Abstract
Curing Alzheimer’s disease (AD) remains an elusive goal and may prove to be impossible due to the very nature of the disease. While modulating disease progression is an attractive target and will alleviate the burden of the most severe disease stages, this strategy will not reduce disease prevalence. Preventing or, as will be described, delaying the onset of cognitive impairment and AD will provide the greatest benefit to individuals and society by pushing the onset of disease into later ages. Because of the highly variable age of disease onset, AD prevention studies that do not stratify participants by age-dependent disease risk will be operationally challenging – large in size and of long duration. We present a composite genetic biomarker to stratify disease risk that facilitates clinical studies in high risk people. In addition, we discuss the rationale for the use of pioglitazone to delay disease onset in high risk people.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that progresses over an extended time course, with much of the neuropathological damage occurring silently, without overt clinical symptoms. The disease gradually robs people of their independence and dignity and it is, inescapably, fatal. The vast majority of AD cases, and the majority of all dementia, is due to late-onset, sporadic disease rather than the early-onset form that is linked to completely penetrant mutations in the genes encoding amyloid precursor protein (APP), its processing enzymes presenilin 1 (PS1) or presenilin 2 (PS2), or via duplication of APP1. Early onset AD is rare, accounting for <1% of all AD patients. By contrast, late onset AD, which is the topic of this article, is the most common form of dementia and typically affects individuals in or after the 7th decade of life. Although genetics is not determining for the development of late onset AD, it does account for up to 80% of personal risk for eventually developing the disease sometime after the age of ~65 years2. One in 8 people in the United States who are over age 65 are now living with AD, and with more people living to older ages the incidence of AD will continue to climb3. In the absence of an effective therapy that at least delays disease onset, but preferably both delays onset and slows progression, it has been projected that 9–16 million Americans will be living with this disease by 2050, with almost half living with advanced disease3, 4, and worldwide 106 million people will be afflicted with this fatal disease5. The financial costs associated with AD propagate beyond the direct cost of caring for patients to include lost productivity and substantial emotional capital expended by care-givers. It is estimated that US expenditures on AD and other dementias will be $200 billion in 2012. According to one model, this cost will increase more than 5 fold by 20503. In addition, the healthcare systems in the US will have to undergo structural and organizational changes at the State and Federal levels to accommodate the burgeoning numbers of individuals requiring long-term care in facilities or at home.
In recognition of the growing burden of AD, national plans to address this challenge have been advanced in a number of countries including, as of 2011, the United States. The necessity for a national plan was detailed in a 2007 publication, “Developing a National Alzheimer’s Strategy Equal to the Epidemic”6. In 2009, the Alzheimer’s Study Group released its report, “A National Alzheimer’s Strategic Plan: The Report of the Alzheimer’s Study Group” (http://www.alz.org/documents/national/report_ASG_alzplan.pdf). An important pillar of the Alzheimer’s Solution Project proposed by the Study Group was an initiative to develop the means to delay or prevent AD. In January 2011, President Obama signed the National Alzheimer’s Project Act into law (http://www.gpo.gov/fdsys/pkg/PLAW-111publ375/pdf/PLAW-111publ375.pdf;7), and one of the chief aims of this legislation is to “accelerate the development of treatments that would prevent, halt, or reverse the course of Alzheimer’s disease.”
Physicians currently have few therapies to offer AD patients or individuals with predementia cognitive impairment, and those treatments that are available do not alter progression of AD, although they do provide slight or moderate improvement in cognition and/or function and behavior for for some patients8-11. These drugs are the acetylcholinesterase inhibitors, galantamine, rivastigmine, and donepezil, and the N-methyl D-aspartate receptor antagonist, memantine. Pharmaceutical companies are actively pursuing therapies that modify the progression of diagnosed AD, but the development landscape is littered with disappointing failures and unconfirmed successes in small studies12. These failures have led experts in the field to suggest that, once entrenched, disease progression may be inexorable and a better strategy may be to attempt to intercede prior to the onset of clinical symptoms, before development of mild cognitive impairment (MCI). To prove that a therapy “prevents” AD, subjects would have to be followed to the end of their lifetimes, this can never be proven in the context of a limited-term clinical trial. Therefore “delay of onset” of MCI due to AD13 is a more realistic goal although extension studies could be designed to confirm delay of AD dementia or prevention of AD. Delaying AD onset by as little as 5 years is expected to provide significant societal and individual benefit4, 14.
A number of large, multi-center, placebo-controlled, double-blind, randomized AD primary prevention studies have been conducted, enrolling cognitively normal subjects and measuring the development of all-cause dementia or probable AD (Table 1). The Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) investigated naproxen and celecoxib15. ADAPT was halted early because of the potential for non-steroidal anti-inflammatory agents to induce cardiovascular harm; however an analysis of the available data indicated that, rather than preventing AD, celecoxib and naproxen increased the hazard ratio for incident AD or all-cause dementia16. The Women’s Health Initiative Memory Studies (WHIMS) investigated estrogen with and without progestin17, 18. While the estrogen plus progestin study of WHIMS was also halted early because of the potential for health risks, the combination treatment or estrogen alone increased the risk for dementia or MCI incidence in the population tested19. The Ginkgo Evaluation of Memory (GEM) study investigated ginkgo biloba extract but this treatment did not have a significant effect relative to placebo on any of the cognitive or functional endpoints tested20. GuidAge also tested ginkgo biloba extract21 and, like the GEM study, did not show evidence that the treatment lowered the incidence of dementia relative to placebo. These failures could have been due to targeting the wrong disease pathway, choosing a drug that didn’t hit the disease pathway as expected, or because the target population or the risk enrichment scheme was not optimal for a disease prevention trial. There is evidence that the enrichment strategies did not perform as expected, with lower event rates than anticipated. For example, in ADAPT the observed event rate in the placebo group is less than half that which was expected with the enrichment strategy22. Similarly, it was determined that the major limitation of the GuidAge study was that the incidence of dementia was lower than planned for, resulting in limited statistical power to detect a treatment effect23.
