Abstract
Enhanced airway smooth muscle (ASM) contraction is an important component in the pathophysiology of asthma. We have shown that ligand gated chloride channels modulate ASM contractile tone during the maintenance phase of an induced contraction, however the role of chloride flux in depolarization-induced contraction remains incompletely understood. To better understand the role of chloride flux under these conditions, muscle force (human ASM, guinea pig ASM), peripheral small airway luminal area (rat ASM) and airway smooth muscle plasma membrane electrical potentials (human cultured ASM) were measured. We found ex vivo guinea pig airway rings, human ASM strips and small peripheral airways in rat lungs slices relaxed in response to niflumic acid following depolarization-induced contraction induced by K+ channel blockade with tetraethylammonium chloride (TEA). In isolated human airway smooth muscle cells TEA induce depolarization as measured by a fluorescent indicator or whole cell patch clamp and this depolarization was reversed by niflumic acid. These findings demonstrate that ASM depolarization induced contraction is dependent on chloride channel activity. Targeting of chloride channels may be a novel approach to relax hypercontractile airway smooth muscle in bronchoconstrictive disorders.
Keywords: airway smooth muscle, chloride, depolarization, TEA, niflumic acid
Introduction
Asthma remains a global healthcare challenge with recent estimates citing an incidence of 8.2% of the US population in 2009 (1). Although β-agonists remain the mainstay of treatment for acute bronchoconstriction, desensitization to these drugs occurs and concerns remain as to whether the use of long acting β-agonists are associated with increased asthma related deaths (2). In fact, the recent FDA-mandated review of these drugs underscores the pressing need for new therapeutic approaches to treat this disease.
One such potential therapeutic strategy involves targeting ion channels that may influence airway smooth muscle force generation and/or maintenance of contractile tone. One particular class of ion channel that is a promising candidate for such targeting is the chloride channel. While a few seminal studies have established electrophysiologic evidence that chloride channels play an important role in plasma membrane currents (3, 4) the role of these channels in the maintenance of a depolarized-induced contraction in central and peripheral airways remains unknown. This is an important clinical consideration, since most rescue therapies (e.g. β-agonists) for the treatment of bronchospasm are typically administered after the airway smooth muscle has already contracted and are directed at relieving pre-existing bronchoconstriction.
Furthermore, when considering the relevance of chloride channels to the pathophysiology of acute bronchoconstriction two other critical questions remain. The first is the functional relevance of chloride modulation in small peripheral airways since these smaller caliber airways play an important clinical role in human bronchoconstrictive disease and all prior functional studies focusing on chloride flux have only been performed in large, upper airway segments (5). Given the well-established observation that receptor/channel expression can differ greatly between larger proximal airway and smaller peripheral airway segments (6) the effects of chloride modulation in proximal airway tissues may not be relevant to small airways. The second concern we sought to address in this study was the applicability of these findings to human physiology, since all prior studies did not include evidence of functional relevance in intact human airway smooth muscle.
As a result of these considerations, the current study addresses the hypotheses that chloride channels are critical to the maintenance phase of an airway smooth muscle contraction and that chloride channel blockade can relax depolarization-induced pre-contracted guinea pig and human airway smooth muscle. Furthermore, in addition to corroborating our findings in human cells and tissues, we hypothesized that the beneficial functional effects of chloride blockade are preserved in small peripheral airways.
Materials and Methods
Cell culture
Cultures of immortalized human airway smooth muscle cells were a kind gift from Dr. William Gerthoffer (Univ. of S. Alabama) and have previously been characterized (7). The cells were grown to confluence in 96-well black-walled clear bottom plates for fluorescent FLIPR membrane potential assays. For electrophysiology studies, cells were grown in 25 cm2 flasks that were pre-coated with collagen (rat tail collagen type 1, 5 µg/cm2). All cells were maintained in M199 media supplemented with 10% fetal bovine serum, 0.25 µg/ml epidermal growth factor, 1 µg/ml fibroblast growth factor, ITS supplement (1 mg/ml insulin, 0.55 mg/ml transferrin, 0.67 µg/ml sodium selenium) and antibiotics (100 un/ml penicillin G sodium, 100 µg/ml streptomycin sulfate, 0.25 µg/ml amphotericin B) at 37 °C in 5% CO2-95% air as described (7).
