Abstract
Endothelial dysfunction has been associated with the development of atherosclerosis and cardiovascular diseases. Adult endothelial progenitor cells (EPCs) are derived from hematopoietic stem cells and are capable of forming new blood vessels through a process of vasculogenesis. There are studies which report correlations between circulating EPCs and cardiovascular risk factors. There are also studies on how pharmacotherapies may influence levels of circulating EPCs. In this review, we discuss the potential role of endothelial progenitor cells as both diagnostic and prognostic biomarkers. In addition, we look at the interaction between cardiovascular pharmacotherapies and endothelial progenitor cells. We also discuss how EPCs can be used directly and indirectly as a therapeutic agent. Finally, we evaluate the challenges facing EPC research and how these may be overcome.
Keywords: Endothelial progenitor cells, Cardiovascular diseases, Hypertension, Diabetes, Dyslipidemia, Therapy, Stents
Core tip: Our review summarizes the important associations between endothelial progenitor cells, cardiovascular risks, drugs and diseases. Current pharmacotherapies may enhance endothelial progenitor cell numbers and function. These and the evolving endothelial progenitor cell-based therapies may be important in the future treatment of cardiovascular diseases.
INTRODUCTION
Cardiovascular diseases (CVD) are the leading cause of mortality in both developed and developing countries[1]. Angiogenesis, the formation of new blood vessels, has attracted interest in the field of cardiology[2]. It was believed that angiogenesis could only occur by the new blood vessels sprouting out of pre-existing vessels. Under physiological conditions, vascular endothelium secretes substances that alter vascular tone and “defend” the vessel wall from inflammatory cell infiltration, thrombus formation and vascular smooth muscle cell proliferation[3]. Indeed, endothelial damage has been implicated in atherosclerosis, thrombosis and hypertension. The balance between endothelial injury and recovery is important for reducing cardiovascular events[4]. However, mature endothelial cells possess limited regenerative capacity. There is growing interest in circulating endothelial progenitor cells (EPCs) as they may maintain endothelial integrity, function and postnatal neovascularization[4].
EPC
Differentiation of mesodermal cells to angioblasts and subsequent endothelial differentiation was thought to exclusively happen in embryonic development. This concept was overturned in 1997 when Asahara et al[5] published that purified CD34-positive hematopoietic progenitor cells from adults can differentiate ex vivo to an endothelial phenotype. These EPCs showed expression of various endothelial markers and are incorporated into neovessels at sites of ischemia.
EPCs appear to be a heterogeneous group of cells originating from multiple precursors within the bone marrow and present in different stages of endothelial differentiation in peripheral blood. For this reason, the precise characterization of EPCs is difficult because many of the cell surface markers used in phenotyping are shared by hematopoietic stem cells and by adult endothelial cells[6].
Currently, EPCs are defined as cells positive for both a hematopoietic stem cell marker such as CD34 and an endothelial marker protein such as VEGFR2. CD34 is not exclusively expressed on hematopoietic stem cells but also on mature endothelial cells. Other studies have used the more immature hematopoietic stem cell marker CD133 and demonstrated that purified CD133-positive cells can differentiate to endothelial cells in vitro[7]. CD133, also known as prominin or AC133, is a highly conserved antigen with unknown biological activity which is expressed on hematopoietic stem cells but is absent on mature endothelial cells and monocytic cells[7]. Thus, CD133+VEGFR2+ cells more likely reflect immature progenitor cells, whereas CD34+VEGFR2+ may represent shed cells of the vessel wall[8]. Controversy remains with respect to the identification and the origin of endothelial progenitor cells which are isolated from peripheral blood mononuclear cells by cultivation in medium favoring endothelial differentiation.
TYPES OF EPC
Although the markers for identification of EPC populations vary between studies, it has been agreed that there are lineage and functional heterogeneities within the EPC population. There are at least two different types of EPCs: the early and late EPCs. Early EPCs are usually referred to as the angiogenic EPC population obtained from short-term cultures of 4-7 d in vitro. These early EPCs form colony forming units (CFU) and possess many endothelial characteristics, such as harboring markers of CD31, TIE2 and VEGFR2[5]. Hill et al[9] reported a negative correlation between EPCs, measured by CFU and Framingham risk score in 45 men with various cardiovascular risks. They also reported a positive correlation between CFU and brachial flow-mediated dilation, a measure of endothelial function. Late EPCs, often called out-growth EPCs, have different growth patterns and are usually obtained from long term cultures of at least 2-3 wk in vitro. Outgrowth EPCs possess additional endothelial characteristics, such as VE-cadherin and von Willebrand factor, in addition to CD31, CD133, CD34 and VEGFR2[4]. These outgrowth EPCs will further differentiate into mature endothelial cells for angiogenesis and vasculogenesis. These two types of cells have distinct morphology: the early EPCs have a spindle shape (Figure 1) while outgrowth EPCs have a cobblestone-like shape (Figure 2).
Figure 1.
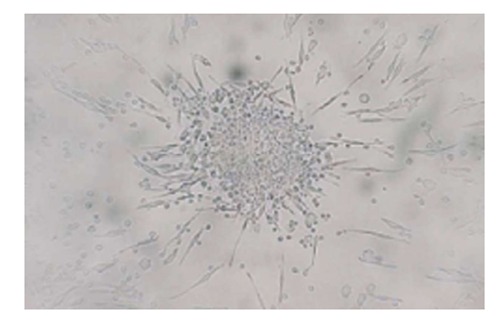
Colony forming unit isolated from human peripheral blood mononuclear cells using commercial colony forming unit-hill assay.
Figure 2.
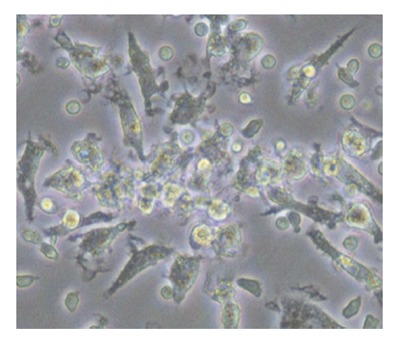
Cobble-shaped outgrowth endothelial progenitor cells from human peripheral blood at day 14.
Although endothelial dysfunction is associated with the development of atherosclerosis[10], the utility of EPCs as a prognostic marker has only recently been demonstrated. In a study with 44 patients with coronary artery disease (CAD) and 33 patients with acute coronary syndrome followed up for a median of 10 mo duration, a reduced number of EPCs was associated with a significantly higher incidence of cardiovascular events[11]. In another larger study with 519 patients with stable CAD, increased levels of endothelial progenitor cells were related to a reduced risk of death from cardiovascular causes, a first major cardiovascular event, revascularization and hospitalization[12].
