Abstract
CHK2 kinase is a key mediator in many cellular responses to genotoxic stresses including ionizing radiation and topoisomerase inhibitors. Upon ionizing radiation, CHK2 is activated by ATM kinase and regulates the S-phase and G1-S checkpoints, apoptosis, and DNA repair by phosphorylating downstream target proteins such as p53 and Brca1. In addition, CHK2 is thought to be a multi-organ cancer susceptibility gene. In this study, we used a tandem affinity purification strategy to identify proteins that interact with CHK2 kinase. CDK11p110 kinase, implicated in pre-mRNA splicing and transcription, was identified as a CHK2-interacting protein. CHK2 kinase phosphorylated CDK11p110 on serine 737 in vitro. Unexpectedly, CHK2 kinase constitutively phosphorylated CDK11p110 in a DNA damage-independent manner. At a molecular level, CDK11p110 phosphorylation was required for homodimerization without affecting its kinase activity. Overexpression of CHK2 promoted pre-mRNA splicing. Conversely, CHK2 depletion decreased endogenous splicing activity. Mutation of the phosphorylation site in CDK11p110 to alanine abrogated its splicing activating activity. These results provide the first evidence that CHK2 kinase promotes pre-mRNA splicing via phosphorylating CDK11p110.
Keywords: CHK2, pre-mRNA splicing, CDK11, DNA damage response pathway
Introduction
The checkpoint kinase 2 (CHK2) was first identified as a mammalian homolog of the Saccharomyces cerevisiae Rad53 and Schizosaccharomyces pombe Cds1 checkpoint genes (1). CHK2 is a key mediator in cellular responses to genotoxic stresses including ionizing radiation and topoisomerase inhibitors. CHK2 kinase is strongly and rapidly activated when DNA is damaged or when DNA breaks are induced by ionizing radiation, chemotherapeutic agents, or other compounds that harm DNA directly or indirectly (2–5). Signals from damaged DNA are transmitted to the ATM kinase, which has a number of substrates involved in the checkpoint cascade. Upon ionizing radiation (IR), CHK2 is activated by phosphorylation at threonine 68 in an ATM-dependent manner. This propagates the checkpoint signal, causing cell cycle arrest in the G1, S, and G2/M phases, activation of DNA repair, and in some cases, programmed cell death (6, 7). In response to DNA damage, the CHK2 protein phosphorylates several proteins including p53, Cdc25A, Cdc25C, Brca1 and PML (8–13). These proteins halt cell division and determine whether a cell will repair the damage or undergo apoptosis. This prevents cells with mutated or damaged DNA from dividing, which helps maintain genomic stability. The ATR kinase similarly phosphorylates CHK2 after high levels of DNA damage or treatment with other agents such as ultraviolet radiation (UV) or the ribonucleotide reductase inhibitor hydroxyurea (14). CHK2−/− ES (embryonic stem) cells are defective in maintaining IR-induced G2 arrest, and mouse CHK2−/− thymocytes exposed to IR fail to stabilize p53 and induce G1 arrest and apoptosis (6, 8). CHK2 loss-of-function mutations occur in various types of cancer, and functionally defective variants of CHK2 cause a predisposition to familial breast and prostate cancer (15). Recently, DNA damage-independent functions of CHK2 were reported to be required for proper progression of mitosis, and for the maintenance of chromosomal stability in human somatic cells (16).
Cyclin-dependent kinase 11 (CDK11) is expressed as two predominant protein isoforms designated by their apparent molecular masses (p110 and p58). These kinases were named CDK11p110 and CDK11p58 because of their interaction with the regulatory subunit cyclin L (17). The CDK11p110 and CDK11p58 isoforms are produced from the same mRNAs through use of an internal ribosome entry site and two different AUG codons located in the coding sequence of the CDK11p110 mRNAs (18). The larger CDK11p110 isoform associates with cyclin L and is involved in pre-mRNA splicing and transcription regulation. The CDK11p110 protein kinases interact with numerous proteins involved in the production of RNA transcripts during proliferation, including the largest subunit of RNA polymerase II and casein kinase II (19). In transcription, the CDK11p110 isoform interacts with the transcriptional elongation factors ELL2, TFIIF, TFIIS (SII), and FACT. CDK11p110 also interacts with multiple proteins involved in RNA splicing, including RNPS1 and 9G8, and phosphorylates the splicing factor 9G8 (20, 21). CDK11p58 is an essential regulator of mitosis, required for centriole duplication, centrosome maturation, bipolar spindle assembly, maintenance of sister chromatid cohesion and cytokinesis (22–25). CDK11p58, in association with cyclin D3, is reported to negatively affect androgen receptor transcriptional activity, whereas CDK11p110 positively affects transcription of numerous reporter genes (26). CDK11 was identified as a positive regulator of hedgehog signaling in both fly and vertebrate cells (27).
To investigate the functions of CHK2, we performed a tandem purification analysis with CHK2. CDK11p110, implicated in pre-mRNA splicing, was identified as a CHK2-interaction protein and was phosphorylated on serine 737 by CHK2 in vitro and in vivo. We demonstrated that CDK11p110 was phosphorylated in a CHK2-dependent and DNA damage-independent manner. Homodimerization of CDK11p110 was significantly reduced in CHK2 knockdown cells, compared to in control cells. In addition, CDK11p110 S737A mutant protein did not form a homodimer, suggesting that CHK2-dependent phosphorylation of CDK11 was required for homodimerization. Overexpression of CHK2 promoted pre-mRNA splicing and pre-mRNA splicing activity was decreased in CHK2 knockdown cells compared to control cells. Wild-type CDK11p110 enhanced pre-mRNA splicing in vivo, but the phosphorylation site mutant CDK11p110 did not. Taken together, our data provide for the first time, evidence for the constitutive functions of CHK2 and its roles in pre-mRNA splicing.
Results
CHK2 specifically interacts with CDK11p110
To identify cellular factors involved in CHK2 function, we performed a TAP purification using CHK2 as bait. The TAP tag contains protein-A- and calmodulin-binding motifs to permit purification of tagged proteins on IgG-agarose and calmodulin-Sepharose, respectively (28). The N-terminally TAP-tagged CHK2 was transiently expressed in 293T cells, and the TAP-CHK2 and its associated proteins were purified by successive chromatography on IgG-agarose and calmodulin-Sepharose to remove nonspecific proteins. The purified preparations were subjected to electrophoresis on a SDS-PAGE gel, which was silver-stained. The identity of protein bands was established by LC-mass/mass spectrometry. A protein with an apparent molecular mass of 110 kDa was identified as CDK11p110, which is implicated in pre-mRNA splicing and transcription (Fig. 1A, Supplementary Fig. S1, Supplementary Table 1). While many other proteins were co-purified with TAP-CHK2 protein, protein bands were hardly observed in TAP protein which we used as a negative control (Supplementary Fig. S1). In addition, CDK11p110 was not detected in TAP-CHK1, TAP-MRE11 or TAP-SMC1 complexes, suggesting the interaction between CHK2 and CDK11p110 was specific.