Membrane potential measurements using fluorescent potentiometric probe (FLIPR)
The membrane potential effects of tetraethylammonium chloride (TEA) and niflumic acid were assessed using the FLIPR membrane potential assay. Human airway smooth muscle cells were grown to 100% confluency in 96 well black-walled clear bottom plates. Protocols for dye loading and cell preparations were previously described (8). The cell fluorescence changes were assessed using a Flexstation 3 microplate reader (Molecular Devices, Carlsbad, CA) using settings previously described (9). Briefly the cells were washed and incubated with 50% blue FLIPR dye and placed in the Flexstation. 100 μM NS1619 (a control potassium channel opener causing hyperpolarization), 40 mM KCl (a control depolarizing agent), 100 μM niflumic acid or 10 mM TEA or buffer were added using the Flexstation's automated injector at single concentrations while fluorescence was continuously measured at a rate of 1 reading every 2 sec for a duration of 10 min and data were expressed as changes in relative fluorescent units (ΔRFU) of the FLIPR dye.
Membrane potential measurements using electrophysiologic whole cell recordings
Immortalized human airway smooth muscle cells were grown to confluence on collagen-treated T25 flasks. Collagenase type IV in basal SmBM2 medium was used to release adherent cells from the collagen matrix in the flask. Cells in media were then harvested in a 10-ml conical tube and centrifuged at 300 × g. Supernatant was removed, and the pellet was resuspended in additive-free SmBM2 media and transferred into collagen-treated glass bottom 1 cm Petri dishes at 10% confluence. Each dish was then incubated at 37 °C 5% CO2 for 1–4 h to allow for reattachment of cells to glass-bottom Petri dishes (serving as a disposable recording chamber). ALA VM-8, (ALA, Farmingdale, NY, USA) an 8-chamber pressure-driven drug application system, was used in a still bath of extracellular salt solution. Whole-cell intracellular voltage recordings under current clamp conditions were performed with a 2-kHz Bessel filter, recording at 10 kHz using an Axopatch 200b amplifier (Axon Instruments, Foster City, CA, USA). Intracellular solutions consisted of (in mM): 140 KCl, 5 MgATP, 5 EGTA, 1 MgCl2, 10 HEPES, and 5 CaCl2 (pH 7.2). Extracellular solution consisted of (in mM): 134 NaCl, 1.4 KCl, 10 HEPES, 1 MgCl2, 1.8 CaCl2, and 10 glucose (pH 7.4). Electrodes were pulled using a P-97 micropipette puller from 1.5-mm OD borosilicate capillary glass (Sutter Instruments, Novato, CA, USA). Glass electrode resistances ranged from 5 to 10 MΩ with intracellular solution. All recordings were analyzed on Clampfit 8.0 software (Molecular Devices).
Isolation of smooth muscle from human trachea and guinea pig trachea
All human airway tissue protocols were reviewed by the Columbia University Institutional Review Board and were deemed not human subjects research under 45 CFR 46. Human tracheal tissue was obtained from discarded airway tissue from healthy lung donors during transplantation surgery at Columbia University. All guinea pig protocols were approved by the Columbia University Institutional Animal Care and Use Committee. Guinea pigs were deeply anesthetized with 100 mg/kg i.p. pentobarbital. Excision and dissection protocols of tracheal tissue were previously described (8). Briefly both human and guinea pig airways were dissected removing both extra-luminal fibrous tissue and intraluminal epithelial tissue.
Functional studies of airway smooth muscle
Two contiguous, closed guinea pig tracheal ring segments (epithelium-denuded) were suspended at 1 g resting tension in oxygenated Kreb-Henseleit buffer at 37 °C as previously described (8). Kreb-Henseleit buffer consisted of 140 mM NaCl / 4.7 mM KCl / 2.5 mM CaCl2 / 1.2 mM MgCl2 / 11 mM HEPES / 10 mM d-glucose at a pH of 7.4. Human tracheal smooth muscle strips (epithelium denuded) containing cartilaginous ends with a strip of airway smooth muscle were suspended at 1.5 g resting tension in oxygenated Kreb-Henseleit buffer at 37 °C as previously described (8). Pre-treatments, pre-contraction protocols and the organ bath set up for human and guinea pig have been previously described (8). Briefly, both human and guinea pig airways were tied under tension to an FT03 transducer (Grass Telefactor, Warwick, RI, USA) while tension was continuously digitally recorded. Smooth muscle was contracted with 10 mM TEA and following the attainment of a stable contraction tissues were treated with either vehicle (DMSO 0.1%, ethanol 0.1%), niflumic acid (100 μM), or 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) (Tocris, Bristol, UK) (100 μM) at 45 or 60 min after TEA for guinea pig or human airway smooth muscle, respectively.