However, issues in terms of isolation and identification of EPCs, especially in regards to the characterization or specific cell surface markers of these cells, are still unresolved. In addition, number and/or functionality of EPCs do not adequately describe cardiovascular disease risks. These limitations may be attributable to the inconsistent definitions of EPCs, the number of existing cardiovascular risk factors in different patient populations and the interaction between EPCs and other hematopoietic progenitor, inflammatory cells or platelets. There is also evidence of varied levels of circulating EPCs that are present in a time dependent manner[13]. Therefore, depending on when sampling occurs, EPC numbers and functions may be different.
Peripheral arterial disease
Peripheral arterial disease (PAD) is a manifestation of advanced atherosclerosis and affects 20% of the population aged over 65 years. PAD is associated with endothelial dysfunction but there have been limited and inconsistent data available on the number and functional capacity of EPCs in PAD. Fadini et al[14] first demonstrated that the number of EPCs marked by CD34+/CD133+/KDR+ is significantly decreased in diabetic patients with PAD compared to diabetics alone. This finding was further supported by another paper from Fadini where they reported significantly lower CD34+/KDR+ EPCs in PAD patients compared to healthy controls[15]. On the other hand, several studies have documented an increased number and functionality of EPCs in PAD patients compared to controls[15] (Table 1). Both studies reported poor angiogenic response to ischemia and EPC differentiation in PAD patients, together with reduced angiogenesis and low EPC levels[14,15]. In PAD, EPC mobilization can occur through inflammation and matrix metalloproteinase-mediated mechanisms[16]. Membrane type 1 matrix metalloproteinase (MT1-MMP) can contribute to vascular remodeling and regulate mobilization of CD34+ progenitor cells, while pentraxin-3 is predominantly produced by vascular endothelium and is considered to reflect inflammatory status of endothelium[16]. There appeared to be an increased number of EPCs and pentraxin-3 and decreased MT1-MMP in PAD patients compared to healthy controls[16]. Furthermore, cardiovascular events were also significantly correlated with decreased EPC levels and increased oxidative stress. In contrast, the number of EPCs was shown to be significantly higher in severe PAD patients compared to healthy subjects[17]. These contrasting results may be present due to the different severity of PAD patients recruited in the study and methodological differences in measuring EPC population which can complicate the interpretation of data. It is also possible that when PAD is only mild, EPC levels correlate to the poor vascular health. However, in severe PAD an elevated number of circulating EPCs may reflect mobilization from the bone marrow to repair endothelial damage. More studies are warranted to investigate these discrepancies in EPC and PAD.
Table 1.
Effect of peripheral arterial disease on endothelial progenitor cells
| Ref. | Subjects | EPCs (number/function) | Findings |
| Fadini et al[14] | 55 diabetic without PAD 72 diabetic with PAD | CD34+/CD133+/KDR+ | CD34+/CD133+/KDR+ is significantly lower in diabetics with PAD compared to diabetics alone |
| Fadini et al[15] | 15 healthy controls 30 PAD | CD34+/KDR+ | CD34+/KDR+ is significantly lower in patients with PAD than controls |
| Delva et al[17] | 24 healthy controls 45 PAD | CFU CD133+, CD34+, CD34+/KDR+ | CFU is significantly increased in patients with PAD compared to controls CD34+ and CD133+ are significantly decreased in patients with PAD compared to controls No difference between groups for CD34+/KDR+ |
| Morishita et al[16] | 22 healthy controls 48 PAD | CD34+/CD133+/ KDR+ | CD34+/CD133+/KDR+ is significantly higher in PAD compared to controls |
EPC: Endothelial progenitor cells; PAD: Peripheral arterial disease; CFU: Colony forming units.
CAD
The presence and extent of endothelial dysfunction predicts the outcome in patients with cardiovascular risk factors and in patients with coronary artery disease. Since endothelial progenitor cells possess the ability to home in on sites of vascular injury, there is emerging interest in the therapeutic use of EPCs related to angiogenesis. In patients with CAD, isolated EPCs had an impaired migratory response and a negative correlation of EPCs with the severity of CAD[18]. This was likely a result of endothelial dysfunction in patients with CAD[18], impaired coronary blood flow regulation and the strong association with risk factors for CAD. These risk factors may interfere with signaling pathways regulating EPC mobilization and differentiation, such as those involving granulocyte-stimulating colony stimulating factor (GS-CSF) or vascular endothelial growth factor (VEGF). Impaired migratory response affected by downregulation of VEGF may be contributed to by VEGFR2. In addition, several studies documented a decreased number of EPCs in CAD patients[19-22]. Circulating EPCs are also significantly lower in patients with progression of CAD angiographically[22]. Exhaustion of endothelial progenitor cells in the bone marrow, reduced nitric oxide bioavailability and long term statin treatment in CAD can also contribute to the reduced number and impairment of EPCs[21]. However, there are contrasting studies that reported an increased number of EPCs in angiographically significant CAD patients. A significant correlation was observed between the maximum stenosis severity and the number of EPCs from these patients[23]. Werner et al[24] also observed an inverse association between the level of circulating EPCs and the risk of cardiovascular events among patients with angiographically documented CAD. The differences in the methodologies are likely to account for the different results. In addition, low frequency of EPCs in circulation and types of EPCs harvested may also contribute to the differences. Moreover, the EPC population may represent a heterogeneous population of endothelial progenitors with differing proliferative capacity. Despite these controversies, the circulating numbers of EPCs appears to predict cardiovascular outcome in patients with CAD[24] (Table 2).
Table 2.