Figure 1. CHK2 interacts with CDK11p110.
A, TAP-CHK2 complexes from 293T cells transfected with pcDNA-TAP-CHK2 were purified using a TAP method. Purified proteins were separated on SDS-PAGE and identified by electrospray ion trap mass spectrometry. B, 293T cells were co-transfected with HA-CDK11p110 and Flag-CHK2. After 48 h, cells were lysed and immunoprecipitated with anti-HA antibody. Immunoprecipitates were analyzed by immunoblotting with anti-HA or anti-FLAG antibody. C, Co-immunoprecipitation assay with anti-CHK2 antibody or IgG control and blotted with antibodies against CHK2 and CDK11. D, Co-immunoprecipitation assay with anti-CDK11 antibody or IgG control and blotted with antibodies against CHK2 and CDK11.
To confirm the results of TAP purification, the interaction of CHK2 with CDK11p110 was assessed using co-immunoprecipitation. HA-CDK11p110 and FLAG-CHK2 or empty vector were co-transfected into 293T cells and cell lysates were immunoprecipitated with anti-HA antibody, followed by western blot analysis with anti-FLAG and anti-HA antibodies. As shown in Figure 1B, Flag-CHK2 co-immunoprecipitated with HA-CDK11p110. In addition, endogenous CDK11p110 and CHK2 proteins were co-precipitated with anti-CHK2 antibodies (Fig. 1C). This was also confirmed with a reverse immunoprecipitation with anti-CDK11 antibodies (Fig. 1D).
CDK11p110 is an in vitro target of CHK2 kinase
Previously, we reported a phosphorylation site motif for CHK2 kinase (29). As CDK11p110 interacts with CHK2 and has six potential CHK2 phosphorylation sites (Fig. 2A), we assessed whether CHK2 phosphorylated CDK11p110 in vitro. To test whether the sites could be phosphorylated by CHK2, GST-fusion CDK11p110 peptide expression vectors were constructed using oligonucleotides encoding 11 amino acids of CDK11p110 that were cloned into pGEX-4T-1. In vitro kinase assays with immunoprecipitated FLAG-CHK2 was performed with GST-peptides as substrates. Of six GST-peptide proteins, three were phosphorylated by CHK2 in vitro (Fig. 2B). To further verify the phosphorylation of CDK11p110 by CHK2, we constructed two truncated GST- CDK11p110 proteins including these three sites. While GST-CDK11 (amino acids 22–204) was little phosphorylated by CHK2, GST-CDK11 (amino acids 699–780) was phosphorylated by CHK2 (Fig. 2C). Because of multiple CDK11 transcripts, amino acid residues are numbered based on the amino acid sequence of CDK11 isoform 1 [NP_076916]. To further confirm the phosphorylation site of CDK11p110 by CHK2, we constructed a truncated GST-CDK11p110 protein with an alanine substitution at serine 737, the potential CHK2 phosphorylation site. While wild-type CDK11p110 was well phosphorylated, the S737A mutant was minimally phosphorylated by CHK2 (Fig. 2D). This suggested that serine 737 was the major phosphorylation site for the CHK2 kinase in vitro. CHK2 and another checkpoint kinase, CHK1 contain highly conserved kinase domains and target some overlapping substrates. These kinases showed similar substrate specificity in vitro (30). We then tested whether CHK1 phosphorylates CDK11p110 in vitro. While CHK1 phosphorylated well-known substrate, GST-CDC25A peptides, it did not phosphorylate GST-CDK11p110 (amino acids 699–780) proteins (Supplementary Fig. S2). Taken together, these results suggested that CDK11p110 was a substrate for CHK2 and that serine 737 of CDK11p110 was specifically phosphorylated by CHK2 in vitro.
Figure 2. CHK2 phosphorylates CDK11p110 in vitro on serine 737.
A, Potential phosphorylation sites of CDK11p110 by CHK2. B, Immunoprecipitated FLAG-CHK2 were incubated with a fixed amount of Escherichia coli-expressed fusion proteins between GST and peptides from various regions of CDK11p110. Amino acid positions corresponding to each peptide are in the upper part of the panel. In vitro kinase assays were performed as described in Materials and Methods. C, In vitro kinase assays with immunoprecipitated FLAG-CHK2 wild-type or kinase-inactive proteins were performed with GST-truncated CDK11p110 (amino acids 44–204 or 699–780) as substrates. D, Immunoprecipitated wild-type or kinase-inactive FLAG-CHK2 were incubated with GST or GST-CDK11p110 (amino acids 699–780) wild-type or S737A.
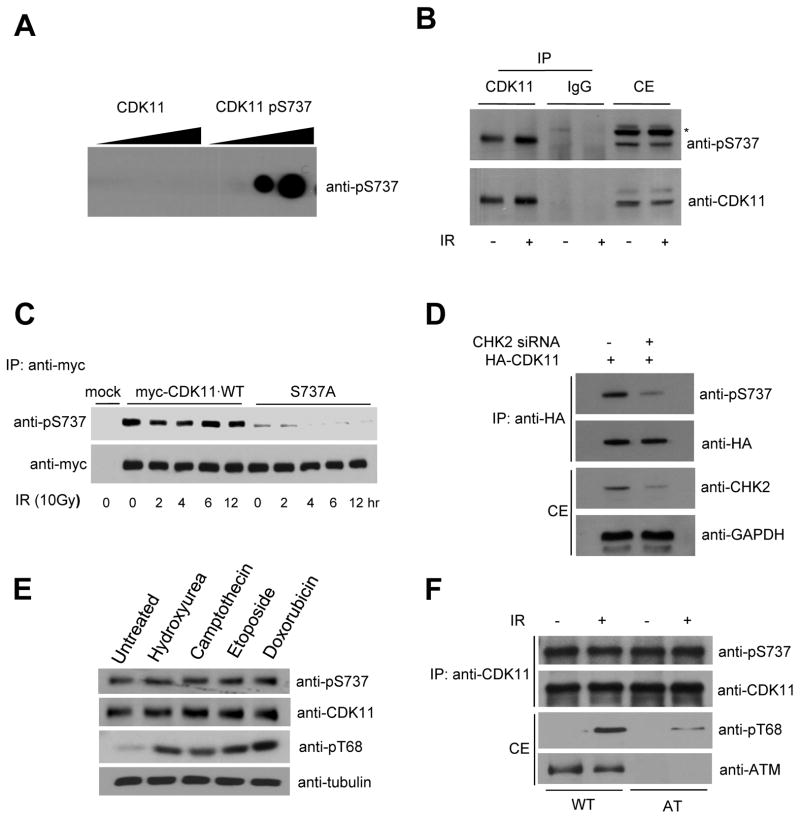
CDK11p110 is constitutively phosphorylated on serine 737 in a CHK2-dependent and DNA damage-independent manner
We then raised a phosphospecific antibody that specifically recognized CDK11p110 when serine 737 was phosphorylated. The specificity of the phosphospecific pS737 antibody was demonstrated by its ability to recognize a peptide sequence surrounding the target serine when phosphorylated, but not when dephosphorylated (Fig. 3A). To investigate whether serine 737 was phosphorylated in vivo and whether phosphorylation was DNA damage-dependent, we examined the phosphorylation of this residue in endogenous CDK11p110 in untreated or IR-treated 293T cells. Unexpectedly, CDK11p110 was constitutively phosphorylated and no changes in the phosphorylation states of CDK11p110 protein were seen after IR (Fig. 3B). The protein levels of CDK11p110 were also not changed by IR. To confirm these results, the 293T cells were transfected with myc-CDK11p110 wild-type or S737A mutant expression vectors and irradiated with 10 Gy. Cells were lysed after irradiation and lysates were immunoprecipitated with anti-myc and phosphorylated CDK11p110 was detected using anti-pS737 antibody. The anti-pS737 antibody recognized wild-type, but not CDK11p110 S737A. No changes in CDK11p110 phosphorylation upon DNA damage were detected (Fig. 3C). Phosphorylation on serine 737 of CDK11p110 was also detected in unirradiated 293T cells by LC-Mass/Mass spectrometry (Supplementary Fig. S2). In addition to serine 737, serine 222, 283, 285, 381 748, threonine 736 and tyrosine 747 were phosphorylated in vivo (Supplementary Table 2). To determine whether the absence of endogenous CHK2 affected phosphorylation of CDK11p110, 293T cells were transfected control siRNAs or CHK2 siRNAs with HA-CDK11p110 plasmids. CHK2 depletion by siRNA against CHK2 abolished CDK11p110 phosphorylation but control siRNAs did not block CDK11p110 phosphorylation (Fig. 3D).
