Investigational Drug Service placed 250 mg of the Pure Encapsulations® ginger or lactose powder in size “0” red animal gelatin capsules made by Gallipot®. Based on high-performance liquid chromatography (HPLC) analysis, a 250-mg capsule of ginger extract contained 5.38 mg (2.15%) 6-gingerol, 1.80 mg (0.72%) 8-gingerol, 4.19 mg (1.78%) 10-gingerol, and 0.92 mg (0.37%) 6-shogaol. Content of gingerols and 6-shogaol in the study medication were independently verified using appropriate HPLC methods (Integrated Biomolecule; Tuscon, AZ, USA) [2]. Participants were told to take the study medication twice per day with water and to bring all unused capsules to the final (72 h) study visit. The first study drug dose was taken within 1 h of the completion of chemotherapy. Patients were seen at the study clinic at the time of their chemotherapy treatment and 3 days after the end of their chemotherapy treatment.
Objectives and outcomes
Our primary objective was to compare the effect of a low-dose (1.0 g) and a high-dose (2.0 g) powdered ginger root extract versus placebo for reducing the prevalence and severity of delayed nausea and vomiting using a 2-day patient diary based on a modified “Morrow Assessment of Nausea and Emesis” (MANE; we replicated questions for days 2 and 3, not just the first 24 h after chemotherapy treatment). The MANE is a validated questionnaire used to assess the prevalence and severity of vomiting and nausea in a given time period, i.e. 24 h. Patients, along with being asked whether they experienced nausea or vomiting during or after their chemotherapy treatment, are also asked how long in minutes or hours their nausea lasted and how they would describe their nausea or vomiting “at its worst” using a six-point Likert scale (very mild to intolerable) as well as the time period when the “nausea or vomiting was the worst”, e.g., 4–8 h after treatment [26]. Delayed chemotherapy-induced nausea or vomiting was defined as any nausea or vomiting that occurred greater than 24 h after receiving chemotherapy.
The secondary objectives included: (1) comparing the effect of a low-dose (1.0 g) and a high-dose (2.0 g) powdered ginger root extract versus placebo for reducing the prevalence and severity of acute (within 24 h of receiving chemotherapy) nausea and vomiting (assessed with the MANE); (2) assessing the safety of different doses (low versus high) of oral powdered ginger root; and (3) determining if study participants are blinded to study assignment, as well as determining which variables may unblind participants (taste, smell, and decrease in nausea and emesis) during the 3-day study period.
We assessed safety by querying participants verbally about any hospitalizations or adverse events that occurred during any of the 3 days of the study. We also reviewed hospital records to assess the cause of hospitalizations and gather information about laboratory abnormalities. Toxicities were graded based on National Cancer Institute Common Toxicity Criteria version 3.0 for Adverse Events [8]. All study participants had access to 24-h nursing support at their local institution. Any adverse events reported by the participants to their local health care providers or CCOP research staff were promptly reported to UM study personnel and followed up by a direct query to the CCOP research site for additional information.
Randomization, blinding, and allocation
Eligible participants were randomized equally to one of three groups: placebo, ginger extract 1.0 g, or ginger extract 2.0 g. The randomization code blocked by research site was computer-generated by the study biostatistician. Study participants were also stratified at randomization into one of two strata [strata 1=5-HT3 antagonist; strata 2=aprepitant (NK1 antagonist)]. Participants who received a 5-HT3 antagonist plus aprepitant were placed into the aprepitant strata, while those participants that received only a 5-HT3 antagonist were placed in the 5-HT3 strata. The stratification allowed for an equal distribution of the NK1 antagonist (aprepitant) to be distributed equally between the treatment arms.
All study participants as well as all study personnel who assessed outcomes, worked with study data, or administered tests or questionnaires were unaware of the randomization list or treatment assignment.
Statistical methods and sample size
Baseline characteristics were reported, stratified by treatment group, using means and SDs for continuous variables and counts and percentages for categorical variables. Balance between treatment groups on baseline characteristics was tested using Kruskal–Wallis statistics for continuous variables and Pearson’s chi-square and Fisher exact tests, as appropriate, for categorical variables.