Effect of coronary artery disease on endothelial progenitor cells
| Ref. | Subjects | EPCs (number/function) | Findings |
| Vasa et al[18] | 9 healthy controls | CD34+/KDR+ (flow cytometry) | Both CD34+/KDR+ and migratory activity were impaired in patients with CAD compared to controls |
| 45 CAD | Migratory activity | ||
| Eizawa et al[19] | 36 healthy controls | CD34+ (flow cytometry) | CD34+ is significantly decreased in patients with stable CAD |
| 34 stable CAD | |||
| Wang et al[20] | 44 controls | CD34+/KDR+ (flow cytometry) | CD34+/KDR+ is the lowest in severe CAD followed by mild CAD Migratory activity is also impaired in CAD patients |
| 35 mild CAD | Migratory activity | ||
| 25 severe CAD | |||
| Liguori et al[21] | 15 healthy controls | CFU | CFU, CD34+ and migratory capacity were significantly impaired in patients with CHD CHD is the main predictor which impairs CFU capacity |
| 40 CHD | CD34+ (flow cytometry) | ||
| Migratory activity | |||
| Briguori et al[22] | 136 CAD | CFU | Low levels of CFU and CD34+/KDR+ predict CAD progression |
| CD34+/KDR+ (flow cytometry) | |||
| Güven et al[23] | 24 controls | CD34+ (flow cytometry) | CD34+ EPC is significantly elevated in CAD patients compared to controls |
| 24 CAD | EPCs is also positively correlated with maximum stenosis | ||
| Werner et al[24] | 90 CHD | CFU | CD34+/KDR+ and CFU positively correlate with endothelium-dependent vasodilation (acetylcholine infusion) |
| CD34+/KDR+ (flow cytometry) |
CAD: Coronary artery disease; CFU: Colony forming unit; CHD: Coronary heart disease; EPC: Endothelial progenitor cells.
Congestive heart failure
It has been shown that endothelial dysfunction occurs in patients with congestive heart failure (CHF)[25-27]. Despite these observations, limited data are available regarding the pattern of mobilization of EPCs and CD34+ cells during HF. In a study of EPC in HF, HF was associated with higher circulating EPC levels compared to healthy controls[28]. However, the severity of heart failure correlates with circulating EPCs inversely with significantly higher CD34+ counts in mild HF compared to severe HF[29]. CHF may result in hematopoietic progenitor cells migrating to the sites of damage to undergo progenitor cell differentiation. However, a depletion of progenitor cells in the chronic stage of the disease could contribute to the biphasic bone marrow pattern of response to heart failure[29]. Consistent with numbers, the colony forming unit, one of the functional capacities of EPCs, is an independent predictor for outcomes in CHF and is also negatively correlated with New York Heart Association functional class[30]. Pertinent studies are summarized in Table 3.
Table 3.
Summary of clinical trials: Effect of heart failure on endothelial progenitor cells
| Ref. | Subjects | EPCs (number/function) | Findings |
| Valgimigli et al[28] | 45 healthy controls 91 CHF | CD34+, CD34+/CD133+/KDR+ (flow cytometry) | CD34+ and CD34+/CD133+/KDR+ are significantly elevated in CHF patients compared to controls EPC number is negatively correlated with NYHA functional class |
| Nonaka-Sarukawa et al[29] | 22 healthy controls 16 mild CHF 10 severe CHF | CD34+ (flow cytometry) | CD34+ is significantly higher in mild CHF compared to severe CHF |
| Michowitz et al[30] | 107 CHF | CFU | CFU is the independent predictor for CHF CFU is also negatively correlated with NYHA functional class |
EPC: Endothelial progenitor cells; CHF: Congestive heart failure; CFU: Colony forming unit; NYHA: New York Heart Association.
EFFECTS OF CARDIOVASCULAR-RELATED PHARMACOTHERAPIES ON EPC
The presence of conventional cardiovascular risk factors, such as hypertension, dyslipidemia, diabetes and cardiovascular diseases, are associated with endothelial injury and dysfunction. Experimental and clinical studies evaluate endothelial dysfunction as alterations of vasomotor function, such as endothelium-dependent relaxations[31,32]. Recent research on cell biology has identified circulating EPCs as a useful biomarker of endothelial function and integrity. Cardiovascular pharmacotherapies (Figure 3) have been shown to improve overall numbers and function of EPCs in patients with cardiovascular risks in clinical studies.
Figure 3.
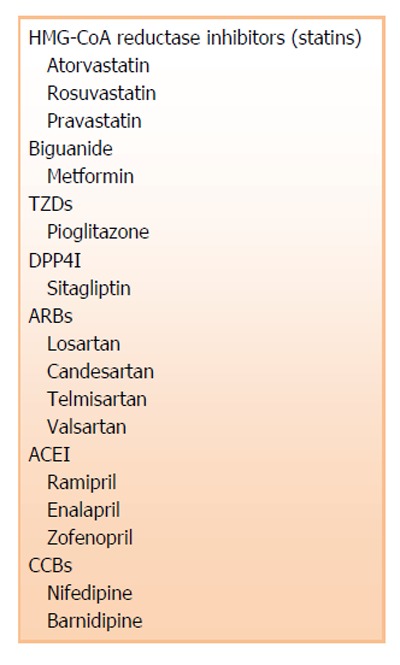
Cardiovascular-related pharmacological therapies which may affect numbers and function of endothelial progenitor cells. TZDs: Thiazolidinedione; DPP4I: Dipeptidyl peptidase 4 inhibitors; ARBs: Angiotensin II receptor blockers; ACEI: Angiotensin converting enzyme inhibitors; CCBs: Calcium channel blockers.
Antihypertensive medication
There are many classes of antihypertensives which lower blood pressure by different mechanisms. Among the most widely used are angiotensin II receptor blockers (ARBs), angiotensin converting enzyme (ACE) inhibitors and calcium channel blockers (CCBs). They all have been shown to modulate EPC number and/or functions.
ARBs: Their main mechanism of action is to act on the renin-angiotensin-aldosterone system for treatment of hypertension. Several studies have explored the effect of ARBs in influencing the number and/or function of EPCs in both experimental and clinical hypertension. Three experimental studies using spontaneous hypertensive rats successfully demonstrated improved EPC numbers and function with ARB treatment[33-35]. In hypertension, endothelial damage can be caused by reactive oxygen species (ROS) secondary to the increased production of tissue angiotensin II. Since vascular NAD(P)H oxidase is a major source of ROS in the cardiovascular system, ARBs can significantly inhibit major components of NAD(P)H oxidase. Inhibition of oxidative stress in hypertension by ARBs correlated with improvement in EPC numbers and function[33-35]. These findings were separately validated in the clinical setting where a similar improvement in EPC numbers and function were observed in healthy subjects and those with CAD[36,37] (Table 4). EPCs cultivated from healthy volunteers treated with telmisartan had a significantly higher number and improved function of EPCs compared to cells not treated with telmisartan[36]. However, the increase of numbers and function of EPCs was inhibited by specific peroxisome proliferator-activated receptor-gamma (PPAR-γ) inhibitor, GW9662. This suggests that telmisartan-induced EPC proliferation is likely via the PPAR-γ-dependent pathway. Furthermore, it has also been shown that telmisartan is a ligand of PPAR-γ[38]. In a double-blinded study, CAD patients with no history of hypertension receiving 80 mg of telmisartan for 4 wk had a significantly higher absolute number of EPCs compared to the placebo group. This was further supported by improvement in endothelial function in the treatment group[37]. The improvement on EPCs in these patients with CAD was independent from the antihypertensive action of telmisartan as reduction in blood pressure was not statistically different between the groups. Therefore, ARBs may be able to induce improvement in numbers and function of EPCs via pleiotropic effects. The several mechanisms include inhibition of NAD(P)H oxidases and stimulation through the PPAR-γ pathway.