Measurement of airway luminal area in rat lung slices
The Texas Tech University Health Sciences Center Institutional Animal Care & Use Committee (IACUC) approved the rat animal studies. Rat lung slices were prepared following a modification of a protocol for the preparation of mouse lung slices (10). Briefly, Charles River Sprague-Dawley rats (180–320 g) were euthanized with i.p. sodium pentobarbital (Fatal Plus, 40 mg/kg) and transferred to a clean dissection board in a dedicated sterile environment. Each animal was rinsed with 90% ethanol, the chest cavity opened, and the trachea exposed and cannulated with an intravenous (IV) catheter tube. The lungs were inflated with a warm solution of 2% agarose (low-melting temperature agarose) (USB Corporation, Santa Clara, CA, USA) in sterile Hank's balanced salt solution (sHBSS) by slowly injecting approximately 5.0 ml of agarose solution per 100 g of body weight followed by 2.5 ml of air. The air injection was used to flush the agarose out of the airways and into the distal alveolar space. The agarose was solidified by cooling the lungs with a cotton ball soaked in ice-cold sHBSS and maintaining the rat body temperature at 4 °C for 20 min. The lungs and heart were removed from the animal and held in ice-cold sHBSS for 15 min. The left lung was separated and trimmed near the main bronchus and along the long axis to create a base and then cut in three pieces. Each piece was transferred to the specimen syringe tube of a tissue slicer (Compresstome™ VF-300, Precisionary Instruments, San Jose, CA, USA) with the lobe base sitting on the tube. The lung lobe was embedded first into ∼1 ml 2% agarose and then fully covered with 6% gelatin. After the agarose and gelatin solidified, the block was cut into serial sections of 150 µm starting at the peripheral edge of the lung lobe. The entire procedure was performed in a safety cabinet under sterile conditions. Lung slices were observed on an inverted phase-contrast microscope and checked for the presence of airways. The first few slices usually lacked well-defined airways and were discarded. The subsequent 15–20 slices containing small terminal airways were collected and stored briefly in sHBSS. The slices were then incubated in low-glucose Dulbecco's Modified Eagle Medium supplemented with 1X antibiotic solution containing L-glutamine, penicillin and streptomycin and 50 µg/ml of gentamicin at 37 °C and 10% CO2 in a cell culture incubator. Lung slices were maintained for 8 to 48 h; no significant changes in airway contractility in response to 0.5 µM acetylcholine (ACh) were detected during this period. For experiments we only used lung slices that contained airways with a lumen diameter of 200–300 µm, completely lined by active ciliated epithelial cells, and fully attached to the surrounding lung parenchyma.
Measurement of small airway contraction
Airway contraction and relaxation was accessed in rat lung slices by calculating the changes in the airway cross-sectional luminal area (lumen area hereafter) using phase-contrast video microscopy as previously described (10). Briefly, rat lung slices were mounted in a custom-made perfusion chamber and held in place with a small sheet of nylon mesh. The lung slice was perfused continuously with sHBSS or the indicated experimental solutions. The chamber was placed on the stage of an inverted phase-contrast microscope (Diaphot TMD, Nikon Melville, NY, USA) and lung slices were imaged with a 10× objective. Digital images were recorded to a hard drive in time-lapse (0.5 Hz) using a CCD camera and image acquisition software (Video Savant™, IO Industries London, Ontario, Canada). The cross-sectional area the airway lumen was calculated from each image using a custom-written script in Video Savant. The lumen area was normalized to the area before stimulation and the changes in lumen area were plotted versus time. All experiments were performed at room temperature.
Materials
SmBM2 cell culture media was purchased from Lonza (Walkersville, MD, USA). All other cell culture reagents were purchased from Invitrogen (Grand Island, NY, USA). The blue FLIPR reagent was purchased from Molecular Devices. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Statistical analysis
Fluorescent potentiometric studies (FLIPR assays), relaxation of contractile force in organ bath and measurement of changes in luminal area in rat peripheral lung slices were analyzed by ANOVA with Bonferroni correction. Statistical significance was established at P<0.05 and all values are expressed as means ± S.E.M.
Results
Membrane potential changes in human airway smooth muscle cells following niflumic acid and TEA
In order to confirm that 10 mM TEA (a non-selective potassium channel blocker) depolarizes airway smooth muscle and to compare the magnitude to the depolarization induced by KCl, a potentiometric fluorescent probe was used to measure the membrane potential during 10 mM TEA and 40 mM KCl treatments. Furthermore, to demonstrate that 100 μM niflumic acid hyperpolarizes airway smooth muscle its effect was compared to a positive control, 10 μM NS1619 (a K+ channel opener known to hyperpolarize the plasma membrane). 10 mM TEA and 40 mM KCl caused an increase in fluorescence change consistent with membrane depolarization (152 ± 16.6 and 195 ± 11.8 maximum ΔRFU, respectively) (P<0.001 compared to buffer control). 100 μM niflumic acid and NS1619 demonstrated directional congruency as hyperpolarizing agents with a decrease in fluorescence (–195 ± 22 and –341 ± 37 maximum ΔRFU (P<0.01 and P<0.001 compared to buffer control respectively). (n=4–12) (Fig. 1).