Figure 3. CDK11p110 is phosphorylated on serine 737 in a CHK2-dependent and DNA damage-independent manner.
A, Immunoblots with 0–1 μg of phosphorylated or unphosphorylated synthetic peptides probed with rabbit polyclonal antibodies against peptides containing phosphoserine 737. B, Endogenous phosphorylation of CDK11p110. For immunoprecipitation of CDK11p110, cell extracts from untreated and IR-treated 293T cells were incubated with anti-CDK11 antibody bound to protein G/A beads. Phosphorylation of endogenous CDK11p110 on serine 737 was monitored by immunoblotting with anti-pS737. The level of immunoprecipitated CDK11p110 and input CDK11p110 protein is shown. To induce DNA damage, cells were irradiated with 10 Gy of IR and recovered for 4 hr. C, Antibody specificity was verified by substitution of the phosphorylation site. After IR, 293T cells transfected with myc-CDK11p110 wild-type or S737A expression vectors were probed with anti-pS737 antibody (top panel). Bottom panel shows myc-CDK11p110 level determined by immunoblotting with anti-myc antibody. D, CHK2-dependent phosphorylation of CDK11p110 in vivo. 293T cells were co-transfected with HA-CDK11p110 and control siRNA or CHK2 siRNA. At 48 hr after transfection, cells were immunoprecipitated with anti-HA and western blotting performed with indicated antibodies. E, Immunoblot analyses using phospho-specific antibody (pS737) or anti-CDK11 antibody on CDK11 protein from human diploid fibroblast treated with hydroxyurea (1mM, 12 hrs), camptothecin (2 μM, 12 hrs), etoposide (2 μM, 12 hrs) or doxorubicin (0.5 μM, 12 hrs). pT68 CHK2 phospho-specific antibody was used to show the phosphorylation states of CHK2 under these treatments. F, Immunoblot analyses for CDK11 phosphorylations 4hr after exposure to 0 (−) or 10 (+) Gy of ionizing irradiation in lymphoblasts, GM0536 (WT) and GM01526 (A–T).
Since some endogenous DNA damages or other stresses in 293T cells can slightly activate CHK2 kinase without exogenous DNA damage and phosphorylate CDK11p110, we examined CDK11p110 phosphorylation in early passage normal, diploid, non-immortalized and unstressed human fibroblast (HF) cells. In consistent with above results, constitutive phosphorylation of CDK11p110 could be observed in these cells (Fig. 3E). In addition, other cellular stresses such as replication blockage or other DNA damages also did not affect CDK11p110 phosphorylation. CHK2 proteins were normally phosphorylated on T68 by these treatments.
ATM kinase is upstream of CHK2 kinase in a DNA damage response pathway, so we determined whether ATM is required for the CHK2-dependent CDK11p110 phosphorylation. CDK11p110 was constitutively phosphorylated in A-T cells at a similar level as in normal cells, suggesting that ATM is dispensable for CDK11p110 phosphorylation (Fig. 3F). Taken together, these results suggested that serine 737 of CDK11p110 was specifically phosphorylated in vivo and CHK2 was required for this phosphorylation in a DNA damage-independent manner.
CHK2-dependent phosphorylation is required for CDK11p110 homodimerization
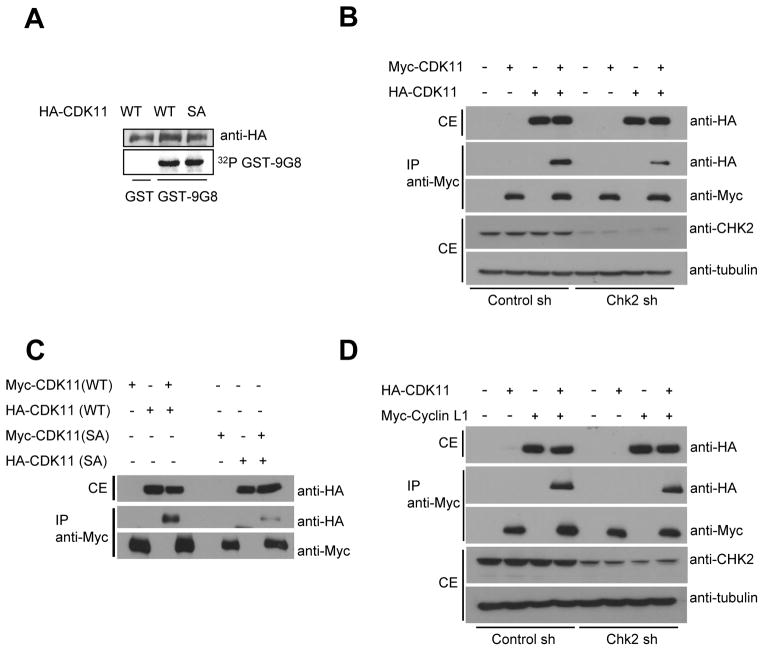
The functional consequences of CDK11p110 phosphorylation were explored. To test whether CHK2-dependent phosphorylation of serine 737 of CDK11p110 affected CDK11p110 kinase activity, HA-tagged CDK11p110 wild-type or S737A vectors were transfected into 293T cells, and immunoprecipitated HA-tagged CDK11p110 activity was assessed using GST-9G8 as a substrate. CDK11p110 S737A mutant protein phosphorylated GST-9G8 at a rate similar to wild-type CDK11p110 (Fig. 4A), suggesting that CHK2-dependent phosphorylation of CDK11p110 did not affect CDK11p110 kinase activity. We then assessed whether phosphorylation affected homodimerization of CDK11p110. The CDK11 mitotic form, CDK11p58, is reported to form a homodimer (31). We assessed whether the phosphorylation of CDK11p110 affected homodimerization. Myc-tagged CDK11p110 and HA-tagged CDK11p110 were co-transfected into a CHK2 knockdown and control cell lines, and proteins were evaluated for co-immunoprecipitation. Dimerization of CDK11p110 proteins was significantly reduced in CHK2 knockdown cell lines compared to control cell lines, suggesting that CHK2 was required for CDK11p110 dimerization. To test whether CHK2-dependent phosphorylation of serine 737 of CDK11p110 affected dimerization, Myc-tagged CDK11p110 and HA-tagged CDK11p110 wild-type or S737A mutant plasmids were co-transfected into 293T cells. Dimerization between Myc-tagged CDK11p110 S737A and HA-tagged CDK11p110 S737A was completely abrogated (Fig. 4C). Cyclin L is known to bind CDK11p110 and be required for CDK11p110 catalytic activity. We investigated whether CHK2 was required for interaction between CDK11p110 and cyclin L. In CHK2 knockdown cell lines, CDK11p110 interacted with Cyclin L to an extent similar to control cell lines.