Prevalence of delayed and acute nausea or vomiting was calculated as a binary variable, i.e., “yes” if the patient had nausea or vomiting (of any severity) or “no” if the patient had no nausea or vomiting. If participants vomited and/or retched at least once, it was counted as a “yes” for vomiting for that time period. The prevalence of nausea and vomiting was compared separately by treatment arm using Cochran Mantel–Haenszel tests stratified for aprepitant (“yes” or “no”). Logistic regression was also used to model the effect of treatment while controlling for covariates, including: emetogenicity of chemotherapeutic agent (high, moderate, low); aprepitant (“yes” or “no”); and presence or absence of baseline nausea or vomiting (“yes” or “no”) as appropriate for delayed values and presence or absence of acute nausea or vomiting (“yes” or “no”). Analyses of severity of delayed and acute nausea and vomiting were examined as an ordinal outcome. Severity of nausea and vomiting and/or retching was graded on a six-point scale, where 1 equaled very mild and 6 equaled intolerable. These severity analyses were only performed on participants who had experienced nausea or vomiting. Analyses of severity were performed using Cochran Mantel–Haenszel tests between treatment groups (placebo, ginger=1.0 g and ginger=2.0 g) and stratified by aprepitant (yes/no). Analyses were conducted according to the intention-to-treat principle; however, no imputation was performed for missing values at day 1, 2, or 3. Data were entered into a central database located at Dartmouth College (Hanover, NH, USA). For all analyses, two-sided tests and a significance level of 0.05 were used. The experiment-wise type I error rate was protected only for the principal outcome measure. No adjustments were made for multiple-hypothesis testing, as the secondary outcomes were viewed as hypothesis-generating.
The sample size was justified in terms of the analysis of between-treatment differences in delayed nausea. The prevalence of delayed nausea in patients taking standard antiemetic therapy was assumed to be 51% for lower dose chemotherapy and 74% for higher dose chemotherapy. If the highest ginger dose caused a 30% relative reduction from placebo in the prevalence of delayed nausea, the study had 75% power to reject the null hypothesis of no treatment effect at a 5% significance level.
Main results
Screening, enrollment, and withdrawals
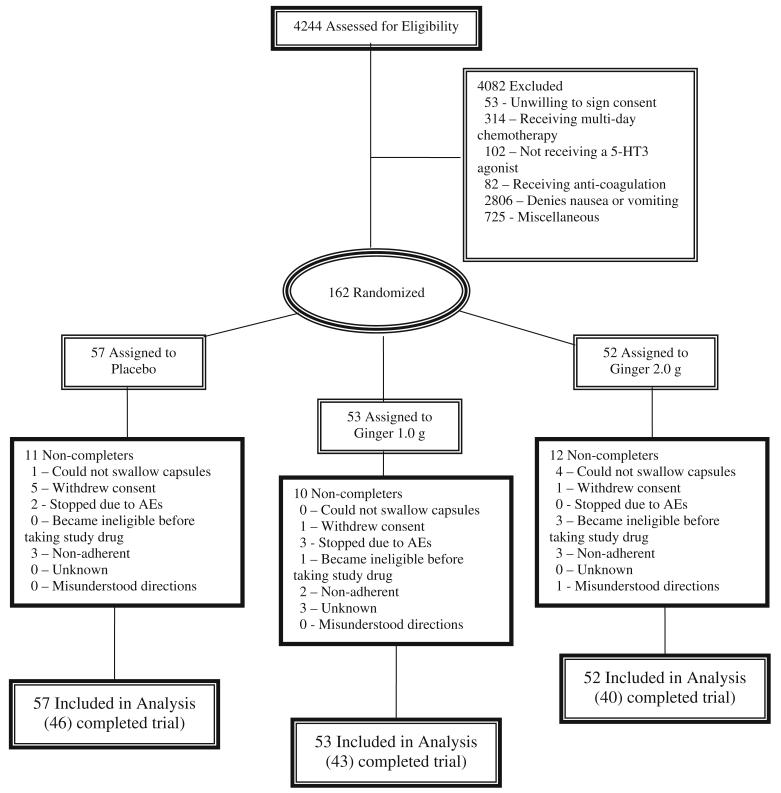
We screened 4,244 patients, of whom 162 met all eligibility criteria and were randomized: 57 to the placebo, 53 to the 1.0-g ginger dose, and 52- to the 2.0-g ginger dose. Figure 1 documents sources of recruitment for potential participants, reasons for exclusions, and reasons for discontinuing the intervention. The low proportion of recruited patients reflects the broad screening of unselected patients presenting to oncology clinics at all CCOP sites. Forty-six participants in the placebo group, 43 participants in the 1.0-g ginger dose, and 40 participants in the 2.0-g ginger dose arm completed all study visits. Adherence to study medications was moderate to high, with 79% of all participants taking greater than 80% of all study medication and with no significant differences between groups (p=0.80).