Fig. 1.

Plasma membrane potential in human airway smooth muscle. Left: Representative tracings of real-time FLIPR potentiometric dye fluorescence emissions after cultured human airway smooth muscle cells were treated with either buffer, 100 μM niflumic acid, 40 mM KCl, 10 mM TEA or 10 μM NS1619. These tracings display directionality of the fluorescence change in relation to depolarization (upward deflection: TEA, KCl) and hyperpolarization (downward deflection: niflumic acid, NS1619). Right: FLIPR potentiometric dye fluorescence emissions (RFU), measures at maximum change values, after cultured airway smooth muscle cells were treated with either buffer (control), 40 mM KCl, 10 μM NS1619, 100 μM niflumic acid or 10 mM TEA. * P<0.01 for niflumic (–195 ± 22 maximum ΔRFU) (n=11); ** P<0.001 for NS1619 (–341 ± 37.7 maximum ΔRFU), TEA (152 ± 16.6 maximum ΔRFU) or KCl (195 ± 20.5 maximum ΔRFU) compared to control (n=4).
Membrane potential changes of TEA and niflumic acid measured by single cell voltage recordings
To confirm the effects of TEA and niflumic acid on plasma membrane potential of smooth muscle cells using traditional electrophysiologic whole cell patch clamp techniques, whole cell current clamp recordings of individual airway smooth muscle cells were performed. The average resting membrane potential was –56.4 ± 6.5 mV (n=3) (Fig. 2, Table 1 ). Exposure to TEA (10 mM) depolarized the plasma membrane (Fig. 2, Table 1). Following a plasma membrane depolarization with TEA, the addition of 100 μM niflumic acid resulted in a complete reversal of the depolarization back to membrane potentials near baseline resting values (Fig. 2, Table 1).
Fig. 2.

Airway smooth muscle whole cell electrical recording of membrane potential. A representative tracing of voltage recordings in a cultured human airway smooth muscle cell under current clamp in a single whole cell recording. Resting membrane potential was –56.4 ± 6.5 mV (n=3). 10 mM TEA depolarized cultured human airway smooth muscle cells and 100 μM niflumic acid treatment after 10 mM TEA returned membrane potential to near baseline resting values (n=3).
Table 1. Membrane potentials of individual human airway smooth muscle cells.
| Treatment | Membrane potential (mV) | ||
|---|---|---|---|
| none | TEA | TEA + niflumic acid | |
| Cell 1 | -58.1 | -16.3 | -60.6 |
| Cell 2 | -45.7 | -34.0 | -39.8 |
| Cell 3 | -68.2 | -37.6 | -81.4 |
Inhibitors of chloride channels directly relax depolarization-induced contractions in guinea pig airway smooth muscle
To investigate the role of chloride channels in depolarization induced contractions, we initially contracted guinea pig tracheal rings in an organ bath using 10 mM TEA. TEA induces a reliable and consistent contraction in both human and guinea pig airway smooth muscle. To demonstrate that chloride channel inhibition can relax an established TEA mediated airway smooth muscle contraction, niflumic acid was added to the buffer in organ baths after the contracted muscle force of guinea pig tracheal rings had reached a stable plateau of increased force. To complement these studies, a second structurally distinct pharmacologic inhibitor of chloride channels, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), was also evaluated. Guinea pig tracheal rings treated with 100 μM niflumic acid or 100 μM NPPB demonstrated a significant decrease in muscle force measured at 30 min after their addition (36.1 ± 8.8% and 59.8 ± 5.8%, respectively), compared to the original TEA-induced force (P<0.001). Vehicle controls (0.1% ethanol for niflumic acid and 0.1% DMSO for NPPB) were without significant effect (108 ± 3.2% and 109 ± 5.1%, respectively) over the same time period (Fig. 3) (n=4–6).
Fig. 3.