Table 4.
Effect of antihypertensive medications on endothelial progenitor cells
| Ref. | Subjects | Drugs | Duration | EPCs (number/function) | Findings |
| Angiotensin II receptor blockers | |||||
| Yao et al[33] | 42 SHR-SP rats | Losartan (10 mg/kg per day) vs Placebo | 2 wk | CFU CD34+ (flow cytometry) Migratory activity | Losartan improved EPC number and function from SHR-SP rats compared to WKY rats |
| Yu et al[34] | 18 SHR-SP rats | Candesartan (1 mg/kg per day) vs Tempol, Trichlormethiazide | 2 wk | CFU | The highest CFU count was observed in candesartan treatment group |
| Yoshida et al[35] | 12 SHR-SP rats | Valsartan (300 mg/L) vs Hydralazine | 2 wk | CFU Migratory activity | Treatment with valsartan stimulated increase in CFU and migration activity in SHR-SP rats compared to hydralazine-treated rats |
| Honda et al[36] | 15 healthy controls | Telmisartan (1 μmol/L) vs Valsartan | 4 d | CFU Proliferation activity | CFU and EPC proliferative activity are significantly increased in cells treated with telmisartan in vitro |
| Pelliccia et al[37] | 40 CAD | Telmisartan (80 mg/d) vs Placebo | 4 wk | CD34+/CD45-/ KDR+ (flow cytometry) | CD34+/CD45-/KDR+ is significantly elevated in patients treated with telmisartan |
| Angiotensin converting enzyme inhibitors | |||||
| Min et al[39] | 20 CAD | Ramipril (5 mg/d) vs Placebo | 4 wk | EPC number Migratory activity Proliferation activity Adhesion activity | There was 1.5 fold increase in EPC number after 1 wk of treatment Followed by 2.5 fold increase in EPC count after 4 wk Migration, proliferation and adhesion activities were also significantly improved with ramipril |
| Cacciatore et al[40] | 36 HT | Enalapril (20 mg/d) vs Zofenopril (30 mg/d) | 1 yr and 5 yr | CFU Migratory activity | Increased CFU count for both treatment groups at 1 yr and 5 yr No difference for migratory activity |
| Calcium channel blockers | |||||
| Sugiura et al[43] | 37 HT | Nifedipine (20 mg/d) vs Untreated | 4 wk | CD34+/CD133+ (flow cytometry) Migratory activity | EPC number and function were significantly improved in the nifedipine group |
| de Ciuceis et al[44] | 29 essential HT | Barnidipine (20 mg/d) vs Hydrochlorothiazide (25 mg/d) | 3 and 6 mo | EPC number | EPC number was significantly elevated in patients treated with barnidipine compared to hydrochlorothiazide |
CAD: Coronary artery disease; CFU: Colony forming unit; EPC: Endothelial progenitor cells; HT: Hypertension; SHR-SP: Spontaneous hypertensive rats-stroke prone; WKY: Wistar-Kyoto.
ACE inhibitors: Similar to ARBs, ACE inhibitors are used to treat hypertension and congestive heart failure through inhibition of angiotensin converting enzyme which is part of the renin-angiotensin-aldosterone system. Generally, there was a positive trend towards improvement in EPC numbers[39] and function[39,40] with ACE inhibitors (ACEI) in patients with stable CAD and in hypertensive patients (Table 4). The administration of ramipril increased the number and improved the functional capacity of EPCs in patients with CAD within 1 wk of treatment. The improvement was further enhanced after 4 wk. Bradykinin B2 receptor pathway which activates endothelial nitric oxide synthase (eNOS) and is involved in neovascularization of EPCs may have contributed to the beneficial effects of ramipril. Indeed, nitric oxide levels were increased via activation of bradykinin. This effect was independent of any impact on blood pressure[39]. Further comparison between enalapril and zofenopril demonstrated that EPCs were increased after 1 and 5 years of follow up[40]. ACE inhibition is reported to stimulate nitric oxide (NO) activity and decreases oxidative stress in human endothelial cells[41]. Zofenopril increases NO production in endothelium, decreases atherosclerotic development and reduces ROS[42]. Similar to ARBs, ACE inhibition improves the number and function of EPCs independently of the blood pressure lowering effect and acts via the endothelial NO pathway.
CCBs: CCBs decrease blood pressure by inhibiting L-type voltage-gated calcium channels to decrease intracellular calcium. It acts on vascular smooth muscle to induce vasodilation and therefore decreases blood pressure. Preliminary results from two studies reported favorable outcomes on EPC numbers and function with CCBs in patients with essential hypertension[43,44] (Table 4). Men with stage 1 hypertension who were treated with nifedipine had a significantly improved number and angiogenic-related function of EPCs[43]. The improvement may have been driven by increased VEGF release from vascular smooth muscle cells by nifedipine. It was also shown that nifedipine-treated EPCs had greater resistance to ROS-mediated oxidative stress and apoptosis. In addition, improvement of endothelial function by nifedipine may be partially due to increased proliferation and angiogenic activities of EPCs. Another CCB, barnidipine, also demonstrated a similar beneficial effect on EPCs in patients with essential hypertension[44]. Thus, CCBs along with ARBs and ACEI may result in better vascular health in CAD patients with and without hypertension.
CHOLESTEROL LOWERING MEDICATION
HMG-CoA reductase inhibitors (statins)
Statins or HMG-CoA reductase inhibitors reduce cholesterol levels through inhibition of HMG-CoA reductase, an important enzyme in the synthesis of cholesterol in the liver. There is evidence to demonstrate that statins play an important role in the primary prevention of CVD[45]. The data on statins in primary and secondary therapy in CVD is overwhelmingly consistent. Different doses of different statins have been reported to be useful in increasing EPC numbers[46-51] and function[46,52] for a treatment period of 3-16 wk. Studies have reported that statins exert beneficial effects on EPCs by enhancing EPC proliferation and differentiation via the Akt pathway. This can result in activation of the eNOS pathway and VEGF-induced endothelial cell migration[46,50]. However, there was a study which reported a contrasting outcome where 40 mg/d of statin long term resulted in a decrease in EPC numbers and continuous statin therapy is inversely correlated with EPC numbers[53] (Table 5). It was put forth that EPCs may be unable to adequately respond to a continuous stimulus of a chronic dose of statins. This may result in desensitization. However, function of EPCs measured by CFU was not altered by statin treatment. Although long term statin therapy may result in a reduced EPC count, the beneficial effects of statin therapy in improving EPC and endothelial function is consistently documented.