Fig. 1.
Patient flow in the randomized controlled trial
Sociodemographic and clinical characteristics
In Table 1, we present the sociodemographic and clinical characteristics of participants by treatment group. There was no significant difference between treatment groups for any demographic or clinical characteristics.
Table 1.
Baseline characteristics of the randomization groups
| Characteristics | Placebo (n=57) | Ginger 1.0 g (n=53) | Ginger 2.0 g (n=52) |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 14 (24.6) | 14 (26.4) | 12 (23.1) |
| Women | 43 (75.4) | 39 (73.6) | 40 (76.9) |
| Race, n (%) | |||
| White | |||
| Age, mean (SD, years) | 55.5 (11.2) | 53.3 (12.0) | 58.3 (12.3) |
| Chemotherapeutic agent, n (%)a | |||
| High (>90%) emetic risk | 10 (17.5) | 9 (17.0) | 11 (21.2) |
| Cisplatin | 6 (10.5) | 8 (15.1) | 8 (15.4) |
| Cyclophosphamide ≥1,500 mg/m2 | 2 (3.5) | 0 (0.0) | 3 (5.8) |
| Dacarbazine | 2 (3.5) | 1 (1.9) | 0 (0.0) |
| Moderate (30 to 90%) emetic risk | 36 (63.2) | 36 (67.9) | 33 (63.5) |
| Oxaliplatin | 2 (3.5) | 1 (1.9) | 3 (5.8) |
| Capecitabine | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Carboplatin | 8 (14.0) | 12 (22.6) | 15 (28.8) |
| Cyclophosphamide <1,500 mg/m2 | 0 (0.0) | 2 (3.8) | 1 (1.9) |
| Cyclophosphamide and Epirubcin | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Doxorubicin | 4 (7.0) | 6 (11.3) | 1 (1.9) |
| Doxorubcin and Carboplatin | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Doxorubcin and Cyclophosphamide | 18 (31.6) | 13 (24.5) | 11 (21.2) |
| Epirubicin | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Irinotecan | 1 (1.8) | 1 (1.9) | 2 (3.8) |
| Low (10 to 30%) emetic risk | 11 (19.3) | 8 (15.1) | 8 (15.4) |
| Docetaxel | 3 (5.3) | 3 (56.6) | 0 (0.0) |
| Docetaxel and Gemcitabine | 1 (1.8) | 1 (1.9) | 0 (0.0) |
| Fluorouracil | 4 (7.0) | 2 (3.8) | 2 (3.8) |
| Gemcitabine | 2 (3.5) | 1 (1.9) | 4 (7.7) |
| Paclitaxel | 1 (1.8) | 1 (1.9) | 1 (1.9) |
| Trastuzumab | 0 (0.0) | 0 (0.0) | 1 (1.9) |
| Concomitant 5-HT3 receptor antagonists and NK-1, n (%) | |||
| Apripetant | 18 (31.6) | 19 (35.8) | 15 (28.8) |
| Dolasetron | 8 (14.0) | 4 (7.5) | 5 (9.6) |
| Granistron | 13 (22.8) | 25 (47.2) | 7 (13.5) |
| Ondansetron | 1 (1.8) | 13 (24.5) | 18 (34.6) |
| Palonosetron | 28 (43.9) | 13 (24.5) | 22 (42.3) |
5-HT3 5-hydroxytryptamine type 3, NK-1 neurokinin-1 receptor antagonist
Emetic risk of intravenously administered antineoplastic agents based on the American Society of Clinical Oncology (ASCO) guidelines for antiemetics in 2006 [21]. When patients received more than one antineoplastic agent, only the agent that possessed the highest emetic risk is listed. If more than one antineoplastic agent of the same emetic risk was administered, then both antineoplastic agents are listed.