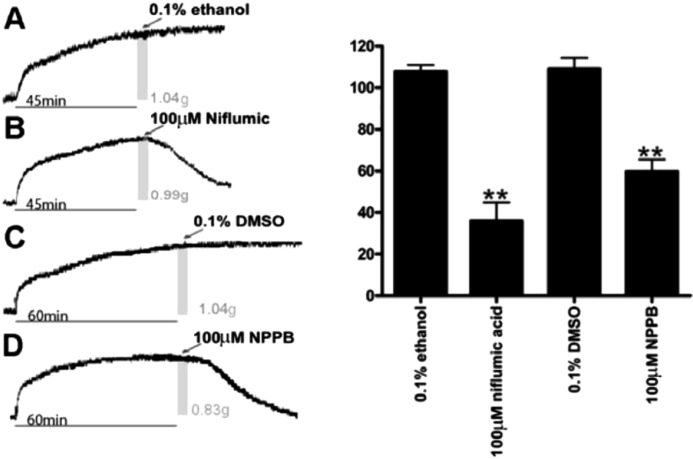
TEA induced contractions of guinea pig airway smooth muscle are relaxed by chloride channel blockers NPPB and niflumic acid. Left: Representative tracings of guinea pig tracheal rings contracted with 10 mM TEA with the addition of either vehicle controls (0.1% ethanol or 0.1% DMSO) or chloride channel antagonists. A and B, the gray bars represent the 45 min time point when treatments were applied. The associated muscle force at the time of treatment is also noted as 1.04 and 0.99 grams, respectively. C and D, the gray bars represent the 60 min time point when treatments were applied. The associated muscle force at the time of treatment is also noted as 1.04 and 0.88 grams respectively. Right: Muscle force expressed as a percent of the plateau TEA-induced contraction. Airway smooth muscle demonstrated a significant reduction in muscle force 30 mins after treatment with 100 μM niflumic acid (n=6) (36.1 ± 8.8% of the initial TEA-induced force) (**P< 0.001 compared to ethanol control). Airway smooth muscle demonstrated a significant reduction in muscle force 30 mins after treatment with 100 μM NPPB (n=6) (59.8 ± 5.8% of the initial TEA-induced force at 30 min) (**P< 0.001 compared to DMSO control). Thirty minutes after treatment with ethanol (n=6) or DMSO (n=4) vehicles the resulting muscle force was not significantly different from the initial TEA-induced force (108 ± 3.2% and 109 ± 5.1%, respectively).
An inhibitor of chloride channels directly relaxes depolarization-induced contractions in human airway smooth muscle
To demonstrate that chloride channel inhibition can relax a TEA mediated contraction in human airway smooth muscle, niflumic acid was added to the buffer in organ baths after human airway strips reached a plateau of muscle force induced by TEA. Niflumic acid decreased airway smooth muscle tension to 29% ± 9.7% of the initial TEA-induced force at 30 min (P<0.001 compared to 0.1% ethanol vehicle control). Ethanol vehicle treated airway smooth muscle strips did not demonstrate a significant change in muscle force over the same time period (Fig. 4) (n=6).
Fig. 4.
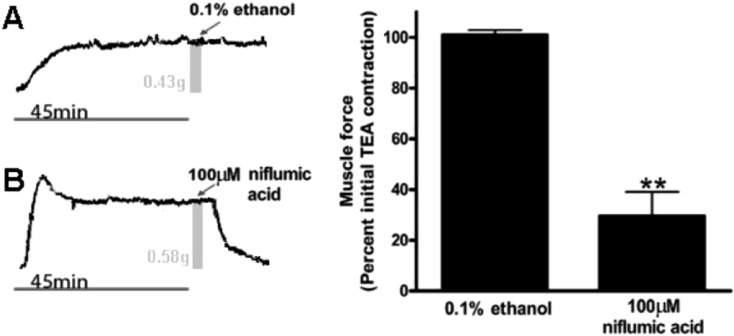
TEA induced contractions in human airway smooth muscle are relaxed by the chloride channel blocker niflumic acid. Left: Representative tracings of human tracheal strips muscle force with the addition of TEA and subsequently treated with either ethanol vehicle control or 100 μM niflumic acid. A and B, the gray bars represent the 45 min time point when treatments were applied. The associated muscle force at the time of treatment is also noted as 0.43 and 0.58 grams, respectively. Right: Muscle force expressed as a percent of plateau TEA-induced contraction. Human airway smooth muscle demonstrated a significant reduction in TEA-induced muscle force at 30 min after the addition of 100 μM niflumic acid (n=6) (29.6 ± 9.7% of the initial TEA-induced force) (** P<0.001 compared to ethanol control). Thirty minutes after treatment with ethanol vehicle (n=6) the resulting muscle force was not significantly different from the initial TEA-induced force (101 ± 1.9%).