Table 5.
Effect of HMG-CoA reductase inhibitors (statins) on endothelial progenitor cells
| Ref. | Subjects | Drugs | Duration | EPCs (number/function) | Findings |
| Vasa et al[46] | 15 CAD | Atorvastatin (40 mg/d) | 4 wk | CD34+/KDR+ (flow cytometry) Migratory activity | CD34+/KDR+ was significantly increased after 4 wk of therapy Migration activity was also significantly improved after 4 wk of treatment |
| Leone et al[47] | 40 STEMI | Atorvastatin (80 mg/d) vs Atorvastatin (20 mg/d) | 16 wk | CD34+/KDR+ (flow cytometry) | Patients who took 80 mg of atorvastatin had higher CD34+/KDR+ than those who took 20 mg atorvastatin |
| Spadaccio et al[48] | 50 CAD | Atorvastatin (20 mg/d) vs Placebo | 3 wk | CD34+/CD133+ (flow cytometry) | Atorvastatin has significantly elevated EPC count after 3 wk |
| Erbs et al[49] | 42 CHF | Rosuvastatin (40 mg/d) vs Placebo | 12 wk | CD34+/KDR+ (flow cytometry) | Rosuvastatin significantly increased EPC count compared to placebo |
| Tousoulis et al[50] | 60 SHF | Rosuvastatin (10 mg/d) vs Allopurinol (300 mg/d) | 4 wk | CD34+/KDR+, CD34+/CD133+/KDR+ (flow cytometry) | CD34+/KDR+ and CD34+/CD133+/KDR+ are improved with rosuvastatin treatment compared to allopurinol |
| Huang et al[51] | 100 healthy controls 100 ICM | Atorvastatin (10 mg/d) vs Atorvastatin (40 mg/d) | 1 yr | CD34+ (flow cytometry) | CD34+ count was significantly elevated in patients under 40 mg atorvastatin after 1 yr |
| Paradisi et al[52] | 20 healthy controls | Pravastatin (40 mg/d) vs Placebo | 8 wk | CFU Tubule formation assay | CFU was increased by 31% in pravastatin group compared to placebo No difference was observed for tubule formation assay between groups |
| Hristov et al[53] | 209 CAD (without statin, n = 65, statin 10/20 mg/d, n = 101, statin 40 mg/d, n = 43) | Statin (10/20 mg/d) or 40 mg/d vs Untreated | 8 wk | CFU CD34+/KDR+ (flow cytometry) | 40 mg/d of statin treatment has significantly decreased EPC numbers Continuous statin therapy inversely correlated with EPC numbers |
CAD: Coronary artery disease; CFU: Colony forming unit; CHF: Congestive heart failure; EPC: Endothelial progenitor cells; ICM: Ischemic cardiomyopathy; SHF: Systolic heart failure; STEMI: ST-elevated myocardial infarction.
ANTI-DIABETIC MEDICATION
Thiazolidinedione/metformin
Thiazolidinedione (TZD) and metformin are important oral medications in the management of type 2 diabetes mellitus. TZD activates peroxisome proliferator-activated receptors, while metformin is a biguanide which is effective in reducing glucose production in the liver. Many clinical trials have compared the effects of both TZD and metformin on EPC numbers and/or function. Overall, TZD, metformin or a combination of both drugs has been shown to be beneficial in improving EPC numbers and/or function in diabetic patients[54-58] (Table 6). In addition, pioglitazone was reported to decrease C-reactive protein (CRP) levels. Since an increased EPC number was significantly correlated to lower CRP, pioglitazone may increase EPC numbers by attenuating the detrimental effects of CRP on EPCs[54,56,57]. Similar to ARBs, pioglitazone, a PPAR-γ agonist, may directly affect EPCs through PPAR-γ receptors[54].
Table 6.
Effect of anti-diabetic medications on endothelial progenitor cells
| Ref. | Subjects | Drugs | Duration | EPCs (number/function) | Findings |
| Thiazolidinedione/metformin | |||||
| Wang et al[54] | 36 type 2 diabetes | Metformin + Pioglitazone (30 mg/d) (n = 24) vs Metformin (n = 12) | 8 wk | CD34+/KDR+ (flow cytometry) Migratory activity | Both EPC number and migration activity improved with combination of metformin and pioglitazone |
| Werner et al[55] | 54 CAD | Pioglitazone (45 mg/d) vs Placebo | 4 wk | CFU CD34+ (flow cytometry) | Improved EPC number and CFU count with pioglitazone treatment |
| Makino et al[56] | 34 type 2 diabetes | Pioglitazone (15-30 mg/d) | 24 wk | CD34+ (flow cytometry) | Number of CD34+ increased steadily at 12 wk and continued to increase after 24 wk of pioglitazone |
| Esposito et al[57] | 110 type 2 diabetes | Pioglitazone (15-45 mg/d) (n = 55) vs Metformin (1000-2000 mg/d) (n = 55) | 24 wk | CD34+/KDR+ (flow cytometry) | Significant improvement in CD34+/KDR+ in patients who took pioglitazone compared to metformin |
| Liao et al[58] | 51 healthy controls 46 type 2 diabetes | Metformin (1700-2550 mg/d) | 16 wk | CD45-/CD34+/KDR+ (flow cytometry) | EPC number is significantly lower in type 2 diabetic patients and significantly improved after metformin |
| Dipeptidyl peptidase 4 inhibitors | |||||
| Fadini et al[59] | 32 type 2 diabetes | Sitagliptin (100 mg/d) (n = 16) vs Metformin (n = 16) | 4 wk | CD34+/KDR+ (flow cytometry) | EPC number in sitagliptin group significantly improved compared to metformin group by 2 fold |
CAD: Coronary artery disease; CFU: Colony forming unit; EPC: Endothelial progenitor cells.