All participants received a 5-HT3 receptor antagonist. Fifty-two participants (32.1%) received aprepitant. Of the 52 participants given aprepitant, 49 (94.2%) were receiving moderate or high emetic risk antineoplastic compared to only three (5.8%) who were being administered low emetic risk antineoplastic agents.
Prevalence and severity of acute and delayed nausea and vomiting
Fifty-eight percent (n=94) of study participants reported experiencing both acute and delayed nausea, while 30.9% (n=50) of participants reported acute vomiting and/or retching and 24.7% (n=40) reported delayed vomiting and/or retching
There was no significant difference between either of the ginger doses compared to placebo in the prevalence of acute or delayed nausea or vomiting. This observation was consistent when participants were stratified by whether or not aprepitant was prescribed as part of their treatment for CINV (Table 2). Although not significant, participants who received aprepitant and either dose of ginger had more treatment failures compared to those who received aprepitant in addition to placebo for both acute and delayed nausea and vomiting.
Table 2.
Prevalence of acute and delayed nausea and vomiting
| Prevalence of treatment failures | Number (%) | P valuea | P valueb | ||
|---|---|---|---|---|---|
|
|
|||||
| Placebo group (n=46) | Ginger 1.0 g group (n=43) | Ginger 2.0 g group (n=40) | |||
| Nausea | |||||
| Acute | |||||
| No apripetant | 23 (50.0) | 21 (48.8) | 20 (50.0) | 0.76 | 0.86 |
| Yes apripetant | 8 (17.4) | 12 (27.9) | 10 (25.0) | ||
| Delayed | |||||
| No apripetant | 23 (50.0) | 26 (60.5) | 17 (42.5) | 0.15 | 0.16 |
| Yes apripetant | 8 (17.4) | 11 (25.6) | 10 (25.0) | ||
| Vomiting | |||||
| Acute | |||||
| No apripetant | 11 (23.9) | 14 (26.4) | 11 (27.5) | 0.35 | 0.47 |
| Yes apripetant | 3 (6.5) | 5 (11.6) | 6 (15.0) | ||
| Delayed | |||||
| No apripetant | 8 (52.6) | 14 (32.6) | 7 (17.5) | 0.35 | 0.07 |
| Yes apripetant | 1 (2.2) | 5 (11.6) | 5 (12.5) | ||
P values were calculated using Cochran Mantel-Haenszel tests stratified by apripetant (yes or no)
P values are Pearson chi-squares calculated using logistic regression adjusting for emetic risk of the chemotherapeutic agent (high, moderate, low), apripetant, presence or absence of baseline nausea or vomiting (yes or no) as appropriate for delayed values and presence or absence of acute nausea or vomiting (yes or no) as appropriate for acute measures
When not stratified by use of aprepitant, there was no significant difference in severity between either the low dose or the high dose of ginger and placebo, except for delayed nausea. Participants who received the high dose of ginger (2.0 g) reported having significantly more severe episode of delayed nausea compared to both placebo and low-dose ginger (1.0 g; mean±SD: placebo=2.8±1.2, ginger 1.0 g=2.9±1.1, ginger 2.0 g=3.4±1.1; p=0.03). Table 3 presents results of the severity of both acute and delayed nausea and vomiting stratified by the use of aprepitant. For those participants who did not receive aprepitant, we observed no significant difference in severity of nausea or vomiting between either dose of ginger or placebo (Table 3). However, participants who were prescribed aprepitant and either dose of ginger had significantly more severe delayed nausea (Table 3).
Table 3.