Table 1. Comparison of previous AD primary prevention studies.
The listed studies represent the intervention, primary prevention trials that were of large size, were multi-center, placebo-controlled, double-blind and randomized. A number of smaller prevention studies have been undertaken, but these are not summarized here.
| Study (Location) |
Age | Eligibility | Study size | Enrichment strategy |
Planned (actual) study duration in years |
Intervention | Primary endpoint [criteria] |
Results |
|---|---|---|---|---|---|---|---|---|
| GEM (USA) | ≥75 | Normal cognition or MCI |
3,069
(2,587 cognitively normal & 483 MCI) |
Very old age | 5 (6.1) | Ginkgo biloba extract |
Dementia (all-cause) incidence [DSM-IV and CDR] |
No difference between groups |
| ADAPT (USA) | ≥70 | Normal cognition |
2,128 | Alzheimer-like dementia in a first degree relative |
7 (1.5) | Naproxen or celecoxib |
AD incidence [DSM- IV, NINCDS- ADRDA] |
No difference between groups (trial terminated early) |
| WHIMS (USA) | 65-79 | Normal cognition |
4,532 | Postmenopausal women |
8.5 (5.6) | Estrogen and progestin |
Dementia incidence [DSM-IV] |
Greater risk of dementia in treatment versus placebo arms (trial terminated early) |
| WHIMS (USA) | 65-79 | Normal cognition |
2,947 | Postmenopausal women |
5 | Estrogen | Dementia incidence [DSM-IV] |
Greater risk of dementia in treatment versus placebo arms (trial terminated early) |
| GuidAge (France) |
≥70 | Self-reported memory complaint |
2,854 | Spontaneous memory complaint to the general practitioner |
5 | Ginkgo biloba extract |
AD incidence [DSM- IV, NINCDS- ADRDA, NINDS- AIREN] |
No difference between groups. Positive result on a pre-specified secondary endpoint (news release, IPSEN Jun10) |
Abbreviations: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders 4th edition; CDR, Clinical Dementia Rating; MCI, mild cognitive impairment; NINCDS-ADRDA, National Institute of Neurological Disorders and Communicative Disorders, Alzheimer’s Disease and Related Disorders; NINDS-AIREN, National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences; GEM, Ginkgo Evaluation of Memory study; ADAPT, Alzheimer’s Disease Anti-inflammatory Prevention Trial; WHIMS, Women’s Health Initiative Memory Study
Most of the cited prevention studies employed some form of disease risk enrichment because the variability in age of disease onset and rate of decline in cognition in the predementia phase, and the relatively low annual rate of conversion to disease in the general population, would otherwise necessitate very large subject numbers and extended time-lines. Employing an enrichment strategy increases study power and reduces the number of trial participants and trial duration24. For example, some studies increased the odds for incident cases of AD by enrolling individuals of advanced age, those who had a family history of disease, or had an existing memory complaint (Table 1). These strategies are ineffective for predicting risk at the level of the individual and in some cases subjects are likely to have already stepped into the AD continuum13, 25. There is a pressing need for a robust, simple, and qualified26 prognostic enrichment strategy that will identify subjects at increased personal risk of developing mild cognitive impairment due to AD (MCI due to AD); that is, to identify people who are at increased risk of developing the earliest symptoms of probable AD but do not already have the disease13. By increasing the number of events (diagnosed cases of MCI due to AD), using a prognostic biomarker will increase study power within a clinical trial.
A biomarker to enable primary prevention – evolution of understanding
We are approaching the 20th anniversary of the publication of a series of papers describing the association between APOE and risk of development of AD and age of onset (AOO) of the disease27. Everyone possesses two copies of the APOE gene, one on each of their two copies of chromosome 19. Two non-synonymous single nucleotide polymorphisms (SNPs) in APOE give rise to three possible alleles, APOE ε2, ε3, and ε4, each of which codes for a different protein isoform. APOE ε3 is by far the most common allele in every human population. APOE ε4 is associated with increased risk and earlier disease onset relative to APOE ε3; APOE ε2 is associated with reduced risk of AD and later disease onset relative to APOE ε3. Although incompletely penetrant, homozygous ε4 carriers (APOE ε4/4, approximately 2% of Caucasians) have the highest risk of developing AD. In one study of autopsy-confirmed AD, the APOE ε4/4 subjects (<2% of Caucasians) had ~20 (95% CI, 10.4– 41) times greater odds of developing AD than APOE ε3/3 subjects (the neutral reference genotype). APOE ε3/4 (~20% Caucasians) and APOE ε2/4 (~2% Caucasians) subjects had 5.5 (95% CI, 4.2–7.2) and 3.2 (95% CI, 1.7–6.2) times greater odds, respectively, of developing the disease28, 29. Although only advanced age is a more significant risk factor than the APOE ε4 allele, the ε4 allele is potentially informative of the genetic risk for developing AD for only a quarter of Caucasians (i.e. for APOE ε4/4, APOE ε3/4, and APOE ε2/4). The relative infrequency of APOE ε4 in any population limits its usefulness as a marker for the stratification of population-based clinical studies and APOE ε4 carriage, alone, does not provide sufficient sensitivity, selectivity or predictive power to be used as a diagnostic or prognostic tool for AD30. Simply put, APOE ε4 can’t address risk for those non-ε4 carriers who are at risk of developing AD