Figure 4. Phosphorylation of CDK11p110 inhibits homodimerization, but not kinase activity or cyclin L binding.
A, Mutation of serine 737 to alanine did not affect kinase activity. In vitro kinase assays with immunoprecipitated HA-CDK11p110 wild-type or S737A proteins were performed with GST-9G8 as a substrate. B, Dimerization of CDK11p110 decreased in stable CHK2 knockdown cell lines, compared to control cells. C, Mutation of serine 737 to alanine abrogated homodimerization. D, Knockdown of CHK2 did not affect CDK11p110 binding to cyclin L. Knockdown efficiencies of CHK2 in CHK2 knockdown cells were by immunoblot using anti-CHK2 antibodies.
Phosphorylation of CDK11p110 by CHK2 promotes pre-mRNA splicing
To understand the roles of phosphorylation of CDK11p110 in cells, we purified CDK11p110 complexes. Nuclear extracts were subjected to immunoprecipitation with anti-CDK11 antibodies, followed by SDS-PAGE electrophoresis. Protein bands were visualized with silver stain (Supplementary Fig. S4) and identified by tryptic peptide MS analysis. Many splicing factors including snRNP and SR proteins were identified as components of CDK11p110 complexes (Supplementary Table 3), suggesting that splicing may be a major function of CDK11p110. CDK11p110 is known to activate pre-mRNA splicing and regulate alternative splicing both negatively and positively through interaction with associated factors in vivo. Thus, we tested whether phosphorylation of CDK11p110 affected its pre-mRNA splicing activity. For the in vivo splicing assay, fragments of the rabbit β-globin gene containing two exons and one intron from pBSAL4 were amplified by PCR and subcloned into a pcDNA3 vector. The pre-mRNA transcribed from this reporter plasmid contains an intron of 319 nucleotides and 5′- and 3′-exon lengths of 56 and 53 nucleotides, respectively. Empty vector and FLAG-CHK2 plasmid were co-transfected with rabbit β-globin reporter plasmid into 293T cells. After 48 hours, the extent of splicing was detected by RT-PCR. Overexpression of CHK2 increased splicing of the rabbit β-globin gene pre-mRNA (Fig. 5A). We then assessed whether CDK11p110 phosphorylation affected pre-mRNA splicing activity. Wild-type CDK11p110 promoted rabbit β-globin pre-mRNA splicing but the S737A mutant did not (Fig. 5B). Recently, luciferase-based reporter systems have been developed for high efficiency in vivo splicing assay (32, 33). Two different in vivo splicing reporter vectors, pTN24 and Luc-I, with different exon-intron combinations were used to investigate the effects of CDK11p110 phosphorylation on pre-mRNA splicing in vivo. The pTN24 vector contains reporter genes for β-galactosidase and luciferase, which are fused in-frame via a recombinant fragment of Ad2 and human αs-tropomyosin genes (32). The pTN24 reporter vector and different amounts of CHK2 expression vector were co-transfected into 293T cells. After 24 hr, β-galactosidase and luciferase activities were measured. Unspliced transcripts produced by the pTN24 construct yielded a protein with only β-galactosidase activity, whereas spliced intronless transcripts yielded a fusion protein with both β-galactosidase and luciferase activities. Transient expression of CHK2 augmented the endogenous intron splicing activity in a dosage-dependent manner (Fig. 5C). We then assessed whether CHK2 depletion affected splicing activity in vivo, using the Luc-I splicing reporter vector which contains different exon-intron structures from the pTN24 vector. In the Luc-I system, the ORF of luciferase is interrupted by a chimeric β-globin/inmmunoglobulin intron (33). The unspliced form produces a truncated protein without enzyme activity because of several stop codons in the intron region. The Luc-I vector and CMV-β-gal vector were co-transfected into CHK2 knockdown and control cells. The endogenous splicing activity was dramatically reduced in CHK2 knockdown cells compared to control cells (Fig. 5D). The knockdown efficiency of CHK2 in CHK2 knockdown cells was shown in Supplementary Fig. S5. The knockdown of CHK2 did not affect the endogenous protein level of CDK11p110.
Figure 5. CHK2-dependent phosphorylation of CDK11p110 promotes pre-mRNA splicing.
A, 293T cells were co-transfected with empty vector or CHK2 expression vector and rabbit β-globin reporter plasmid. After 48 hrs, total RNA was prepared and cDNA was synthesized. Unspliced or spliced forms of rabbit β-globin were detected by PCR. B, 293T cells were transfected with empty vector or CDK11p110 wild-type or S737A expression vector and rabbit β-globin reporter plasmid. Spliced bands were detected by PCR. C, FLAG-CHK2 and empty vectors were transiently co-expressed in 293T cells with the intron-splicing double reporter plasmid pTN24 for 24 hr. Ratios of luciferase to β-galactosidase activities were calculated and expressed as relative splicing activities with the activity of the empty vector cells set at 1. D, Luc-I plasmid and CMV-β-gal plasmids were co-transfected into a CHK2 knockdown HeLa cell line or control HeLa cell line. Luciferase assay was performed 24 hr after transfection. E, siRNA-resistant CHK2 expression vector or empty vector with Luc-I reporter and CMV-β-gal vectors were co-transfected into CHK2 knockdown cells. Luciferase assay was performed 48 hr after transfection. F, Reporter plasmid and pcDNA, CDK11p110 wild-type or S737A mutant plasmids were co-transfected into 293T cells. Luciferase assay was performed 24 hr after transfection. Error bars denote standard deviations for more than three independent experiments. ** p bold> 0.005, * p < 0.01.