Severity of acute and delayed nausea and vomiting
| Severitya | Mean±SD | P valueb (apripetant=no) |
P valueb (apripetant=yes) |
||
|---|---|---|---|---|---|
|
|
|||||
| Placebo group (n=46) |
Ginger 1.0 g group (n=43) |
Ginger 2.0 g group (n=40) |
|||
| Nausea | |||||
| Acute | |||||
| No apripetant | 2.8±1.3 (n=23) | 3.1±1.2 (n=21) | 3.0±1.1 (n=20) | 0.47 | 0.55 |
| Yes apripetant | 3.1±1.5 (n=8) | 2.8±1.1 (n=12) | 2.8±1.5 (n=10) | ||
| Delayed | |||||
| No apripetant | 3.0±1.3 (n=23) | 3.0±1.1 (n=25) | 3.2±1.1 (n=17) | 0.69 | 0.01 |
| Yes apripetant | 2.2±0.7 (n=9) | 2.9±1.3 (n=11) | 3.9±0.9 (n=9) | ||
| Vomiting | |||||
| Acute | |||||
| No apripetant | 3.6±1.4 (n=11) | 3.1±1.4 (n=14) | 2.9±0.9 (n=11) | 0.61 | 0.91 |
| Yes apripetant | 4.0±1.7 (n=3) | 3.4±0.6 (n=5) | 3.7±1.5 (n=6) | ||
| Delayed | |||||
| No apripetant | 4.0±1.3 (n=7) | 2.7±0.9 (n=12) | 3.7±1.0 (n=7) | 0.88 | 0.77 |
| Yes apripetant | 3.0±0.0 (n=1) | 3.0±1.4 (n=5) | 3.6±1.3 (n=5) | ||
Severity of nausea and vomiting graded on a six-point Likert scale graded as 1=very mild, 2=mild, 3=moderate, 4=severe, 5=very severe, and 6=intolerable
P values were calculated using Cochran Mantel–Haenszel tests stratified by apripetant (yes or no)
Participant and assessor blinding
Both patients and research personnel who were responsible for collecting study endpoints from patients (outcome assessors) were asked to evaluate which treatment (1.0 g, 2.0 g, or placebo) the patient received. Outcome assessors were not able to correctly identify which treatment the patient received (p=0.27). Patients, however, were significantly (p=0.01) more likely to correctly guess the treatment they were given. Patients indicated that it was the “the way the capsule worked” (16%placebo, 51%1.0 g, 33%2.0 g for each treatment group; p=0.12) as the most common reason for knowing which treatment they were taking. The taste of the capsule was the second most likely reason given for being able to know what treatment a participant received (9%placebo, 25%1.0 g, 33%2.0 g for each treatment group; p=0.01).
Adverse events
We divided adverse events into those that most commonly occurred in the trial, e.g., laboratory abnormalities events, and side effects most often associated with ginger consumption, e.g., GI events. There were no significant differences in total adverse events, non-serious adverse events, dyspnea, gastrointestinal events, or laboratory abnormalities between treatment groups, although laboratory abnormalities were close to being significantly higher in the placebo and 1.0-g dose (8placebo vs. 81.0-g dose vs. 12.0-g dose; p=0.06) compared to the 2.0-g dose. There were significantly more fatigue (5placebo vs. 11.0-g dose vs. 02.0-g dose; p=0.03) and miscellaneous adverse events (8placebo vs. 31.0-g dose vs. 12.0-g dose; p=0.02) in the placebo group compared to either ginger dose. Nearly all of the adverse events were non-serious and graded as either a 1 or a 2 on the NCI toxicity version 3. Out of the 42 patients who experienced an adverse event, only three were serious: One patient was hospitalized with an upper extremity deep vein thrombosis, another patient was hospitalized after experiencing severe diarrhea and abdominal pain, and another patient hospitalized for anemia, low platelets, and white blood cells. We found no significant difference in serious adverse events between treatment groups (p=0.07).
Discussion
We found no benefit of a ginger extract, in the doses and formulation used, on our primary end point, the prevalence of delayed nausea and vomiting, when added to contemporary standard antiemetic therapy in cancer patients receiving chemotherapy. These results are consistent whether evaluated in unadjusted analyses or in analyses that adjusted for presence or absence of acute nausea, or vomiting, emetogenicity of chemotherapeutic agent, and use of aprepitant. Likewise, ginger extract caused no decrease in the severity of delayed nausea or vomiting. The 2.0-g dose of ginger extract, however, did increase the severity of delayed nausea (p=0.03), although when stratifying by use of aprepitant, severity of delayed nausea was only increased when the 2.0-g dose of ginger was taken with aprepitant (p=0.01) and not when taken without aprepitant (p=0.69).
Similar to delayed CINV, we found that ginger extract did not decrease the prevalence of acute nausea or vomiting compared to placebo. Further, ginger extract at both doses did not affect the severity of acute nausea or vomiting. However, when ginger was taken with aprepitant, there was an increase in the prevalence of delayed vomiting, although this did not reach statistical significance (p=0.07). Otherwise, these results were consistent whether or not a participant had been prescribed aprepitant.