An inhibitor of chloride channels directly relaxes depolarization-induced contractions in rat bronchioles
To demonstrate that TEA induced potassium channel blockade can induce a sustained contraction in smaller airways and to show that this mechanism is conserved between species, rat lung slices were exposed to TEA in a tissue chamber and airway luminal areas were quantified by continuous digital video. 10 mM TEA reduced luminal area comparable to the classical contractile agonists 50 mM KCl and 100 nM ACh (Fig. 5A, 5B). Additionally niflumic acid 100 μM added during the sustained contractile phase of TEA showed direct relaxation (Fig. 5C). Consistent with a contraction, 10 mM TEA treated bronchioles showed a 64.2% ± 7.9% reduction in luminal area. The magnitude of this contractile response was significantly attenuated by 100 μM niflumic acid, diminishing the reduction in luminal area to 7.9% ± 2.6% percent of its original baseline area (n=4, P<0.01) (Fig. 5C).
Fig. 5.

Rat lung slice luminal area measurements. A) Representative images of video recorded rat lung slices. Images (1)–(4) correlate to graphically represented luminal area tracing demarcated by arrows ()–(4) in panel B. Photograph (1) is a rat bronchiole without treatment. Photograph (2) is the same rat bronchiole treated with 100 nM ACh. Photograph (3) is the same rat bronchiole after a buffer wash and treated with 50 mM KCl. Photograph (4) is the same rat bronchiole after a buffer wash and treated with 10 mM TEA. B) Left: A representative tracing of bronchiolar luminal area extracted from video recorded images expressed as a percent of baseline luminal area (denoted by (1)). Right: Airway luminal area was measured as a percentage of baseline luminal areas at points (2), (3) and (4). (n=5 for all groups). Mean intraluminal area values of TEA was not significantly different between ACh and KCl groups (n=5, P>0.05). C) Left: A representative tracing of bronchiolar luminal area measurements showing niflumic acid (NFA) direct relaxation of TEA contraction. Right: Airway luminal area as a percentage of baseline luminal area at points before and after niflumic acid were significantly different (n=4, *P<0.01).
Digital video recordings of single bronchioles contracting and relaxing were obtained. The bronchiole within the lung slice was treated with contractile agonists; 100 nM ACh, 50 mM KCl and 10 mM TEA. Contractions were recorded and HBSS was used to washout the contractile agonist which correlates with smooth muscle relaxation between the contractile challenges (see supplemental video available at the Journal website).
Discussion
The major finding of this study is that functional relaxation of an established depolarization-induced contraction is achieved by chloride channel blockade. It should be noted that this is the first demonstration of this effect in intact human tissues taken from proximal tracheal and first generation bronchial ring preparations. While other investigators have shown that drugs targeting chloride channels are capable of modulating ionic currents, electrical slow waves and airway smooth muscle force generation (3,4,5), their studies have been limited to non-human models and primarily focused on the initiation phase of airway smooth muscle contraction since the drugs were administered before the contractile agonist. In this manuscript we have expanded our experimental model to examine the effect of chloride channel blockade on pre-contracted airway smooth muscle, since most therapeutic interventions used to treat acute bronchoconstriction rely on relaxing established tone (i.e. β agonists). Therefore, the current group of studies extends our knowledge about how chloride flux functions to modulate the maintenance phase of an airway smooth muscle contraction. Additionally, we demonstrate correlative changes by electrophysiology and potentiometric dyes that illustrate for the first time in human cells the ability of these chloride channel antagonists to reverse TEA-induced depolarization. Lastly, we validate that chloride channel blockade also achieves relaxation of smooth muscle in peripheral lung sections with real-time video of a bronchiolar TEA-induced contraction. This is an important distinction since diseases like asthma are thought to involve small airways and differential receptor expression is known to occur between proximal and distal airways (6).
While the relative importance of electromechanical coupling in airway smooth muscle has been controversial, recent evidence demonstrates that proteins critical to airway smooth muscle contraction are indeed influenced by membrane potential. At the plasma membrane, voltage changes have been shown to influence muscarinic, G-protein coupled receptors by increasing the binding affinity of ACh (11). Recently the mechanism of bitter taste receptor mediated airway smooth muscle relaxation has been attributed to blockade of voltage sensitive calcium channels (12). Intracellularly, membrane potential has been shown to induce agonist-independent M3 muscarinic receptor generation of IP3 in vascular smooth muscle (13). Rho kinase activity, a protein that regulates myosin light chain phosphorylation, is enhanced by plasma membrane depolarization, thereby linking membrane potential to increases in activated myosin (14, 15). Finally, at the organelle level, ryanodine- and IP3-receptor mediated calcium release has been shown to be augmented by airway smooth muscle depolarization (16). Thus, plasma membrane electrical potential and classic second messenger control of calcium may not operate in isolation.