In addition, transient expression of shRNA-resistant CHK2 in CHK2 knockdown cells restored splicing activity to a level similar to control cells (Fig. 5E). Co-transfection of CDK11p110 S737A plasmid with Luc-I reporter was used to evaluate the effects of CDK11p110 phosphorylation on splicing compared to co-transfection of Luc-I with CDK11p110 wild-type plasmid. CDK11p110 wild-type enhanced splicing more than two fold, but this effect was significantly reduced with CDK11p110 S737A mutant expression (Fig. 5F). Taken together, these results suggested that phosphorylation at serine 737 of CDK11p110 by CHK2 was important for pre-mRNA splicing activity.
Discussion
CHK2 is an important multifunctional player in the DNA-damage response signaling pathway. Since the discovery of CHK2, much effort has focused on identifying in vivo target proteins in the DNA damage response pathway. As a result, many target proteins including p53 have been identified and the functions of these phosphorylation events have been studied (Fig. 6A). In addition to studies on the functions and regulation of CHK2 kinase in the DNA damage-response pathway, mutations of CHK2 gene in both somatic and hereditary human cancers have been investigated. CHK2 1100delC, a truncated variant that abrogates kinase activity, was found to be a low-penetrance breast cancer susceptibility allele in individuals who do not carry mutations in BRCA1 or BRCA2 (34). Mutations of CHK2 are found in prostate cancer patients, suggesting that mutations in CHK2 may contribute to prostate cancer risk (35). The missense variant I157T of CHK2 was associated with an increased risk of various types of cancers, including breast, colon, kidney, prostate, and thyroid cancers (36). From many studies, CHK2 is thought to be a multi-organ cancer susceptibility gene. Here we report the identification of CDK11p110 as a new binding partner for CHK2 kinase. To identify cellular factors involved in this new function of CHK2, we performed a TAP purification method using CHK2 as bait and found that CDK11p110 co-immunoprecipitated with CHK2. CDK11p110 is reported to associate with multiple proteins involved in RNA splicing, including RNPS1, 9G8 and cylin L, and to phosphorylate the splicing factor 9G8 in vitro (17, 20, 21). Overexpression of CDK11p110 in cultured cells increases in vivo splicing, whereas overexpression of a catalytically inactive form of CDK11p110 inhibits splicing (17). Immunodepletion of the CDK11p110 kinase from nuclear extracts greatly reduces the in vitro splicing activity, whereas re-addition of the CDK11p110 immunoprecipitates rescues splicing activity (20). Recently, CDK11p110 and 9G8 were shown to interact with the eukaryotic initiation factor 3 subunit f (eIF3f) and together alter the 3′ processing of the HIV-1 pre-mRNA (37).
Figure 6. Schematic representation of functions of CHK2 kinase in a DNA damage-dependent pathway and independent pathway.

In DNA damage-dependent pathway, Chk2 is activated by ATM kinase in response to ionizing radiation. Activated CHK2 phosphorylates a number of downstream target proteins, which results in the activation of cell cycle arrest, DNA repair or apoptosis. CHK2 can constitutively phosphorylate CDK11 and promotes pre-mRNA splicing in a DNA damage-independent and ATM-independent manner. During mitosis, CHK2 phosphorylates BRCA in absence of DNA damage and contributes chromosomal stability.
As CDK11p110 has several potential phosphorylation sites for CHK2, we determined whether CHK2 phosphorylates CDK11p110. In vitro kinase assays showed that serine 737 of CDK11p110 is phosphorylated by CHK2 in vitro. Surprisingly, immunoblot analysis with phospho-specific antibody against pS737 of CDK11p110 showed that CDK11p110 was constitutively phosphorylated in a CHK2-dependent but DNA damage-independent manner. Why DNA damage did not affect CDK11p110 phosphorylation is currently unclear. A possible explanation is that the CHK2 kinase responsible for CDK11p110 phosphorylation might be not activated by DNA damage, possibly because of its localization, interaction between CHK2 and CDK11p110, or requirement for other regulatory proteins. These possibilities are still under investigation. Until recently, most studies on CHK2 have focused on its functions in DNA damage-related signaling pathways. Although CHK2 has a relatively high constitutive kinase activity, compared to other kinases whose activities are activated by signals, the role of CHK2 in normal cells is poorly understood. A recent paper reported that the DNA damage-independent functions of CHK2 are required for proper progression of mitosis, and for the maintenance of chromosomal stability in human somatic cells (16).
To help understand the functions of CDK11p110 phosphorylation at the molecular level, we examined whether the phosphorylation of CDK11p110 affected its kinase activity, homodimerization, or binding to cyclin L. While kinase activity or binding to cyclin L was not affected by phosphorylation states of serine 737 of CDK11p110, phosphorylation on serine 737 was required for homodimerization of CDK11p110. However, whether or how CDK11p110 dimerization affects its function requires further investigation. To understand role of phosphorylation of CDK11p110 in cells, CDK11p110 complexes were purified using anti- CDK11 antibody. Many splicing-related factors including snRNP and SR proteins such as 9G8 were identified as components of CDK11p110 complexes. The fact that most of the CDK11p110-interacting proteins were splicing-related proteins, suggested that splicing could be a major function of CDK11p110. To determine whether phosphorylation of CDK11p110 affected pre-mRNA splicing activity, we used three different in vivo splicing assays (β-globin, pTN24, and Luc-I). Overexpression of CHK2 increased splicing of the pre-mRNA of the rabbit β-globin gene. In the pTN24 splicing assay, CHK2 promoted endogenous splicing in a dosage-dependent manner. Conversely, the endogenous splicing activity was dramatically reduced in CHK2 knockdown cells compared to control cells. In addition, transient expression of a shRNA-resistant CHK2 in CHK2 knockdown cells restored splicing activity to a level similar to control cells. These data suggested that CHK2 promoted pre-mRNA splicing in vivo. To determine whether CHK2 promoted splicing via phosphorylation of CDK11p110 on serine 737, the effects of CDK11p110 S737A on splicing were evaluated and compared to wild-type. While wild-type CDK11p110 enhanced pre-mRNA splicing more than two fold, S737A mutant CDK11p110 did not enhance endogenous splicing activity. We conclude that CHK2 protein kinase activity directly influences pre-mRNA splicing in vivo through phosphorylation of CDK11p110, thus providing a potential link between CHK2 deficiency and cancer.