Ginger appeared to be well tolerated. There was no difference between placebo and ginger for all adverse events, for common AE categories including dypsnea and gastrointestinal complaints, or serious adverse events. Despite the lack of statistical significance difference in serious AEs, all of the serious AEs did occur in the low-dose ginger group and approached statistical significance (p=0.07). There were significantly fewer complaints of fatigue (p=0.03) and miscellaneous other adverse events (p=0.02) in the ginger treatment groups versus placebo, and there were borderline significantly fewer laboratory abnormalities in the high-dose ginger arm (p=0.06). The occurrence of all serious adverse events in the low-dose ginger group should be viewed with caution, but given the small number of serious events (three), this result could be due to chance alone. Similarly, the positive effects of ginger on fatigue could also be attributed to chance due to the small number of events. The wide variety of events in the miscellaneous other and laboratory AE groups appear unlikely to be explicable by a pharmacologic effect of ginger. However, possible pharmacological effects such as ginger root’s anti-inflammatory [14, 19, 20, 23] and antioxidant effects [1, 22, 34] could be responsible for some of the decreased AEs. As a consequence, future studies could be considered to examine the protective effect of ginger on fatigue and laboratory abnormalities experienced during chemotherapy.
Our results are in contrast to three other randomized controlled trials (RCTs) [28, 29, 33] examining the safety and efficacy of ginger root extracts or powder for acute CINV. Two of these studies [28, 29] are only available in abstract form, allowing for no more than limited comparisons with our study. Pace [28] found that ginger significantly decreased an acute nausea symptom score, and Pecoraro et al. [29] determined that participants who received ginger compared to placebo appeared to have a greater complete treatment response of acute CINV, although no statistical analysis is provided for the later study. The results of these studies could differ from ours for numerous reasons, including overestimation of treatment effects owing to the studies’ small sample sizes (41 and 12 participants, respectively), lack of blinding, use of different and non-validated outcome measures to assess the prevalence and severity of CINV, different doses and ginger formulations, and lack of examination of delayed CINV.
The third study that found results in contrast to ours was a crossover RCT comparing ginger to metoclopramide and ondansetron for controlling the incidence of CINV for 24 h after treatment. Ginger powder was found to be as successful as metoclopramide in complete control of CINV, but ondansetron was found to be superior to both of the other two treatments (complete control of nausea was 62% in ginger, 58% in metoclopramide, and 86% with ondansetron) [27]. Unlike our study, the 1.0-g dose of ginger powder was not co-administered with antiemetics, e.g., 5-HT3 receptor antagonists, but instead was given instead of standard antiemetic medications.
In contrast, another RCT crossover study in gynecologic oncology patients receiving cisplatin comparing 1.0 g ginger to placebo and metoclopramide (placebo during the first 24 h and metoclopramide during the next 4days) found results similar to ours [24].
There was evidence in this study that ginger, when co-administered with aprepitant, increased the severity of delayed nausea. In addition, while not statistically significant, participants prescribed aprepitant and ginger (either dose) had consistently higher prevalence of both acute and delayed nausea and delayed vomiting compared to those participants who received only aprepitant. It is possible that ginger root, similar to other of food and beverages, can alter the rate and extent of drug absorption. Ginger could decrease the absorption of aprepitant by increasing gastric emptying time and intestinal motility and thus decreasing aprepitant’s anti-nausea effects. Ginger extracts and their constituents can shorten food transit time [30], enhance gastrointestinal motility [36], and reverse pyrogallol-induced delay in gastric emptying in rats [15].
Our study had several limitations. First, we were limited by inadequate power to detect small effect sizes for secondary outcomes. Second, we lacked adequate sample sizes to detect differences in the primary and secondary outcomes by treatment with or without aprepitant. Third, we found that participants were able to determine if they were randomized to either of the ginger treatment arms, indicating that how the capsule tasted allowed them to determine which treatment they had received. Three meta-analyses of clinical trials found that when participants are not blinded, there tends to be a moderate overestimate of the effect of the new treatment [7, 9, 11]. Lack of blinding is of particular concern when the outcomes are subjective or “soft”, such as with severity of nausea where ascertainment bias can play a large role. Fourth, we also had a very heterogeneous patient sample. The variability in our patient sample was beneficial, making the results more generalizable to a wide variety of oncology patients. However, our sample heterogeneity also made it more difficult to detect any effects of ginger in any subset of cancer patients or chemotherapy treatment combinations.