As mentioned, a central observation in this study was the ability of chloride channel blockade afforded by niflumic acid to relax a TEA induced contraction. We chose TEA as a contractile agonist because it allowed us to compare its functional effects in the organ bath with known electrophysiologic effects. TEA also allows for the study of the effect of membrane depolarization on contractile tone in isolation, devoid from the complex second-messenger signaling events that accompany classic G protein coupled contractile pathways (i.e. ACh, histamine, substance P, leukotrienes). TEA (a non-selective potassium channel blocker) was specifically chosen over KCl as an effective agonist of airway smooth muscle contraction. One rationale for using TEA is that KCl creates a high potassium gradient to suppress potassium flux, which essentially never occurs in normal or pathologic physiology. In contrast, TEA mimics a more physiologic depolarization via blockade of endogenous potassium channels. Potassium channel inhibition and modulation occurs in physiologic and pathologic conditions. One example is the phosphorylation of the potassium channel by G protein coupled receptors, which has been described as a function of the M2 receptor on airway smooth muscle (17). Secondly, mutations associated with certain types of asthma occur in certain potassium channel families that have been described as inhibitory towards normal potassium channel function (18). If TEA induced contractions act as a model for these naturally occurring phenomena then chloride flux may have an influential role on the contractile response in normal and possibly diseased tissues. Furthermore, TEA is important because of its effects on membrane potential that has been associated with changes in baseline tone and spontaneous force oscillation (3). Previous studies demonstrated that TEA-induced increases in spontaneous electrical slow wave in airway smooth muscle were abolished by chloride channel blockade with niflumic acid (3). These findings agree with our current tension studies indicating that niflumic acid inhibits both spontaneous slow waves and myogenic tone, and that chloride flux plays a key role in electromechanical coupling.
Additionally, our present study shows that TEA increases membrane potential of a single cell by approximately 30 mV which agrees with previous studies (19). Moreover, we extend the electrophysiologic characterization of human airway smooth muscle cells by demonstrating that niflumic acid induces a relative hyperpolarization following this TEA-induced depolarization. While this phenomenon was not observed in every cell studied, it was observed in the majority of cells studied (60%). This raises the question as to whether there are differential electrophysiologic characteristics between individual smooth muscle cells within the airway. It should be noted that electrophysiologic differences among a population of airway smooth muscle cells has been described before and may allude to a electrophysiologic heterogeneity in airway smooth muscle cells that has not yet been fully appreciated.
While airway smooth muscle is classically thought to be a uniform syncytium, evidence does exist that supports the possibility that individual muscle cells display distinct electrophysiologic properties. Janssen and Simms demonstrated in the same study that ACh elicited transient inward current oscillations in one group of cells, while in other cells ACh elicited a single large slowly rectifying inward current, suggesting different channel activity or expression in these different cells (20). Furthermore, in another study of primary disassociated airway smooth muscle cells, some cells demonstrated spontaneous transient inward and outward currents while other cells demonstrated only spontaneous transient outward currents while others were electrophysiologically quiescent at resting membrane potential (3, 19). Furthermore, in vivo spontaneous electrical and peristaltic contractile activity of developing airway smooth muscle is critical to lung bud development. The origin of this spontaneous electrical signal appears to be in sub-specialized cells of airway smooth muscle beds (21).
The current study demonstrates the ability of niflumic acid to relax the maintenance phase of a depolarization-induced contraction in large and small airways of multiple species, including human. Although airway hyperresponsiveness is complex, and does not depend solely on membrane depolarization, improving our understanding of the role of membrane potential and chloride flux in airway smooth muscle contraction may expand our therapeutic options for hypercontractile airway smooth muscle.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Material
Digital video recording of single bronchiole constriction. A representative digital video recording of a single bronchiole during luminal airway measurements. Treatments shown in figure 5B (ACh 100 nM, KCl 50 mM and TEA 10 mM) were exposed to a single bronchiole. The luminal areas were decreased, representing contraction with each treatment, confirming a contractile response to all three agonists. Note, buffer washes in between each contractile treatment, and that luminal areas return to baseline size during buffer washes.
Acknowledgments
This study was supported by the Grants GM065281 for CWE, GM008464 for PDY and GM093137 for GG from National Institutes of Health.