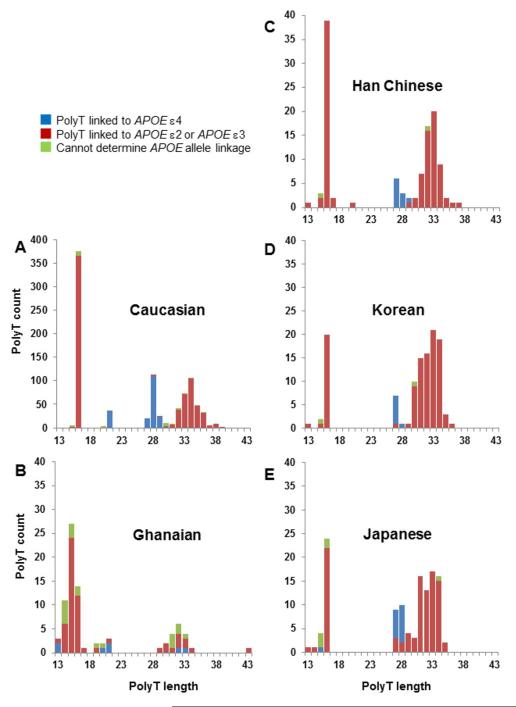
Another genetic locus in the vicinity of APOE is also associated with age of development of AD and stratifies AOO of cognitive impairment31. The second risk locus is a polymorphic, deoxythymidine (polyT) tract, rs10524523 (523), located in intron 6 of the TOMM40 gene. This gene encodes the channel subunit of the outer mitochondrial membrane protein import complex. TOMM40 is not only adjacent to APOE on chromosome 19 but the two genes reside within an extended region of linkage disequilibrium (LD). The discovery of 523 hinged upon the structure of a phylogenetic tree developed from the DNA sequences of a 10 kilobase genomic region encompassing parts of TOMM40 and APOE. This detailed mapping technique described the co-inheritance of specific lengths of the polyT tract, 523, with different alleles of APOE 31. The different tract lengths were categorized as three alleles, short (S, <19 T residues; L, >19 T but < 29 T residues; VL, > 29 T residues). The linkages that are most common in Caucasians are as follows; APOE ε4 is almost always linked to 523 L; APOE ε3 and APOE ε2 may be linked to either a 523 S or 523 VL allele. The key observation was that an excess of disease cases mapped onto one of the two major branches of the phylogenetic tree. This branch was home to all of the APOE ε4-containing DNA sequences (haplotypes) and a subset of the APOE ε3-containing haplotypes. The distinguishing feature of the APOE ε3 haplotypes that segregated to the higher risk branch was that the APOE ε3 was linked to a 523 VL allele. By contrast, those APOE ε3 haplotypes that were in the lower risk branch almost always contained 523 S alleles. With relatively few exceptions (only ~2% of ε4 haplotypes), ε4 was connected to an L allele. Therefore, the APOE ε4 and the 523 L alleles reliably report the presence of the other in Caucasians. Note that the 523 allele frequencies vary across different ethnic groups (Figure 1 and 32), as do APOE allele frequencies. In addition, the linkage between APOE ε4 and the 523 L allele is not as invariant in other groups as it is for Caucasians. Genotyping samples of African-American and Ghanaian subjects revealed that APOE ε4 is inherited with L alleles approximately 50% of the time, compared to 98% in Caucasians, and is otherwise inherited with S alleles32, 33. This may provide an explanation for the variable genetic association of APOE ε4 with African and African American study populations over the years34-37.
Figure 1. Frequencies of TOMM40 523 polyT lengths in different groups.
PolyT length is in number of T residues. The Caucasian samples (n=463) in panel A were obtained from the Wisconsin Registry for Alzheimer’s Prevention64. The Ghanaian samples (B, n=41) were from elderly, non-demented subjects. The Far Eastern samples (C–E, n=60 in each case) were from young, healthy subjects. 523 allele lengths were determined by sequencing. Blue bars, 523 alleles connected to APOE ε4; red bars, 523 alleles linked to APOE ε3 (or APOE ε2); green bars indicate when the linkage between 523 and an APOE allele could not be unambiguously assigned knowing only the genotypes at the two loci.
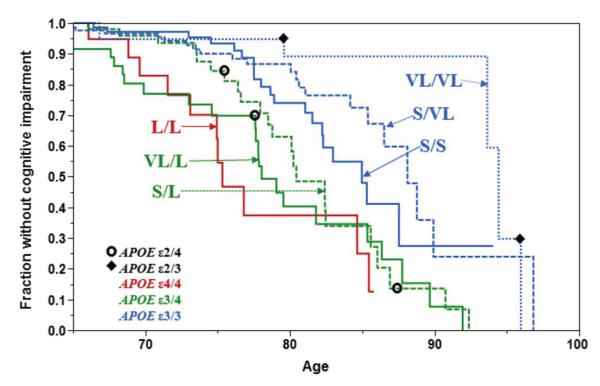
The distribution of APOE ε3 across the phylogenetic tree suggested that the heterogeneity in AOO of AD in individuals who carry APOE ε3 might be explained by linkage to alternative 523 alleles (S or VL). The hypothesis was first assessed with a simple association test in a group of APOE ε3/4 (i.e. 523 VL/L and S/L) subjects. The average age of disease onset in this small sample differed according to 523 genotype, with VL/L subjects developing disease ~ 7 years earlier than S/L subjects (70 versus 77 years, respectively)31. Prior to the discovery of the association between 523 and age of AD onset, the average age of disease onset for APOE ε3/4 subjects was estimated to be 75 years38, but there was a great deal of variation in the actual age of diagnosis. The relationship between all 523 genotypes and AOO has now been explored in a larger cohort (Figure 2).
Figure 2. Age of onset of cognitive impairment as a function of TOMM40 523 genotype.