In eukaryotes, pre-mRNA splicing is often precisely regulated in response to tissue-specific, physiologic, or developmental signals (38). Gene-specific regulatory elements and their trans-acting factors have been individually studied. However, the general mechanisms of constitutive and alternative splice site selection are still poorly understood. In addition, a number of RNA-binding proteins with roles in alternative splicing regulation, as well as other RNA-processing events, have been identified as disease-associated genes, particularly in neurodegenerative disorders and cancer (38, 39). Regulatory mechanisms of splicing machinery are not fully understood. Many studies have suggested the functional connection between pre-mRNA splicing and the cell cycle (40). Like transcription of many genes, pre-mRNA splicing is also repressed during mitosis. Since CHK2 is activated during mitosis and activates CDK11 p110 by an unknown mechanism, it is not likely that CHK2-dependent regulation of CDK11 p110 is involved in the mitotic repression of pre-mRNA splicing. However, we can not rule out the possibility that CDK11 may be repressed during mitosis by other regulatory mechanisms. While CDK11p110 is expressed at constant levels during the cell cycle, the shorter CDK11p58 is expressed only during G2 and M phases. Since CDK11p58 also contain serine 737, it is conceivable that CDK11p58 is also regulated by CHK2. During mitosis, CDK11p58 plays many roles such as centriole duplication, centrosome maturation, bipolar spindle assembly, maintenance of sister chromatid cohesion and cytokinesis (22–25). It will be interesting to examine whether CHK2 regulates the functions of CDK11p58 during mitosis. This work will give important clues about the mitotic functions of CHK2 kinase.
Here we presented evidence supporting a molecular signaling mechanism governing the activation of CDK11p110, which enables modulation of splicing activity. These results provide the first evidence that Chk2 protein kinase activity directly influences pre-mRNA splicing in vivo through phosphorylation of CDK11p110 (Fig. 6B). Our study provides new clues to the understanding of Chk2-mediated pre-mRNA splicing. Much remains to be learned about the molecular mechanisms and the regulatory network of pre-mRNA splicing. How phosphorylation of CDK11p110 or Chk2 kinase activity affects pre-mRNA splicing remains to be investigated. Chk2 is not likely to nonspecifically regulate splicing events. Future work should focus on the identification of endogenous target genes whose splicing is regulated by Chk2 kinase. Elucidating these will help understand the functions of Chk2 kinase in cancer and develop a new strategy for anti-cancer drugs.
MATERIALS AND METHODS
Cell Culture and Antibodies
HEK293T, HeLa and early passage human diploid fibroblast cell lines were cultured in Dulbecco’s modification of Eagle’s medium (Gibco-BRL) supplemented with 10% fetal bovine serum at 37°C in 5% CO2. Transient transfection of HEK293T cells was performed using Effectene transfection reagent (Qiagen) according to the manufacturer’s instructions. Epstein-Barr virus-immortalized lymphoblastoid cell lines from healthy persons (GM0536; NIGMS Human Mutant Cell Repository) and from persons who were homozygous for the ATM mutation (GM 1526) were cultured in RPMI 1640 supplemented with 15% fetal bovine serum. Cells were irradiated with a 137Cs source at a dose rate of 0.9 Gy per min.
Monoclonal anti-HA and anti-c-myc were from Roche Applied Science. Anti-FLAG was from Sigma, anti-CDK11 was from Bethyl and anti-CHK2 was from Upstate. The polyclonal anti-pS737 antibody was prepared by immunization of rabbit with a peptide containing phosphoserine 737 (EQQRVKRGTpSPR).
Construction of plasmids and GST-fusion protein purification
For the construction of the TAP-CHK2 expression plasmid, the open reading frame (ORF) of CHK2 was obtained by PCR amplification and cloned into the pcDNA3-TAP vector. To construct the entry vector of CDK11, the ORF of CDK11 was obtained by PCR amplification using HA-CDK11p110 (a gift from Dr. R. Vaillancourt) as a template, and cloned into pENTR1A (Invitrogen). For construction of CDK11 expression plasmids, pENTR1A-CDK11p110 underwent LR recombination with pDEST-SG5-HA and pDEST-SG5-flag destination vectors. Truncated CDK11p110 genes were obtained by PCR amplification and subcloned into pGEX4T-1. GST-peptides and GST-truncated proteins were induced by 0.5 mM isopropyl-1-thio-β-D-galactopyranoside and purification carried out using a bulk GST purification module (GE Healthcare) according to the manufacturer’s instructions. Substitution of serine 737 with alanine was achieved by site-directed mutagenesis PCR with the HA-CDK11p110 wild-type construct as a template and the mutagenesis primers 5′-gtgaagcggggcaccgccccgaggccccct-3′ and 5′-gcctcctcagggggcctcggggcggtgcc-3′. The mutation was confirmed by DNA sequencing. For the construction of pcDNA-Rb-β-globin, fragments of the rabbit β-globin gene containing two exons and one intron from pBSAL4 (41) were amplified by PCR and subcloned into a pcDNA3 vector.
Purification and identification of TAP-CHK2 complexes
Complexes of TAP-CHK2 were purified using the TAP purification method as described previously (28). 293T cells in 10 × 100 mm cell culture dishes were transfected with pcDNA3-TAP-CHK2 or pcDNA3-TAP as a negative control, using the calcium phosphate precipitation method. Cells were harvested at 2 days post transfection, and cell lysates were prepared by resuspending cells in IPP150 lysis buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% NP-40 and 1 mM dithiothreitol) containing protease inhibitors (10 μg/ml PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 10 μg/ml pepstatin A). TAP-CHK2 and the interacting proteins were purified by overnight incubation of cell lysates with 100 μl (packed volume) of immunoglobulin G (IgG)–Sepharose (Amersham) at 4°C. IgG-Sepharose was washed three times with IPP150 lysis buffer and twice with TEV cleavage buffer (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.1%NP-40, 1 mM dithiothreitol, and 0.5 mM EDTA). CHK2 and interacting proteins were released by cleavage with TEV protease (Invitrogen). The supernatant was retrieved and mixed with three volumes of calmodulin binding buffer (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, 10 mM β-mercaptoethanol, 1 mM magnesium acetate, 1 mM imidazole, 2 mM CaCl2, and 0.1% NP-40) and calmodulin beads (Stratagene, La Jolla, CA). This was incubated for 4 hr at 4 °C, with rotation. After incubation, beads were washed three times with calmodulin binding buffer and eluted twice with calmodulin elution buffer (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, 10 mM β-mercaptoethanol, 1 mM imidazole, 2 mM EGTA, and 0.1% NP-40). The eluent was precipitated with trichloroacetic acid and the pellet was washed twice with ice-cold acetone. TAP-CHK complexes in the eluent were separated by SDS-PAGE electrophoresis. To identify proteins, gels were stained with silver and protein bands excised, destained and digested in the gel with trypsin. Extracted peptides were analyzed by LC-mass/mass spectrometry.