References
- 1.Sondik EJ, Madans JH, Gentleman JF. Summary Health Statistics for U.S. Adults: National Health Interview Survey 2011, U.S. Department of Health and Human Services. Vital and Health Statistics ed. Center for Disease Prevention and Control, 2012: 1–218. [PubMed] [Google Scholar]
- 2.Wijesinghe M, Weatherall M, Perrin K, Harwood M, Beasley R. Risk of mortality associated with formoterol: a systematic review and meta-analysis. Eur Respir J. 2009; 34(4): 803–11. doi: 10.1183/09031936.00159708 [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007; 30(1): 114–33. doi: 10.1183/09031936.00147706 [DOI] [PubMed] [Google Scholar]
- 4.Janssen LJ, Sims SM. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflugers Arch. 1994; 427(5-6): 473–80. doi: 10.1007/BF00374263 [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Trimble N, Pertens E, Janssen LJ. Intracellular Cl- fluxes play a novel role in Ca2+ handling in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006; 290(6): L1146–53. doi: 10.1152/ajplung.00393.2005 [DOI] [PubMed] [Google Scholar]
- 6.Yamakage M, Chen X, Tsujiguchi N, Kamada Y, Namiki A. Different inhibitory effects of volatile anesthetics on T- and L-type voltage-dependent Ca2+ channels in porcine tracheal and bronchial smooth muscles. Anesthesiology. 2001; 94(4): 683–93. doi: 10.1097/00000542-200104000-00024 [DOI] [PubMed] [Google Scholar]
- 7.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006; 291(3): L523–34. doi: 10.1152/ajplung.00013.2006 [DOI] [PubMed] [Google Scholar]
- 8.Yim PD, Gallos G, Xu D, Zhang Y, Emala CW. Novel expression of a functional glycine receptor chloride channel that attenuates contraction in airway smooth muscle. FASEB J. 2011; 25(5): 1706–17. doi: 10.1096/fj.10-170530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen AA. Functional characterisation of human glycine receptors in a fluorescence-based high throughput screening assay. Eur J Pharmacol. 2005; 521(1-3): 39–42. doi: 10.1016/j.ejphar.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee S, Trice J, Shinde P, Willis RE, Pressley TA, Perez-Zoghbi JF. Ca2+ oscillations, Ca2+ sensitization, and contraction activated by protein kinase C in small airway smooth muscle. J Gen Physiol. 2013; 141(2): 165–78. doi: 10.1085/jgp.201210876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003; 278(25): 22482–91. doi: 10.1074/jbc.M301146200 [DOI] [PubMed] [Google Scholar]
- 12.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013; 11(3): e1001501. doi: 10.1371/journal.pbio.1001501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ureña J, del Valle-Rodríguez A, López-Barneo J. Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle. Cell Calcium. 2007; 42(4-5): 513–20. doi: 10.1016/j.ceca.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 14.Janssen LJ, Tazzeo T, Zuo J, Pertens E, Keshavjee S. KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004; 287(4): L852–8. doi: 10.1152/ajplung.00130.2004 [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Zuo J, Pertens E, Helli PB, Janssen LJ. Regulation of Rho/ROCK signaling in airway smooth muscle by membrane potential and (Ca2+)i. Am J Physiol Lung Cell Mol Physiol. 2005; 289(4): L574–82. doi: 10.1152/ajplung.00134.2005 [DOI] [PubMed] [Google Scholar]
- 16.Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci USA. 2009; 106(27): 11418–23. doi: 10.1073/pnas.0813307106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J Physiol. 2011; 589(Pt 7): 1803–17. doi: 10.1113/jphysiol.2010.204347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao K, Niu T, Xu X, Fang Z, Xu X. Single-nucleotide polymorphisms of the KCNS3 gene are significantly associated with airway hyperresponsiveness. Hum Genet. 2005; 116(5): 378–83. doi: 10.1007/s00439-005-1256-5 [DOI] [PubMed] [Google Scholar]
- 19.Snetkov VA, Hirst SJ, Twort CH, Ward JP. Potassium currents in human freshly isolated bronchial smooth muscle cells. Br J Pharmacol. 1995; 115(6): 1117–25. doi: 10.1111/j.1476-5381.1995.tb15926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen LJ, Sims SM. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992; 453: 197–218. doi: 10.1113/jphysiol.1992.sp019224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD, Burdyga TV. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am J Respir Cell Mol Biol. 2005; 33(2): 153–60. doi: 10.1165/rcmb.2005-0137OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Digital video recording of single bronchiole constriction. A representative digital video recording of a single bronchiole during luminal airway measurements. Treatments shown in figure 5B (ACh 100 nM, KCl 50 mM and TEA 10 mM) were exposed to a single bronchiole. The luminal areas were decreased, representing contraction with each treatment, confirming a contractile response to all three agonists. Note, buffer washes in between each contractile treatment, and that luminal areas return to baseline size during buffer washes.