The Bryan ADRC, Memory, Health and Aging cohort65 (n=508, 106 conversion events) was followed prospectively at the Bryan ADRC at Duke University. Cognitive status was determined using standard neuropsychological tests66, 67. Age at which cognitive impairment occurred was retrospectively stratified by TOMM40 genotype and Kaplan-Meier curves were constructed. TOMM40 genotype was determined using sequencing-based genotyping method (Polymorphic DNA Technologies, Inc). TOMM40 genotypes and the corresponding APOE genotypes are indicated on the figure. The red line corresponds to APOE ε4/4; the two green lines correspond to APOE ε3/4, and the three blue lines correspond to APOE ε3/3. Note that, within this cohort, there are 78 individuals who carry an APOE ε2 allele, but only 5 convert to cognitive impairment during the study (VL/L (APOE ε2/4), n=1; S/L (APOE ε2/4), n=2; VL/VL (APOE ε3/3), n=2 ). These individuals are indicated as points on the appropriate TOMM40 genotype curve; open circles and closed diamonds indicate the age of symptom onset for the APOE ε2/4 and APOE ε2/3 cases, respectively. Number of subjects per 523 genotype and (number converted to case status): L/L, n=23(11); VL/L, n=54(24); S/L, n=72(23); S/S, n=100(20); S/VL, n=138(22); VL/VL, n=51(6).
The Kaplan-Meier curves of Figure 2 present a retrospective stratification, by TOMM40 genotype, of the ages of onset of MCI for subjects, all Caucasian, that were followed in the Joseph & Kathleen Bryan Alzheimer’s Disease Research Center (Bryan ADRC) at Duke University. For this cohort, cognitive changes were monitored over time using a battery of neuropsychological tests to determine the age of onset of cognitive impairment and probable AD dementia. This is an important point – the subjects were followed prospectively to catch the earliest clinical symptoms of the disease process, and ascertainment variability was minimized because all subjects were assessed at one research center using validated tests and standardized practices and definitions of cognitive status.
Each curve shows the proportion of subjects of each 523 genotype that remains unaffected by MCI at each age. The APOE genotype that corresponds to each 523 genotype is also shown on the graph. The L/L curve in Figure 2 corresponds closely to APOE ε4/4 since 98% of all APOE ε4 alleles are linked to L in Caucasians. In the Bryan ADRC cohort, 50% of the L/L subjects were diagnosed with probable AD by age 76. For the APOE ε3/4 subjects, stratification by 523 genotype gives two, separable, age-of-onset distributions, corresponding to VL/L and S/L. For these subjects, it is the 532 allele (S or VL) linked to the APOE ε3 that is driving the difference in AOO. Instead of one curve for APOE ε3 homozygotes, three distinct curves can be constructed, for S/S, S/VL and VL/VL. Differences in AOO for these subjects are completely independent of carriage of APOE ε4. As with APOE ε3, APOE ε2 may be linked to an S or a VL allele. Like previous reports, carriage of at least one allele of APOE ε2 typically results in later AOO39, except when it is paired with an APOE ε4 (i.e. also an L allele) in the genotype. Since APOE ε2 is rare, it is difficult to test the combined effect of carriage of ε2 and the 523 alleles. The cohort used to develop the AOO distributions in Figure 2 contained only 78 APOE ε2 carriers. Of these, only 5 subjects developed cognitive impairment (shown in Figure 2 as diamonds (APOE ε2/3) and open circles (APOE ε2/4)).
Close inspection of the AOO curves suggests that VL has a different effect depending on whether it is in the context of APOE ε3/4 or APOE ε3/3. In the first case, VL is associated with earlier AOO, whereas in the latter case it is associated with later AOO. This conundrum is partially addressed by functional experiments in people who are still in the normal cognition range, which suggest that carriage of VL by APOE ε3 homozygotes adversely affects cognition and brain volume prior to the development of cognitive impairment. Johnson et al. demonstrated that cognitively normal, late middle aged, APOE ε3/3 subjects who were homozygous for the VL allele performed worse on a word retrieval task, a test of episodic memory, than their S/S counterparts40. Furthermore, in a magnetic resonance imaging study of a sample of these APOE ε3/3 subjects, there was an inverse relationship between number of VL alleles and grey matter volume in two regions of the brain that are affected early in the progression of AD, the ventral posterior cingulate and medial ventral precuneus40. Caselli et al., analyzed the episodic memory performances of cognitively normal people using the Rey Auditory Verbal Learning Test (Rey AVLT)41. Carriage of at least one APOE ε4 and no S allele (i.e. APOE ε3/4 VL/L) was associated with accelerated memory decline after age 60 relative to APOE ε4 carriers with at least one S allele, though the relative contributions of the APOE and TOMM40 loci could not be distinguished. Within the APOE ε3/3 subjects, those who were homozygous for the VL allele demonstrated a reduced test-retest effect (cognitively normal subjects typically perform better on retest because of practice) on the Rey AVLT whereas S/S carriers had normal test-retest measures. This suggested that the VL allele had a deleterious effect with respect to being able to recall the test and thus improve performance. The difference between S/S and VL/VL carriers was particularly evident before age 60. A third study also found an APOE-independent effect of 523 on cognitive performance in cognitively-normal, older adults aged 64-93 years42. In this cohort, the S/S carriers within the APOE ε3/3 sub-group performed better than S/VL heterozygotes on tests of episodic memory, attention, and executive function. We speculate, based upon this functional data, that the VL/VL genotype (in APOE ε3 homozygotes) may be associated with presymptomatic AD processes that are most evident at younger ages when subtle signs of cognitive dysfunction are not masked by later pathology and that, in the presence of APOE ε4 (i.e. in APOE ε3/4 subjects), this early VL effect is exacerbated.
Developing a risk algorithm for clinical trial enrichment
Together the APOE and 523 genotypes are prognostic of age-dependent risk for most Caucasians, and employing knowledge of the two genotypes together provides a better means, relative to those that were used previously, to enrich a clinical trial with at-risk subjects.