In summary, the data from this study indicate that a ginger extract provides no clinical benefit, at the doses evaluated, when given in addition to standard evidence-based contemporary anti-nausea CINV medical therapy to patients receiving chemotherapy. Ginger extract may have a negative interaction when taken with aprepitant on severity of nausea. Ginger may also have positive benefits in decreasing fatigue and non-GI adverse events during chemotherapy.
Acknowledgments
This research was supported by NCI grant 1 KO7 CA102592-01, R21AT0001735 from the National Center for Complementary and Alternative Medicine (NCCAM), NCI CN-55124, and NCI U10CA74648 (CCOP Research Base). Research resources were also provided by the General Clinical Research Center of the University of Michigan (M01-RR00042). The ginger extract was generously donated by Pure Encapsulations® (Sudbury, MA).
Contributor Information
Suzanna M. Zick, Departments of Family Medicine, University of Michigan, Ann Arbor, MI, USA; University of Michigan Medical Center, 715 E. Huron, Suite 2E, Ann Arbor, MI 48104, USA
Mack T. Ruffin, Departments of Family Medicine, University of Michigan, Ann Arbor, MI, USA
Julia Lee, Biostatistics Unit of the Comprehensive Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Daniel P. Normolle, Biostatistics Unit of the Comprehensive Cancer Center, University of Michigan, Ann Arbor, MI, USA
Rivka Siden, Department of Pharmacy Services, University of Michigan, Ann Arbor, MI, USA.
Sara Alrawi, Departments of Family Medicine, University of Michigan, Ann Arbor, MI, USA.
Dean E. Brenner, Division of Hematology/Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA
References
- 1.Aeschbach R, Loliger J, Scott BC, et al. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol. 1994;32(1):31–36. doi: 10.1016/0278-6915(84)90033-4. doi:10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- 2.Baranowski JD. High-performance liquid-chromatographic separation of pungency components of ginger. J Chromatogr A. 1985;319(3):471–474. doi:10.1016/S0021-9673(01)90593-X. [Google Scholar]
- 3.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. doi: 10.1200/JCO.2006.05.6382. doi:10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 4.Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12(9):684–701. doi: 10.1016/j.phymed.2004.07.009. doi:10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Coates A, Abraham S, Kaye SB, et al. On the receiving end-patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19(2):203–208. doi: 10.1016/0277-5379(83)90418-2. doi:10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 6.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. doi: 10.1007/s00520-006-0173-z. doi:10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Miller JN, Mosteller F. How study design affects outcomes in comparisons of therapy. I: Medical. Stat Med. 1989;8(4):441–454. doi: 10.1002/sim.4780080408. doi:10.1002/sim.47800804088. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed on March 25th 2008]; http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 9.Devereaux PJ, Manns BJ, Ghali WA, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA. 2001;285(15):2000–2003. doi: 10.1001/jama.285.15.2000. doi:10.1001/jama.285.15.2000. [DOI] [PubMed] [Google Scholar]
- 10.de Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol. 2003;21(22):4105–4111. doi: 10.1200/JCO.2003.10.128. doi:10.1200/JCO.2003.10.128. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson D, Glass K, Waring D, Shapiro S. Turning a blind eye: the success of blinding reported in a random sample of randomized, placebo controlled trials. BMJ. 2004;328:7437. doi: 10.1136/bmj.37952.631667.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feyer P, Kleeberg UR, Steingraber M, Gunther W, Behrens M. Frequency of side effects in outpatient cancer care and their influence on patient satisfaction—a prospective survey using the PASQOC(R) questionnaire. Support Care Cancer. 2008;16:567–575. doi: 10.1007/s00520-008-0422-4. doi:10.1007/s00520-008-0422-4. [DOI] [PubMed] [Google Scholar]