Dipeptidyl peptidase 4 inhibitors
Dipeptidyl peptidase 4 (DPP4) inhibitors are new oral hypoglycemic agents and so there is limited data on their effects on EPCs. There is one study that reported increased EPC numbers with sitagliptin after 4 wk of treatment compared to metformin[59] (Table 6). In addition, besides increased EPC levels, plasma stromal-derived factor-1α (SDF-1α) levels were also increased in patients who were on sitagliptin treatment for 4 wk[59]. The positive effect of DPP4 inhibitors on EPCs is likely driven by SDF-1α, a physiological substrate of DPP4 and a chemokine which can stimulate bone marrow mobilization of EPCs. SDF-1α is upregulated and, upon binding to its receptor CXCR4, stimulates the bone marrow to release EPCs. DPP4 inhibition increases circulating SDF-1α levels.
EPC AS A THERAPEUTIC POTENTIAL CANDIDATE IN CARDIOVASCULAR DISEASES
Endothelial progenitor cell capture stent
The EPC capture stent is a device which uses the ability of bone marrow-derived EPCs to repair damaged arterial segments. The surface of EPC antibody consists of a covalently coupled polysaccharide intermediate coating with anti-human CD34 antibodies and is then attached to a stainless steel stent. Upon stent placement, the anti-human CD34 antibodies will therefore attract circulating EPCs to differentiate into mature endothelial cells to form a functional endothelium layer. This accelerated healing approach aims to decrease the risk of stent thrombosis and restenosis, as well as reduce prolonged dual antiplatelet therapy in these patients. Effectiveness and safety of this EPC capture stent have been tested in patients with de novo CAD[60-63] and STEMI[64-67] and generally these stents are feasible and safe, with major adverse cardiac events reported between 4.2% to 16%. Despite this, there are also contradictory findings which suggest that an EPC capture stent is no better than conventional stents in reducing in-stent restenosis[62,63]. Preliminary results from a new anti-human CD133 coated coronary stent tested on a porcine model have demonstrated no difference in re-endothelialization or neointima formation with the use of CD133-stents. The existing low number of circulating CD133-positive cells may have resulted in the lack of efficacy of these stents[68].
Endothelial progenitor cell therapy
Since the successful isolation of adult EPCs in 1997, we now know that bone marrow-derived EPCs may be mobilized to stimulate angiogenesis and may attenuate tissue ischemia for CAD and PAD. Initial pre-clinical studies have reported favorable improvement in left ventricular function in a rat model of myocardial infarction after intravenous injection of ex vivo expanded human CD34+ cells[69]. Furthermore, another study examined the effect of catheter-based, intramyocardial transplantation in a swine model of myocardial infarction, providing encouraging outcomes in favoring the application of EPCs as a potential therapeutic therapy in clinical trials[70].
Recently, there have been several studies using intramyocardial transplantation of autologous CD34+ cells in patients with cardiovascular diseases to improve cardiovascular outcomes.
In patients with refractory angina despite medical therapy with antianginal medications and undergoing several revascularization options, including coronary artery bypass graft and percutaneous coronary intervention, intramyocardial transplantation of autologous CD34+ cells may be a feasible option. A phase I/II clinical trial[71] of 24 patients followed by phase IIb[72] of 167 patients reported a significant improvement in angina frequency and exercise tolerance. An ongoing RENEW study, a phase III trial of 444 patients, will adequately examine the effect of intramyocardial transplantation of autologous CD34+ cells in patients with refractory angina[73]. Besides CAD, there was also a pilot study on the effect of autologous intramuscular injection of CD34+ in critical limb ischemia. The study found that CD34+ treatment reduced amputation rates[74].
CONCLUSION
Endothelial dysfunction secondary to various cardiovascular risk factors can lead to the development of atherosclerosis. As mature endothelial cells possess limited regenerative capacity, there is growing interest in circulating EPCs due to their acclaimed role in maintenance of endothelial integrity, function and postnatal neovascularization. There have been increasing numbers of studies investigating the effects of pharmacotherapies which cardiac patients tend to take on EPC numbers and functions. EPC behavior and mechanisms are also elucidated in patients with CVD, including CAD, HF and PAD. Some studies showed conflicting results and this may be due to the varying definition of EPCs using different methods of identification, different timing of blood sampling, different severity of native disease and concomitant medication and comorbidities that may affect EPC numbers and functions. Besides a biological marker, EPCs have also been shown to be a useful prognostic marker in predicting events in patients with CAD. Lastly, there are several promising studies to suggest EPCs as a novel therapy for CVDs[74]. However, due to the paucity of circulating cells and the effects of disease on cell quality, investigators need to be mindful of its possible limitations. Possible solutions include enhancing these cell numbers by increasing their mobilization or concentrating them before transplantation and improving their function using ex vivo augmentation. Several pilot studies on animals have already shown encouraging results. Further translation to clinical practice is anticipated.