Based on the data presented, a ‘risk algorithm’, composed of APOE and TOMM40 genotypes, and age at randomization, has been developed. This algorithm is not a diagnostic, but it is a means for enriching a clinical trial with subjects who have higher risk of developing MCI due to AD during the course of a study of approximately 5-year duration. A straightforward classification scheme that constitutes the risk algorithm is summarized in Table 2. For the age range in the trial (68–83 years), the low risk stratum will include APOE ε2/2 and APOE ε2/3 subjects, and a proportion of APOE ε3/3 subjects. All VL/VL subjects will also be classified as low risk, as Figure 2 demonstrates that these subjects are at relatively low risk of developing cognitive impairment in this age range. The rare APOE ε2/4 subjects will be designated as high risk for the purpose of the trial. TOMM40 523 L/L subjects and those with VL/L will also be classified as high risk. There are three common genotypes with risk that changes as a function of age at randomization. The age at which these subjects are classified as high risk is the point on each of the curves where the slope increases. Based on the Bryan ADRC sample, for which we have the most reliable AOO data, S/L, one of the two APOE ε3/4 groups, becomes high risk beginning at age 74 years; S/S subjects and S/VL (sub-groups of APOE ε3/3) enter the high risk category at ages 77 and 76, respectively.
Table 2. Risk stratification scheme.
APOE ε2 carriers are assigned to the high or the low risk strata according to APOE genotype. Risk is assessed for all other subjects according to TOMM40 genotype. APOE ε2/2, APOE ε2/3 and VL/VL subjects are considered to be at low risk. Certain genotypes are considered high risk in the context of the targeted age range for the delay-of-onset clinical trial. Subjects with any of three 523 genotypes, S/L, S/S, and S/VL will be placed in the high risk category if he or she enters the trial at or older than the age indicated. 523 genotype frequencies are from the Cache County Study of Memory cohort (n=2,042)68. APOE genotype frequencies are for Caucasian controls from Farrer et al. (n=6,262)43.
| 523 or
APOE genotype |
Genotype frequency | Risk for the Study |
|---|---|---|
| For APOE ε2 carriers | ||
| APOE ε2/2 | 1% | All low risk |
| APOE ε2/3 | 13% | All low risk |
| APOE ε2/4 | 3% | All high risk |
| For non-APOE ε2 carriers | ||
| 523 L/L | 2% | All high risk |
| 523 L/VL | 13% | All high risk |
| 523 S/L | 14% | ≥74 is high risk |
| 523 S/S | 17% | ≥77 is high risk |
| 523 S/VL | 36% | ≥76 is high risk |
| 523 VL/VL | 18% | All low risk |
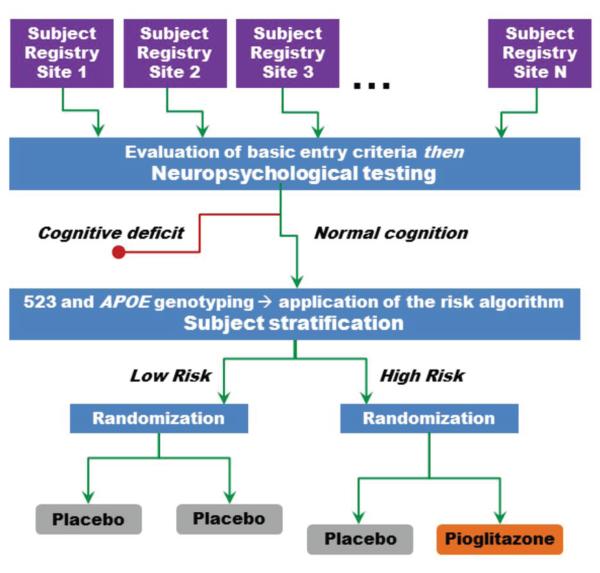
We have designed a pharmacogenetically-enriched, double-blind, delay-of-disease-onset clinical trial of cognitively-normal subjects aged 68–83, inclusive, using the enrichment scheme (see Figure 3). The subjects in this study will be classified as having high or low risk for development of cognitive symptoms over the subsequent 5 years, which we predict will be the duration of the event-driven study, according to the risk algorithm we just described. High risk subjects will be randomized to active therapy or placebo; low risk subjects will be treated with placebo only. The low risk subjects will not be exposed to active treatment because these subjects may not receive benefit from an intervention and the number of subjects required to measure a significant difference between active and placebo treatment in this group, given the expected low event rate, would be very large.
Figure 3. Pharmacogenetically-enriched, primary prevention clinical trial design.
Subject registries are established at a number of international sites that have resources for identifying an epidemiological population of the appropriate age range; competencies for neuropsychological testing are developed at each site. At the start of the trial, entry and exclusion criteria are reviewed for each subject and eligible subjects undergo a battery of neuropsychological tests. Those subjects with normal cognition (age-normed) proceed into the prevention study, are genotyped and segregated to the high and low risk strata of the study according to the risk algorithm (Table 1). Low risk subjects are treated with placebo and high risk subjects are randomized to placebo or PIO.
The risk algorithm presented in Table 2, was developed using data from Caucasian subjects. Before a similar risk algorithm can be developed for non-Caucasians, we need to acquire data on the interaction between APOE and TOMM40 genotypes and disease risk in different races43.
Co-development of a prognostic test and therapeutic drug – the test
Should the treatment prove to be efficacious, the risk algorithm must be qualified in order to support prescribing by physicians. This is accomplished in this co-development program. To validate a prognostic biomarker for patient care, more is needed than demonstration of an association between a biomarker and a phenotype in a cross-sectional or retrospective study. Any prognostic biomarker must be validated in a well-powered, prospective study that clearly establishes the relationship between the biomarker and the clinical or disease outcome26 The risk algorithm described here will be validated during the conduct of a phase 3, delay-of-onset study. Upon completion of the trial, the positive and negative predictive values for the risk algorithm will be calculated by comparing the high risk, placebo-treated group with the low risk group (all treated with placebo). Knowledge of these test performance metrics is necessary for the qualification of the risk stratification algorithm for use as a prognostic biomarker in the clinic.