Immunoprecipitations and immunoblots
Cells were lysed in NETN lysis buffer containing 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium fluoride, 1 mM sodium orthovanadate and 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1μg/ml pepstatin A for 1 hr at 4°C. Following lysis, cells were centrifuged at 13,000 rpm for 10 min at 4°C. Total cell extracts were incubated with appropriate antibodies for 4 hr at 4°C and protein G plus/protein A-agarose (Calbiochem) was added, and the incubation was continued for 2 hr. After incubation, immunoprecipitates were washed three times with NETN lysis buffer and subjected to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with TBS containing 5% skim milk and 0.1% Tween 20 for 30 min at room temperature (RT). Membranes were incubated with appropriate primary antibody for 2 hr at RT. After washing with TBS containing 0.1% Tween 20 three times for 10 min, membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hr at RT. After washing with TBS containing 0.1% Tween 20 three times for 10 min, proteins were detected using the enhanced chemiluminescent (ECL) system (GE Healthcare).
In vitro kinase assay
In vitro kinase assays for CHK2 were performed as described previously (29). 293T cell line were transfected with FLAG-CHK2 or HA-CDK11p110 and extracts prepared by resuspension in NETN buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1μg/ml pepstatin). Cleared supernatants were immunoprecipitated with anti-FLAG antibody (M2) or anti-HA antibody and protein G plus/protein A-agarose (Calbiochem) was added. Beads were washed three times with TGN buffer and twice with 1x kinase buffer (20 mM Hepes, pH 7.5, 50 mM NaCl, 10 mM MgCl2, and 1 mM DTT). Immunoprecipitates were resuspended in 30 μl kinase buffer containing 10 μCi [γ-32P]ATP, and 1 μg GST-peptides or GST-truncated proteins as substrates. Kinase reactions were performed at 30°C for 30 min and stopped by the addition of SDS-PAGE loading buffer. Reaction products were separated by SDS-PAGE and transferred to nitrocellulose membranes. Immunoprecipitated FLAG-CHK2 or HA-CDK11p110 were confirmed by western blotting with anti-FLAG or anti-HA monoclonal antibodies. Radiolabeled proteins were visualized using a phosphorimage analyzer.
Transfection of CHK2 siRNA and Generation of CHK2 knockdown stable cell lines
293T cells (6 × 105 per well) were seeded into a six-well tissue culture plate and were growing at 50% confluence the following day when the siRNA transfections were begun. CHK2 smartpool siRNA (M-003256-00-05), and a negative control (D-001206-13-05) were from Dharmacon Research. The transfections of siRNA were carried out using LipofectAMINE 2000 (Life Technologies).
Stable cell lines exhibiting knockdown of CHK2 expression was generated using Mission shRNA vector (TRCN0000039945, Sigma). PLK0.1 TR control vector (random 18mer) was used for generating control cell lines. 293T cells were seeded at 3 × 106 cells per 10 cm plate and transfected with 10 μg of the shRNA vector together with 5 μg of the VSVg and 5 μg of the Δ8.2 helper plasmids using Effectene (Qiagen). The medium was changed 12 hours after transfection. After 48hr, lentivirus-containing culture media were harvested. 293T cells or Hela cells were infected by 0.45 μm-filtered supernatant from virus-producing cells in the presence of 8 μg/ml polybrene. After 2 days, the puromycin resistant cells were maintained with 2 μg/ml puromycin. Medium containing puromycin was replaced every 2 days until puromycin-resistant stable cell lines were established.
In vivo splicing assay
For the β-globin splicing assay, 293T cells were co-transfected with pcDNA3-Rb-β-globin alone or with plasmids (Flag-CDK11 wild-type, S737A mutant, FLAG-CHK2 wild-type and KD mutant plasmids). Total RNA from transfected cells was extracted by an RNeasy kit (Qiagen) according to the manufacturer’s instruction. cDNA generated by Superscript II enzyme (Invitrogen, NY) were analyzed by PCR.
For the in vivo β-galactosidase/luciferase splicing assay using pTN24 reporter, HEK293T cells were transfected using FuGENE 6 (Roche Applied Science) as described by the manufacturer. FLAG-CHK2 and empty vectors were transiently co-expressed in 293T cells with the intron-splicing double reporter plasmid pTN24 for 24 h. The ratio of luciferase to β-galactosidase activity was calculated and expressed as relative splicing activity with the activity of the empty vector cells set at 1.0. β-galactosidase and luciferase activities were measured using the Dual-Light System (Applied Biosystems), Bright-Glo Luciferase Assay System (Promega), and Beta-Glo Assay System (Promega). All measurements were within the linear range established using luciferase and β-galactosidase standards. All samples were measured in quadruplicate, and 3 to 10 independent transfected cultures were assayed per data point.
For the in vivo splicing assay using Luc-I reporter, Luc-I plasmid and CMV-β-gal plasmids with indicated expression vectors were co-transfected into 293T or HeLa cells. The luciferase and β-galactosidase assays were performed 24 hr after transfection. Error bars denote the standard deviations (SD) for more than three independent experiments. Results are represented as the mean with SD. The comparison of different groups was carried out using a two-tailed unpaired Student’s t-test. Differences were considered statistically significant at p italic> 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Richard R. Vaillancourt for providing HA-CDK11p110 expression vector; Dr. Anglus I. Lamond for pBSAL4 plasmid; Dr. Gideon Dreyfuss for pCMV-Luc 2CP/ARE and pCMV-Luc 2CP/Intron/ARE plasmids; Dr. Eperon for the pTN24 plasmids; Dr. Jin-Hyun Ahn for pDEST-SG5-HA, pDEST-SG5-flag destination vectors and early passage human diploid fibroblast cell lines. This work was supported by NRF Grants 2011-0008174, funded by the Ministry of Science and Technology, Republic of Korea.