Footnotes
Supported by The National Medical Research Council, Singapore, No. NMRC/NIG/1038/2010; and the National University Health System Clinician Scientist Program (NCSP) from the Clinician Scientist Unit, Yong Loo Lin School of Medicine, National University of Singapore
P- Reviewers: Soker S, Zeng LF S- Editor: Song XX L- Editor: Roemmele A E- Editor: Liu SQ
References
- 1.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Poh KK. Gene and cell therapy for chronic ischaemic heart disease. Expert Opin Biol Ther. 2007;7:5–15. doi: 10.1517/14712598.7.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 4.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 7.Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 8.Handgretinger R, Gordon PR, Leimig T, Chen X, Buhring HJ, Niethammer D, Kuci S. Biology and plasticity of CD133+ hematopoietic stem cells. Ann N Y Acad Sci. 2003;996:141–151. doi: 10.1111/j.1749-6632.2003.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 12.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 13.Lee LC, Chen CS, Choong PF, Low A, Tan HC, Poh KK. Time-dependent dynamic mobilization of circulating progenitor cells during percutaneous coronary intervention in diabetics. Int J Cardiol. 2010;142:199–201. doi: 10.1016/j.ijcard.2008.11.198. [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Sartore S, Baesso I, Lenzi M, Agostini C, Tiengo A, Avogaro A. Endothelial progenitor cells and the diabetic paradox. Diabetes Care. 2006;29:714–716. doi: 10.2337/diacare.29.03.06.dc05-1834. [DOI] [PubMed] [Google Scholar]
- 16.Morishita T, Uzui H, Nakano A, Mitsuke Y, Geshi T, Ueda T, Lee JD. Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin-3, malondialdehyde-modified low-density lipoprotein and membrane type-1 matrix metalloproteinase. J Atheroscler Thromb. 2012;19:149–158. doi: 10.5551/jat.10074. [DOI] [PubMed] [Google Scholar]
- 17.Delva P, De Marchi S, Prior M, Degan M, Lechi A, Trettene M, Arosio E. Endothelial progenitor cells in patients with severe peripheral arterial disease. Endothelium. 2008;15:246–253. doi: 10.1080/10623320802487718. [DOI] [PubMed] [Google Scholar]
- 18.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 19.Eizawa T, Ikeda U, Murakami Y, Matsui K, Yoshioka T, Takahashi M, Muroi K, Shimada K. Decrease in circulating endothelial progenitor cells in patients with stable coronary artery disease. Heart. 2004;90:685–686. doi: 10.1136/hrt.2002.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HY, Gao PJ, Ji KD, Shen WF, Fan CL, Lu L, Zhu DL. Circulating endothelial progenitor cells, C-reactive protein and severity of coronary stenosis in Chinese patients with coronary artery disease. Hypertens Res. 2007;30:133–141. doi: 10.1291/hypres.30.133. [DOI] [PubMed] [Google Scholar]
- 21.Liguori A, Fiorito C, Balestrieri ML, Crimi E, Bruzzese G, Williams-Ignarro S, D’Amora M, Sommese L, Grimaldi V, Minucci PB, et al. Functional impairment of hematopoietic progenitor cells in patients with coronary heart disease. Eur J Haematol. 2008;80:258–264. doi: 10.1111/j.1600-0609.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 22.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, Mariani G, D’Andrea D, De Micco F, Rivera NV, et al. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J. 2010;24:1981–1988. doi: 10.1096/fj.09-138198. [DOI] [PubMed] [Google Scholar]
- 23.Güven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–1587. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 24.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 25.Chong AY, Blann AD, Patel J, Freestone B, Hughes E, Lip GY. Endothelial dysfunction and damage in congestive heart failure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 2004;110:1794–1798. doi: 10.1161/01.CIR.0000143073.60937.50. [DOI] [PubMed] [Google Scholar]
- 26.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 27.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 28.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 29.Nonaka-Sarukawa M, Yamamoto K, Aoki H, Nishimura Y, Tomizawa H, Ichida M, Eizawa T, Muroi K, Ikeda U, Shimada K. Circulating endothelial progenitor cells in congestive heart failure. Int J Cardiol. 2007;119:344–348. doi: 10.1016/j.ijcard.2006.07.191. [DOI] [PubMed] [Google Scholar]
- 30.Michowitz Y, Goldstein E, Wexler D, Sheps D, Keren G, George J. Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart. 2007;93:1046–1050. doi: 10.1136/hrt.2006.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 32.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32 Suppl 2:S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao EH, Fukuda N, Matsumoto T, Kobayashi N, Katakawa M, Yamamoto C, Tsunemi A, Suzuki R, Ueno T, Matsumoto K. Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect. Hypertens Res. 2007;30:1119–1128. doi: 10.1291/hypres.30.1119. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Fukuda N, Yao EH, Matsumoto T, Kobayashi N, Suzuki R, Tahira Y, Ueno T, Matsumoto K. Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens. 2008;21:72–77. doi: 10.1038/ajh.2007.5. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida Y, Fukuda N, Maeshima A, Yamamoto C, Matsumoto T, Ueno T, Nojima Y, Matsumoto K, Soma M. Treatment with valsartan stimulates endothelial progenitor cells and renal label-retaining cells in hypertensive rats. J Hypertens. 2011;29:91–101. doi: 10.1097/HJH.0b013e32834000e2. [DOI] [PubMed] [Google Scholar]
- 36.Honda A, Matsuura K, Fukushima N, Tsurumi Y, Kasanuki H, Hagiwara N. Telmisartan induces proliferation of human endothelial progenitor cells via PPARgamma-dependent PI3K/Akt pathway. Atherosclerosis. 2009;205:376–384. doi: 10.1016/j.atherosclerosis.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 37.Pelliccia F, Pasceri V, Cianfrocca C, Vitale C, Speciale G, Gaudio C, Rosano GM, Mercuro G. Angiotensin II receptor antagonism with telmisartan increases number of endothelial progenitor cells in normotensive patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Atherosclerosis. 2010;210:510–515. doi: 10.1016/j.atherosclerosis.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 39.Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–209. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 40.Cacciatore F, Bruzzese G, Vitale DF, Liguori A, de Nigris F, Fiorito C, Infante T, Donatelli F, Minucci PB, Ignarro LJ, et al. Effects of ACE inhibition on circulating endothelial progenitor cells, vascular damage, and oxidative stress in hypertensive patients. Eur J Clin Pharmacol. 2011;67:877–883. doi: 10.1007/s00228-011-1029-0. [DOI] [PubMed] [Google Scholar]
- 41.Jacoby DS, Rader DJ. Renin-angiotensin system and atherothrombotic disease: from genes to treatment. Arch Intern Med. 2003;163:1155–1164. doi: 10.1001/archinte.163.10.1155. [DOI] [PubMed] [Google Scholar]
- 42.Scribner AW, Loscalzo J, Napoli C. The effect of angiotensin-converting enzyme inhibition on endothelial function and oxidant stress. Eur J Pharmacol. 2003;482:95–99. doi: 10.1016/j.ejphar.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, Kimura G, Ueda R, Rabelink TJ, Murohara T. Nifedipine improves endothelial function: role of endothelial progenitor cells. Hypertension. 2008;52:491–498. doi: 10.1161/HYPERTENSIONAHA.108.111914. [DOI] [PubMed] [Google Scholar]