The risk algorithm is intended to fill an important gap in AD clinical research that currently hinders the development of therapeutics to delay or prevent the onset of pathological cognitive decline. The gap is the inability to identify individuals who are at greater risk of developing cognitive impairment, within a defined time-frame, before clinical symptoms are manifest.
Recent guidelines from National Institute on Aging-Alzheimer’s Association workgroups recommend that early symptomatic AD, termed MCI due to AD, can be diagnosed using core clinical criteria that are based in part upon neuropsychological tests and measures of subtle functional changes that will be used in the clinical trial described here13. This set of recommendations also discusses use of an imaging biomarker or a biomarker expressed in cerebrospinal fluid (CSF) for research purposes13. The delay-of-onset trial is designed as a primary prevention trial that uses recommended clinical criteria – operationalized using a battery of neuropsychological tests, informant ratings and measures capturing change in function, neurological evaluation, exclusion of medical causation, and judgment of a clinician – to select subjects clinically unaffected by the disease process and also to evaluate subjects during the course of the study. The trial will not select subjects using imaging or biochemical biomarkers, although imaging may be used to rule out other possible causes of cognitive symptoms. However, the Phase 3 study described here may provide the time course for development of clinically-assessed MCI due to AD against which expressed biomarkers, like CSF beta-amyloid or tau protein, can be evaluated. At present, these markers are not validated by regulatory agencies. It is anticipated that by the time the Phase 3 study completes, one or more expressed markers may be analytically validated at which point it would be instructive to retrospectively assess the biomarkers in the clinical trial population. It should also be noted that while this trial has been powered according to the anticipated enrichment provided by the risk algorithm for Caucasian subjects, and the endpoints will be qualified only in Caucasians, there is a need to generalize the prognostic biomarker to other populations. To this end, the trial will enroll non-Caucasians and, in parallel, independent studies of the relationship between APOE–TOMM40 haplotypes and development of MCI due to AD in non-Caucasian cohorts with accurate age of onset information will be conducted.
Co-development of a prognostic test and therapeutic drug – the drug
In addition to prospectively qualifying the risk algorithm as a companion, prognostic biomarker, the clinical trial will simultaneously test a low dose of pioglitazone (PIO), to delay the onset of mild cognitive symptoms of the AD type (i.e. MCI due to AD)13. This co-development clinical study design was first discussed during a US Food and Drug Administration (FDA) Voluntary eXploratory Data Submission process, and is now the basis of Investigational New Drug and Investigational Device Exemption submissions to the FDA and the European Medicines Agency (pending at the time of publishing) sponsored by a Zinfandel Pharmaceuticals-Takeda Pharmaceuticals alliance. The clinical trial is expected to launch in 2013.
The risk algorithm will be qualified for use as a prognostic biomarker by assessing its performance in the placebo-treated groups for predicting low and high risk subjects. The efficacy of PIO to delay the onset of MCI due to AD will be tested by comparing the incidence of MCI due to AD in the high risk, PIO-treated group versus the high risk, placebo-treated group. While this study will test PIO, the study design is suitable for testing efficacy of any therapeutic with a sound rationale for delaying the onset of MCI due to AD. Enrichment of the clinical trial with subjects at high risk for developing MCI due to AD will increase statistical power to detect a treatment effect in this risk group. PIO is currently marketed under the trade name Actos® for the treatment of type 2 diabetes mellitus (T2DM). In the delay of MCI due to AD study, PIO will be administered at a dose strength that is significantly lower than strengths marketed for treatment of type 2 diabetes mellitus (i.e. 15, 30, or 45 mg daily). We are currently evaluating in vivo changes in neural activity in response to repeated dosing to establish the minimal potentially efficacious dose to delay disease onset. A preliminary rat study showed that as a result of daily administration of very low doses of PIO (0.04 mg/kg – 0.32 mg/kg, resulting in serum exposure roughly equivalent to a daily human dose of 1.5 – 12 mg) elicited rapid changes in neural activity across the brain44. In this experiment, the pharmacodynamic marker for neural activity was resting state functional connectivity in awake rats as measured by blood-oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI)45. fMRI experiments to test the effect of PIO on human brain activity and to establish dosage are currently underway. Unlike the rat study, these experiments will report on brain activity during tasks that challenge specific cognitive functions, e.g. episodic memory, that are of particular interest in the earliest stages of cognitive impairment and in Alzheimer’s dementia46 .
The choice of PIO for the delay-of-onset trial was based on several factors; not least among them was the safety of the drug which is demonstrated by the extensive human use history in T2DM. Moreover, there is a large body of evidence demonstrating that peroxisome proliferator-activated receptor γ (PPARγ) agonists, including PIO, prevent or reverse AD pathology in a number of pre-clinical studies and modulated symptoms in small human AD treatment (not delay-of-onset or prevention) trials. The detailed mechanism underlying the salutary effects of PPARγ agonists on AD-related pathophysiologies has not been determined. However, the PPARγ receptor is widely distributed throughout the brain in all cell types and supports neurogenesis and the normal functions of neurons, microglial cells, and astrocytes. Activation by PPARγ agonists in AD ameliorates a number of pathophysiological processes, including mitochondrial dysfunction, neuroinflammation and increased amyloid burden47, 48. Transgenic mouse models of familial AD and rat models of aging have also provided important insights. PPARγ agonists improved learning and/or memory in every transgenic mouse study where this was evaluated, and also caused decreased Aβ levels and amyloid burden, reduced astrocytic and astroglial activation and, when tested, reduced oxidative stress49,50-55. In addition, Strum et al. demonstrated that orally administered ROSI penetrated the blood brain barrier and induced neuronal mitochondrial biogenesis in mice56.