- 13.Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100(10):2261–2268. doi: 10.1002/cncr.20230. doi:10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 14.Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–132. doi: 10.1089/jmf.2005.8.125. doi:10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 15.Gupta YK, Sharma M. Reversal of pyrogallol-induced delay in gastric emptying in rats by ginger (Zingiber officinale) Methods Find Exp Clin Pharmacol. 2001;23(9):501–503. doi: 10.1358/mf.2001.23.9.662137. doi:10.1358/mf.2001.23.9.662137. [DOI] [PubMed] [Google Scholar]
- 16.Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U. S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. doi:10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman D. The new holistic herbal. Element Books Limited; Shaftesbury, Dorset: 1983. [Google Scholar]
- 18.Huang QR, Iwamoto M, Aoki S, Tanaka N, Tajima K, Yamahara J, Takaishi Y, Yoshida M, Tomimatsu T, Tamai Y. Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull (Tokyo) 1991;39(2):397–399. doi: 10.1248/cpb.39.397. [DOI] [PubMed] [Google Scholar]
- 19.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66(13):1614–1635. doi: 10.1016/j.phytochem.2005.05.007. doi:10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry. 2004;65(13):1937–1954. doi: 10.1016/j.phytochem.2004.06.008. doi:10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24(18):2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 22.Krishnakantha TP, Lokesh BR. Scavenging of superoxide anions by spice principles. Indian J Biochem Biophys. 1993;30(2):133–134. [PubMed] [Google Scholar]
- 23.Lantz RC, Chen GJ, Sarihan M, Solyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14(2-3):123–128. doi: 10.1016/j.phymed.2006.03.003. doi:10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Manusirivithaya S, Sripramote M, Tangjitgamol S, et al. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int J Gynecol Cancer. 2004;14(6):1063–1069. doi: 10.1111/j.1048-891X.2004.14603.x. doi:10.1111/j.1048-891X.2004.14603.x. [DOI] [PubMed] [Google Scholar]
- 25.Mills S, Bone K. Principles and practice of phytotherapy. Churchill Livingstone; Oxford: 2000. [Google Scholar]
- 26.Morrow GR. A patient report measure for the quantification of chemotherapy induced nausea and emesis: psychometric properties of the Morrow assessment of nausea and emesis (MANE) Br J Cancer. 1992;19(Suppl):S72–S74. [PMC free article] [PubMed] [Google Scholar]
- 27.Ody P. The complete medicinal herbal. Dorling-Kindersley; New York: 1993. [Google Scholar]
- 28.Pace J. Oral ingestion of encapsulated ginger and reported self-care action for the relief of chemotherapy-associated N & E. Dissertations Abstracts International. 1987;47:3297-B. [Google Scholar]
- 29.Pecoraro A, Patel J, Guthrie T, Ndubisi B. Efficacy of ginger as an adjunctive anti-emetic in acute chemotherapy-induced nausea and vomiting; ASHP Mid-Year Clinical Meeting; 1998.p. 429E. [Google Scholar]
- 30.Platel K, Srinivasan K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung. 2000;44(1):42–46. doi: 10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. doi:10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Sharma SS, Gupta YK. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale) J Ethnopharmacol. 1998;62(1):49–55. doi: 10.1016/s0378-8741(98)00053-1. doi:10.1016/S0378-8741(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 32.Shibata C, Sasaki I, Naito H, Ueno T, Matsuno S. The herbal medicine Dai-Kenchu-Tou stimulates upper gut motility through cholinergic and 5-hydroxytryptamine 3 receptors in conscious dogs. Surgery. 1999;126(5):918–924. [PubMed] [Google Scholar]
- 33.Sontakke S, Thawani V, Naik MS. Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: a randomized, cross-over, double-blind study. Indian J Pharmacol. 2003;35:32–36. [Google Scholar]
- 34.Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res. 1998;402(1-2):259–267. doi: 10.1016/s0027-5107(97)00305-9. doi:10.1016/S0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- 35.Yamahara J, Huang QR, Iwamoto M, Kobayash G, Matsuda H, Fujimura H. Active components of ginger exhibiting anti-serotonergic action. Phytother Res. 1989;3:70–71. doi:10.1002/ptr.2650030208. [Google Scholar]
- 36.Yamahara J, Huang QR, Li YH, Xu L, Fujimura H. Gastrointestinal motility enhancing effect of ginger and its active constituents. Chem Pharm Bull (Tokyo) 1990;38(2):430–431. doi: 10.1248/cpb.38.430. [DOI] [PubMed] [Google Scholar]