- 44.de Ciuceis C, Pilu A, Rizzoni D, Porteri E, Muiesan ML, Salvetti M, Paini A, Belotti E, Zani F, Boari GE, et al. Effect of antihypertensive treatment on circulating endothelial progenitor cells in patients with mild essential hypertension. Blood Press. 2011;20:77–83. doi: 10.3109/08037051.2010.535973. [DOI] [PubMed] [Google Scholar]
- 45.Minder CM, Blumenthal RS, Blaha MJ. Statins for primary prevention of cardiovascular disease: the benefits outweigh the risks. Curr Opin Cardiol. 2013;28:554–560. doi: 10.1097/HCO.0b013e32836429e6. [DOI] [PubMed] [Google Scholar]
- 46.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 47.Leone AM, Rutella S, Giannico MB, Perfetti M, Zaccone V, Brugaletta S, Garramone B, Niccoli G, Porto I, Liuzzo G, et al. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: Statins for regeneration after acute myocardial infarction and PCI (STRAP) trial. Int J Cardiol. 2008;130:457–462. doi: 10.1016/j.ijcard.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 48.Spadaccio C, Pollari F, Casacalenda A, Alfano G, Genovese J, Covino E, Chello M. Atorvastatin increases the number of endothelial progenitor cells after cardiac surgery: a randomized control study. J Cardiovasc Pharmacol. 2010;55:30–38. doi: 10.1097/FJC.0b013e3181c37d4d. [DOI] [PubMed] [Google Scholar]
- 49.Erbs S, Beck EB, Linke A, Adams V, Gielen S, Kränkel N, Möbius-Winkler S, Höllriegel R, Thiele H, Hambrecht R, et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling--results from a randomized, double-blind, and placebo-controlled study. Int J Cardiol. 2011;146:56–63. doi: 10.1016/j.ijcard.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Tousoulis D, Andreou I, Tsiatas M, Miliou A, Tentolouris C, Siasos G, Papageorgiou N, Papadimitriou CA, Dimopoulos MA, Stefanadis C. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: the impact of inflammatory process and oxidative stress. Atherosclerosis. 2011;214:151–157. doi: 10.1016/j.atherosclerosis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Huang B, Cheng Y, Xie Q, Lin G, Wu Y, Feng Y, Gao J, Xu D. Effect of 40 mg versus 10 mg of atorvastatin on oxidized low-density lipoprotein, high-sensitivity C-reactive protein, circulating endothelial-derived microparticles, and endothelial progenitor cells in patients with ischemic cardiomyopathy. Clin Cardiol. 2012;35:125–130. doi: 10.1002/clc.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paradisi G, Bracaglia M, Basile F, Di’Ipolito S, Di Nicuolo F, Ianniello F, Quagliozzi L, Donati L, Labianca A, Di Cesare C, et al. Effect of pravastatin on endothelial function and endothelial progenitor cells in healthy postmenopausal women. Clin Exp Obstet Gynecol. 2012;39:153–159. [PubMed] [Google Scholar]
- 53.Hristov M, Fach C, Becker C, Heussen N, Liehn EA, Blindt R, Hanrath P, Weber C. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis. 2007;192:413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Wang CH, Ting MK, Verma S, Kuo LT, Yang NI, Hsieh IC, Wang SY, Hung A, Cherng WJ. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. Am Heart J. 2006;152:1051.e1–1051.e8. doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 55.Werner C, Kamani CH, Gensch C, Böhm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 56.Makino H, Okada S, Nagumo A, Sugisawa T, Miyamoto Y, Kishimoto I, Akie TK, Soma T, Taguchi A, Yoshimasa Y. Pioglitazone treatment stimulates circulating CD34-positive cells in type 2 diabetes patients. Diabetes Res Clin Pract. 2008;81:327–330. doi: 10.1016/j.diabres.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Esposito K, Maiorino MI, Di Palo C, Gicchino M, Petrizzo M, Bellastella G, Saccomanno F, Giugliano D. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes--a randomized controlled trial. Diabetes Obes Metab. 2011;13:439–445. doi: 10.1111/j.1463-1326.2011.01367.x. [DOI] [PubMed] [Google Scholar]
- 58.Liao YF, Chen LL, Zeng TS, Li YM, Fan Yu LJ. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc Med. 2010;15:279–285. doi: 10.1177/1358863X10367537. [DOI] [PubMed] [Google Scholar]
- 59.Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005;45:1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 61.Beijk MA, Klomp M, van Geloven N, Koch KT, Henriques JP, Baan J, Vis MM, Tijssen JG, Piek JJ, de Winter RJ. Two-year follow-up of the Genous™ endothelial progenitor cell capturing stent versus the Taxus Liberté stent in patients with de novo coronary artery lesions with a high-risk of restenosis: a randomized, single-center, pilot study. Catheter Cardiovasc Interv. 2011;78:189–195. doi: 10.1002/ccd.23143. [DOI] [PubMed] [Google Scholar]
- 62.den Dekker WK, Houtgraaf JH, Onuma Y, Benit E, de Winter RJ, Wijns W, Grisold M, Verheye S, Silber S, Teiger E, et al. Final results of the HEALING IIB trial to evaluate a bio-engineered CD34 antibody coated stent (Genous™Stent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis. 2011;219:245–252. doi: 10.1016/j.atherosclerosis.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 63.Klomp M, Beijk MA, Damman P, Woudstra P, Koch KT, Tijssen JG, de Winter RJ. Three-year clinical follow-up of an unselected patient population treated with the genous endothelial progenitor cell capturing stent. J Interv Cardiol. 2011;24:442–449. doi: 10.1111/j.1540-8183.2011.00665.x. [DOI] [PubMed] [Google Scholar]
- 64.Co M, Tay E, Lee CH, Poh KK, Low A, Lim J, Lim IH, Lim YT, Tan HC. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J. 2008;155:128–132. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Lee YP, Tay E, Lee CH, Low A, Teo SG, Poh KK, Yeo WT, Lim J, Lim IH, Lim YT, et al. Endothelial progenitor cell capture stent implantation in patients with ST-segment elevation acute myocardial infarction: one year follow-up. EuroIntervention. 2010;5:698–702. doi: 10.4244/eijv5i6a115. [DOI] [PubMed] [Google Scholar]
- 66.Low AF, Lee CH, Teo SG, Chan MY, Tay E, Lee YP, Chong E, Co M, Tin Hay E, Lim YT, et al. Effectiveness and safety of the genous endothelial progenitor cell-capture stent in acute ST-elevation myocardial infarction. Am J Cardiol. 2011;108:202–205. doi: 10.1016/j.amjcard.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 67.Chong E, Poh KK, Liang S, Soon CY, Tan HC. Comparison of risks and clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronary interventions. J Interv Cardiol. 2010;23:451–459. doi: 10.1111/j.1540-8183.2010.00581.x. [DOI] [PubMed] [Google Scholar]
- 68.Sedaghat A, Sinning JM, Paul K, Kirfel G, Nickenig G, Werner N. First in vitro and in vivo results of an anti-human CD133-antibody coated coronary stent in the porcine model. Clin Res Cardiol. 2013;102:413–425. doi: 10.1007/s00392-013-0547-4. [DOI] [PubMed] [Google Scholar]
- 69.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 70.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 71.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 72.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Povsic TJ, Junge C, Nada A, Schatz RA, Harrington RA, Davidson CJ, Fortuin FD, Kereiakes DJ, Mendelsohn FO, Sherman W, et al. A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J. 2013;165:854–861.e2. doi: 10.1016/j.ahj.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]