Several small human trials have examined the effectiveness of a PPARγ agonist for the treatment (not the delay) of probable AD or mild cognitive impairment. These small studies indicated that ROSI or PIO could improve or stabilize cognition, cerebral blood flow, and plasma Aβ40/Aβ4257-61.
Three phase 3 studies examined the efficacy of ROSI-XR (rosiglitazone-extended release tablets) for AD. In two of these studies, REFLECT–2 and –3, ROSI-XR was adjunctive to donepezil or any acetylcholinesterase inhibitor, respectively, for 48 weeks62. In REFLECT–1, ROSI-XR was tested as monotherapy for 24 weeks63. None of the studies provided statistically significant evidence for efficacy of ROSI for treatment of frank AD. However, these studies revealed positive trends on some cognitive tests which suggest that PPARγ agonists warrant further study for AD treatment. It should also be noted that the phase 2b study of ROSI59 was limited to Caucasian subjects whereas the phase 3 studies were not.
The primary prevention, or delay-of-onset, trial described here is an important and necessary experiment. It expands our understanding of how to co-develop medicines and companion prognostic (broadly designated as “diagnostic”) tests, how to ethically and practically conduct primary prevention studies through the use of a prognostic enrichment strategy, it may prove the effectiveness of a safe drug for a new indication and, most importantly, if successful this product of this trial will significantly reduce the future burden of AD.
Acknowledgement
Dr. Welsh-Bohmer receives research support from the National Institutes of Health (NIA P30AG-028377).
Footnotes
There is a conflict of interest
Dr. Roses is CEO and owner of Zinfandel Pharmaceuticals, Inc which is in an alliance with Takeda Pharmaceuticals to test the efficacy of pioglitazone in a pharmacogenetically-stratified delay of onset clinical trial. Dr. Roses also has an appointment at Duke University Medical Center. Drs. Crenshaw, Saunders, Lutz, Gottschalk and Grossman are paid consultants for Zinfandel Pharmaceuticals. Dr. Burns is a Zinfandel Pharmaceuticals employee and Dr. Brannan is employed by Takeda Pharmaceuticals International, Inc.
References
- 1.Bateman R, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 2011;3:1–13. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashford JW, Mortimer JA. Non-familial Alzheimer’s disease is mainly due to genetic factors. J. Alzheimers Dis. 2002;4:169–77. doi: 10.3233/jad-2002-4307. [DOI] [PubMed] [Google Scholar]
- 3.Association A.s. Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–68. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 6.Gingrich N, Egge R. Developing a national Alzheimer’s strategy equal to the epidemic. Alzheimers Dement. 2007;3:239–42. doi: 10.1016/j.jalz.2007.04.378. [DOI] [PubMed] [Google Scholar]
- 7.Khachaturian ZS. Prospects for designating Alzheimer’s disease research a national priority. Alzheimers Dement. 2011;7:557–61. doi: 10.1016/j.jalz.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Birks J, Harvey R. Donepezil for dementia due to Alzheimer’s disease (Review) Cochrane Database Syst Rev. 2009 Jan 25;(1):CD001190. doi: 10.1002/14651858.CD001190. (2009) [DOI] [PubMed] [Google Scholar]
- 9.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–16. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 10.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2009 Jan 25;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McShane R, Schneider LS. Meta-analysis of memantine: Summary and commentary on the Cochrane Collaboration’s systematic review. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2005;1:67–71. doi: 10.1016/j.jalz.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012;73:504–17. doi: 10.1111/j.1365-2125.2011.04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Changing the Trajectory of Alzheimer’s Disease: A National Imperative (2010) Alzheimer’s Association; Chicago, Il: http://www.alz.org/documents_custom/trajectory.pdf. [Google Scholar]
- 15.Martin BK, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch. Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group AR. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 17.Espeland MA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 18.Rapp SR, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 19.Shumaker SA, et al. Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 20.Snitz BE, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–70. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrieu S, et al. GuidAge study: a 5-year double blind, randomised trial of EGb 761 for the prevention of Alzheimer’s disease in elderly subjects with memory complaints. i. rationale, design and baseline data. Curr. Alzheimer Res. 2008;5:406–15. doi: 10.2174/156720508785132271. [DOI] [PubMed] [Google Scholar]
- 22.Meinert CL, Breitner JC. Chronic disease long-term drug prevention trials: lessons from the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) Alzheimers Dement. 2008;4:S7–S14. doi: 10.1016/j.jalz.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Vellas B, et al. Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11:851–9. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 24.Temple R. Enrichment of Clinical Study Populations. Clin. Pharmacol. Ther. 2010;88:774–8. doi: 10.1038/clpt.2010.233. [DOI] [PubMed] [Google Scholar]
- 25.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodsaid FM, Mendrick DL. Translational Medicine and the Value of Biomarker Qualification. Sci. Transl. Med. 2010;2:47ps4. doi: 10.1126/scitranslmed.3001040. [DOI] [PubMed] [Google Scholar]
- 27.Roses AD. On the discovery of the genetic association of Apolipoprotein E genotypes and common late-onset Alzheimer disease. J. Alzheimers Dis. 2006;9:361–6. doi: 10.3233/jad-2006-9s340. [DOI] [PubMed] [Google Scholar]
- 28.Corneveaux JJ, et al. Association of CR1, CLU and PICALM with Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. Hum. Mol. Genet. 2010;19:3295–301. doi: 10.1093/hmg/ddq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberg DTA, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apolipoprotein E gene: Climate, local adaptations, and evolutionary history. Am. J. Phys. Anthropol. 2010;143:100–11. doi: 10.1002/ajpa.21298. [DOI] [PubMed] [Google Scholar]
- 30.Mayeux R, et al. Utility of the Apolipoprotein E Genotype in the Diagnosis of Alzheimer’s Disease. N Engl J Med. 1998;338:506–11. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 31.Roses AD, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2010;10:375–84. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnertz C, et al. Characterization of the Poly-T Variant in the TOMM40 Gene in Diverse Populations. Plos ONE. 2012;7:e30994. doi: 10.1371/journal.pone.0030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roses A, et al. TOMM40 “523” polyT allele frequencies in different ethnic groups. Alzheimers Dement. 2010;4:e41. [Google Scholar]