References
- 1.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998 Dec 4;282(5395):1893–7. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 2.Ahn J, Prives C. Checkpoint kinase 2 (Chk2) monomers or dimers phosphorylate Cdc25C after DNA damage regardless of threonine 68 phosphorylation. J Biol Chem. 2002 Dec 13;277(50):48418–26. doi: 10.1074/jbc.M208321200. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999 Jul 15;18(28):4047–54. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 4.Darbon JM, Penary M, Escalas N, Casagrande F, Goubin-Gramatica F, Baudouin C, et al. Distinct Chk2 activation pathways are triggered by genistein and DNA-damaging agents in human melanoma cells. J Biol Chem. 2000 May 19;275(20):15363–9. doi: 10.1074/jbc.275.20.15363. [DOI] [PubMed] [Google Scholar]
- 5.Pommier Y, Weinstein JN, Aladjem MI, Kohn KW. Chk2 molecular interaction map and rationale for Chk2 inhibitors. Clin Cancer Res. 2006 May 1;12(9):2657–61. doi: 10.1158/1078-0432.CCR-06-0743. [DOI] [PubMed] [Google Scholar]
- 6.Bartek J, Falck J, Lukas J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001 Dec;2(12):877–86. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- 7.Solier S, Sordet O, Kohn KW, Pommier Y. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol. 2009 Jan;29(1):68–82. doi: 10.1128/MCB.00581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000 Mar 10;287(5459):1824–7. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 9.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000 Feb 1;14(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 10.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000 Feb 1;14(3):278–88. [PMC free article] [PubMed] [Google Scholar]
- 11.Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001 Apr 12;410(6830):842–7. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000 Mar 9;404(6774):201–4. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Kuo C, Bisi JE, Kim MK. PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat Cell Biol. 2002 Nov;4(11):865–70. doi: 10.1038/ncb869. [DOI] [PubMed] [Google Scholar]
- 14.Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem. 2008 Mar 7;283(10):6572–83. doi: 10.1074/jbc.M707568200. [DOI] [PubMed] [Google Scholar]
- 15.Lee SB, Kim SH, Bell DW, Wahrer DC, Schiripo TA, Jorczak MM, et al. Destabilization of CHK2 by a missense mutation associated with Li-Fraumeni Syndrome. Cancer Res. 2001 Nov 15;61(22):8062–7. [PubMed] [Google Scholar]
- 16.Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B, et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol. 2010 May;12(5):492–9. doi: 10.1038/ncb2051. [DOI] [PubMed] [Google Scholar]
- 17.Loyer P, Trembley JH, Grenet JA, Busson A, Corlu A, Zhao W, et al. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: influence of cyclin L isoforms on splice site selection. J Biol Chem. 2008 Mar 21;283(12):7721–32. doi: 10.1074/jbc.M708188200. [DOI] [PubMed] [Google Scholar]
- 18.Cornelis S, Bruynooghe Y, Denecker G, Van Huffel S, Tinton S, Beyaert R. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol Cell. 2000 Apr;5(4):597–605. doi: 10.1016/s1097-2765(00)80239-7. [DOI] [PubMed] [Google Scholar]
- 19.Sachs NA, Vaillancourt RR. Cyclin-dependent kinase 11(p110) activity in the absence of CK2. Biochim Biophys Acta. 2003 Dec 5;1624(1–3):98–108. doi: 10.1016/j.bbagen.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Hu D, Mayeda A, Trembley JH, Lahti JM, Kidd VJ. CDK11 complexes promote pre-mRNA splicing. J Biol Chem. 2003 Mar 7;278(10):8623–9. doi: 10.1074/jbc.M210057200. [DOI] [PubMed] [Google Scholar]
- 21.Trembley JH, Hu D, Hsu LC, Yeung CY, Slaughter C, Lahti JM, et al. PITSLRE p110 protein kinases associate with transcription complexes and affect their activity. J Biol Chem. 2002 Jan 25;277(4):2589–96. doi: 10.1074/jbc.M109755200. [DOI] [PubMed] [Google Scholar]
- 22.Wilker EW, van Vugt MA, Artim SA, Huang PH, Petersen CP, Reinhardt HC, et al. 14-3-3sigma controls mitotic translation to facilitate cytokinesis. Nature. 2007 Mar 15;446(7133):329–32. doi: 10.1038/nature05584. [DOI] [PubMed] [Google Scholar]
- 23.Petretti C, Savoian M, Montembault E, Glover DM, Prigent C, Giet R. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep. 2006 Apr;7(4):418–24. doi: 10.1038/sj.embor.7400639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu D, Valentine M, Kidd VJ, Lahti JM. CDK11(p58) is required for the maintenance of sister chromatid cohesion. J Cell Sci. 2007 Jul 15;120(Pt 14):2424–34. doi: 10.1242/jcs.007963. [DOI] [PubMed] [Google Scholar]
- 25.Franck N, Montembault E, Rome P, Pascal A, Cremet JY, Giet R. CDK11(p58) is required for centriole duplication and Plk4 recruitment to mitotic centrosomes. PLoS One. 2011;6(1):e14600. doi: 10.1371/journal.pone.0014600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zong H, Chi Y, Wang Y, Yang Y, Zhang L, Chen H, et al. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Mol Cell Biol. 2007 Oct;27(20):7125–42. doi: 10.1128/MCB.01753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nybakken K, Vokes SA, Lin TY, McMahon AP, Perrimon N. A genome-wide RNA interference screen in Drosophila melanogaster cells for new components of the Hh signaling pathway. Nat Genet. 2005 Dec;37(12):1323–32. doi: 10.1038/ng1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001 Jul;24(3):218–29. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 29.Seo GJ, Kim SE, Lee YM, Lee JW, Lee JR, Hahn MJ, et al. Determination of substrate specificity and putative substrates of Chk2 kinase. Biochem Biophys Res Commun. 2003 May 2;304(2):339–43. doi: 10.1016/s0006-291x(03)00589-8. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill T, Giarratani L, Chen P, Iyer L, Lee CH, Bobiak M, et al. Determination of substrate motifs for human Chk1 and hCds1/Chk2 by the oriented peptide library approach. J Biol Chem. 2002 May 3;277(18):16102–15. doi: 10.1074/jbc.M111705200. [DOI] [PubMed] [Google Scholar]
- 31.Chi Y, Zhang C, Zong H, Hong Y, Kong X, Liu H, et al. Thr-370 is responsible for CDK11(p58) autophosphorylation, dimerization, and kinase activity. J Biol Chem. 2011 Jan 21;286(3):1748–57. doi: 10.1074/jbc.M110.107367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasim MT, Chowdhury HM, Eperon IC. A double reporter assay for detecting changes in the ratio of spliced and unspliced mRNA in mammalian cells. Nucleic Acids Res. 2002 Oct 15;30(20):e109. doi: 10.1093/nar/gnf108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Younis I, Berg M, Kaida D, Dittmar K, Wang C, Dreyfuss G. Rapid-response splicing reporter screens identify differential regulators of constitutive and alternative splicing. Mol Cell Biol. 2010 Apr;30(7):1718–28. doi: 10.1128/MCB.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002 May;31(1):55–9. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 35.Dong X, Wang L, Taniguchi K, Wang X, Cunningham JM, McDonnell SK, et al. Mutations in CHEK2 associated with prostate cancer risk. Am J Hum Genet. 2003 Feb;72(2):270–80. doi: 10.1086/346094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cybulski C, Gorski B, Huzarski T, Masojc B, Mierzejewski M, Debniak T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004 Dec;75(6):1131–5. doi: 10.1086/426403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valente ST, Gilmartin GM, Venkatarama K, Arriagada G, Goff SP. HIV-1 mRNA 3′ end processing is distinctively regulated by eIF3f, CDK11, and splice factor 9G8. Mol Cell. 2009 Oct 23;36(2):279–89. doi: 10.1016/j.molcel.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002 Apr 4;416(6880):499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- 39.Ward AJ, Cooper TA. The pathobiology of splicing. J Pathol. Jan;220(2):152–63. doi: 10.1002/path.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blencowe BJ. Splicing regulation: the cell cycle connection. Curr Biol. 2003 Feb 18;13(4):R149–51. doi: 10.1016/s0960-9822(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 41.Lamond AI, Konarska MM, Sharp PA. A mutational analysis of spliceosome assembly: evidence for splice site collaboration during spliceosome formation. Genes Dev. 1987 Aug;1(6):532–43. doi: 10.1101/gad.1.6.532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.