- 34.Green RC, et al. Risk of dementia among white and african american relatives of patients with Alzheimer disease. JAMA. 2002;287:329–36. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 35.Gureje O, et al. APOE ε4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann. Neurol. 2006;59:182–5. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maestre G, et al. Apolipoprotein E and Alzheimer’s disease: Ethnic variation in genotypic risks. Ann. Neurol. 1995;37:254–9. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- 37.Tang M-X, et al. The APOE-ε4 Allele and the Risk of Alzheimer Disease Among African Americans, Whites, and Hispanics. JAMA. 1998;279:751–5. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 38.Ashford J. APOE genotype effects on Alzheimer’s disease onset and epidemiology. J. Molec. Neurosci. 2004;23:157–65. doi: 10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- 39.Corder EH, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 1994;7:180–4. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 40.Johnson SC, et al. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE ε3/ε3 genotype. Alzheimers Dement. 2011;7:456–65. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caselli R, et al. Longitudinal Modeling of Cognitive Aging and the TOMM40 Effect. Alzheimers Dement. 2012;8:490–5. doi: 10.1016/j.jalz.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayden KM, et al. A homopolymer polymorphism in the TOMM40 gene contributes to cognitive performance in aging. Alzheimers Dement. 2012;8:381–8. doi: 10.1016/j.jalz.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and Alzheimer disease: A meta-analysis. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 44.Asin KE, et al. Changes in fMRI resting state functional connectivity in rats after acute and repeat dosing with pioglitazone. Presented at Alzheimer’s Association International Conference; Vancouver, Canada. 2012. [Google Scholar]
- 45.Zhang N, et al. Mapping resting-state brain networks in conscious animals. J. Neurosci. Methods. 2010;189:186–96. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wierenga C, Bondi M. Use of Functional Magnetic Resonance Imaging in the Early Identification of Alzheimer’s Disease. Neuropsychol. Rev. 2007;17:127–43. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landreth G, et al. PPARγ agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics. 2008;5:481–9. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicolakakis N, Hamel E. The nuclear receptor PPARγ as a therapeutic target for cerebrovascular and brain dysfunction in Alzheimer’s disease Front. Aging Neurosci. 2010;2:21. doi: 10.3389/fnagi.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer’s disease patients and experimental models. J. Cereb. Blood Flow Metab. 2011;31:1354–70. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du J, Sun B, Chen K, Fan L, Wang Z. Antagonist of peroxisome proliferator-activated receptor γ induces cerebellar amyloid-β levels and motor dysfunction in APP/PS1 transgenic mice. Biochem Biophys Res Commun. 2009;384:357–61. doi: 10.1016/j.bbrc.2009.04.148. [DOI] [PubMed] [Google Scholar]
- 51.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms Underlying the Rapid Peroxisome Proliferator-Activated Receptor-γ-Mediated Amyloid Clearance and Reversal of Cognitive Deficits in a Murine Model of Alzheimer’s Disease. J. Neurosci. 2012;32:10117–28. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen WA, et al. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 2006;199:265–73. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav. Brain Res. 2011;216:255–61. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Searcy JL, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2012;30:943–61. doi: 10.3233/JAD-2012-111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicolakakis N, et al. Intact memory in TGF-β1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazone. J. Cereb. Blood Flow Metab. 2011;31:200–11. doi: 10.1038/jcbfm.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strum JC, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J. Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 57.Abbatecola AM, et al. Rosiglitazone and Cognitive Stability in Older Individuals With Type 2 Diabetes and Mild Cognitive Impairment. Diabetes Care. 2010;33:1706–11. doi: 10.2337/dc09-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geldmacher DS, Fritsch T, McClendon MJ, Lerner AJ, Landreth GE. P2-408: A double-blind, placebo-controlled, 18-month pilot study of the PPAR-gamma agonist pioglitazone in Alzheimer’s disease. Alzheimers Dement. 2006;2:S366–S. [Google Scholar]
- 59.Risner ME, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6:246–54. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 60.Sato T, et al. Efficacy of PPAR-[gamma] agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging. 2010;32:1626–33. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Watson GS, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–8. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 62.Harrington C, et al. Effects of rosiglitazone-extended release as adjunctive therapy to acetylcholinesterase inhibitors over 48 weeks on cognition in APOE4-stratified subjects with mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2009;5:e17–e8. [Google Scholar]
- 63.Gold M, et al. Rosiglitazone Monotherapy in Mild-to-Moderate Alzheimer’s Disease: Results from a Randomized, Double-Blind, Placebo-Controlled Phase III Study. Dement Geriatr Cogn Disord. 2010;30:131–46. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. [Accessed Accessed: October 17, 2012];Wisconsin Registry for Alzheimer’s Prevention (WRAP) < http://www.wai.wisc.edu/research/wrap.html>.
- 65.Joseph, Bryan Kathleen. [Accessed February 13, 2012];Alzheimer’s Disease Research Center: Memory, Health, & Aging Project. 2012 < https://adrc.mc.duke.edu/index.php/research/mha-project>.
- 66.Morris JC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 67.Weintraub S, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis. Assoc. Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welsh-Bohmer KA, et al. Modifying dementia risk and trajectories of cognitive decline in aging: The Cache County Memory Study. Alzheimers Dement. 2006;2:257–60. doi: 10.1016/j.jalz.2006.04.011. [DOI] [PubMed] [Google Scholar]