Abstract
We developed an alcohol self-administration paradigm to model individual differences in impaired control. The paradigm includes moderate drinking guidelines meant to model limits on alcohol consumption, which are typically exceeded by people with impaired control. Possible payment reductions provided a disincentive for excessive drinking. Alcohol use above the guideline, despite possible pay reductions, was considered to be indicative of impaired control. Heavy-drinking 21–25 year-olds (N = 39) were randomized to an experimental condition including the elements of the impaired control paradigm or to a free-drinking condition without these elements. Alcohol self-administration was compared between these two conditions to establish the internal validity of the experimental paradigm. In both conditions, participants self-administered beer and non-alcoholic beverages for 3 hours in a bar setting with 1–3 other participants. Experimental condition participants self-administered significantly fewer beers and drank to lower blood-alcohol concentrations (BACs) on average than those in the free-drinking condition. Experimental condition participants were more likely than free-drinking condition participants to intersperse non-alcoholic beverages with beer and to drink at a slower pace. Although experimental condition participants drank more moderately than those in the free-drinking condition overall, their range of drinking was considerable (BAC range = .024–.097) with several participants drinking excessively. A lower initial subjective response to alcohol and earlier age of alcohol use onset were associated with greater alcohol self-administration in the experimental condition. Given the variability in response, the impaired control laboratory paradigm may have utility for preliminary tests of novel interventions in future studies and for identifying individual differences in problem-drinking risk.
Keywords: laboratory methods, young adult, negative consequences, protective strategies, subjective response
Frequent, heavy drinking is common among young adults (Harrison, Desai, & McKee, 2008) and is associated with negative consequences (e.g., fatal traffic accidents; Hingson, Zha, &Weitzman, 2009). This behavior has clinical ramifications as alcohol use disorders are more common among 18–24 year olds than any other age group (Falk, Yi, & Hiller-Sturmhofel, 2008). While many young adults will “mature out” and desist with heavy drinking by their mid-to-late twenties, a minority will continue and encounter clinically significant problems (Jackson, Sher, Gotham, & Wood, 2001). Thus, additional research is needed to determine which young adults may be at risk for long-term negative outcomes (Courtney & Polich, 2009).
Evidence suggests that impaired control over alcohol use is a construct that may help to identify those at risk for subsequent problem drinking. Impaired control, defined as “a breakdown of an intention to limit consumption” (Heather, Tebbutt, Mattick, & Zamir, 1993, p. 701), refers to a diminished ability to avoid alcohol use altogether or to control alcohol use once initial consumption has begun (Kahler, Epstein, & McCrady, 1995). Converging evidence suggests that impaired control develops relatively early in the natural history of problem drinking (see Leeman, Patock-Peckham, & Potenza, 2012 for a review) and prospectively predicts alcohol-related problems among young adults (Leeman, Toll, Taylor, & Volpicelli, 2009).
Given the importance of impaired control to conceptions of addiction in general (Levine, 1978) and to young adults in particular (Leeman, Fenton, & Volpcelli, 2007; Patock-Peckham & Morgan-Lopez, 2006), a behavioral index of impaired control could be a valuable research tool. Although the Impaired Control Scale (ICS), developed by Heather et al. (1993) is reliable and valid, self-report measures like the ICS essentially assess whether participants believe they have difficulty limiting their alcohol use. Although this is valuable, as verified by multiple findings supporting the validity of the ICS (Heather & Dawe, 2005; Heather, Booth, & Luce, 1998; Leeman et al., 2007; Marsh, Smith, Saunders, & Piek, 2002), laboratory paradigms could be used to measure actual drinking behavior indicative of impaired control. Research on related constructs like impulsivity has demonstrated the utility of both self-report and behavioral assessments (Dougherty, Mathias, Marsh, Moeller, & Swann, 2004) and in some studies, self-report and behavioral measures of self-control-related constructs are weakly related (Krishnan-Sarin, Reynolds et al., 2007; Reynolds, Ortengren, Richards, & de Wit, 2006). Extrapolating from this research, there may be unique variability in problem drinking outcomes that could be explained by self-report and behavioral indices of impaired control.
Human laboratory paradigms have provided valuable behavioral indices of addictive behaviors (McKee, Krishnan-Sarin, Shi, Mase, & O’Malley, 2006; Schuckit, 1985). Benefits of laboratory administration methods include their utility in prospective prediction of problem outcomes (King, de Wit, McNamara & Kao, 2011; Trim, Schuckit, & Smith, 2009) and their time- and cost-efficiency in preliminary tests of novel interventions (Kenna, Leggio, & Swift, 2009; McKee et al., 2009). Prior laboratory studies have demonstrated links between alcohol and difficulties with self-control, including relationships between trait disinhibition and alcohol self-administration (Leeman, Corbin, & Fromme, 2009) and the disinhibiting effects of alcohol (Fillmore, 2003; Loeber & Duka, 2009; Reed, Levin, & Evans, 2012). For these reasons, we endeavored to develop and test an alcohol self-administration paradigm to model individual differences in impaired control over alcohol use. Given the public health problem of young adult heavy drinking, risk for self-control difficulties among young people (Rutherford, Mayes, & Potenza, 2010) and need for novel interventions in this population (Carey, Scott-Sheldon, Carey, & DeMartini, 2007), we developed this paradigm primarily for use with young adults.
The impaired control laboratory paradigm contains two key elements: moderate drinking guidelines and possible payment reductions (Table 1). The moderate drinking guidelines are meant to model limits on alcohol consumption, which are typically exceeded by people with impaired control (Heather et al., 1993). While the moderate drinking guidelines were created by us rather than by participants themselves, recent evidence suggests that self-generated and externally-generated goals are associated with similar reductions in alcohol consumption among college students (Lozano & Stephens, 2010). Payment reductions were included in order to model negative consequences of alcohol consumption. The payment reductions were based on participants’ performance on a battery of cognitive and psychomotor tasks after alcohol self-administration. Poor task performance triggered drawings for possible pay reductions taking place on a date 1–3 days after alcohol self-administration. We implemented probabilistic payment reductions in order to model the uncertain nature of negative consequences. In the “real world,” heavier drinking increases the likelihood of negative consequences but negative consequences do not become a certainty until drinkers consume an amount of alcohol much higher than what is allowed in alcohol self-administration studies. Holding pay reduction drawings at a later date modeled the often distal quality of negative consequences. While opportunities to drink alcohol are typically immediate, the impact of negative consequences is often felt at a later time. For instance, students who opt to drink heavily rather than study for exams can do so right away, while they will not receive their bad grades until days later. While continued alcohol use despite potential negative consequences is not part of impaired control per se, multiple prior findings in undergraduates suggest close relationships between impaired control and alcohol-related problems (e.g., Patock-Peckham & Morgan-Lopez, 2006).
Table 1.
Description of the two key elements of the impaired control alcohol self-administration paradigm
| Paradigm element |
Details | Meant to model | Pragmatic benefit to paradigm |
|---|---|---|---|
| Drinking guideline | Consume no more than 3 drinks (2 for women) during ad-libitum drinking period | A limit on alcohol consumption to which those with impaired control over alcohol use typically have difficulty adhering | Provides participants with a guideline for controlled, moderate drinking |
| Payment reductions | At a follow-up appointment 1–3 days after self-administration session, participants draw from a hat for a possible pay reduction ($0, $6, $12) once for each of 4 cognitive/psychomotor tasks they perform poorly following ad-libitum drinking period | Negative consequences of alcohol use: both their uncertain and often distal nature | Creates a disincentive for participants to drink excessively and a reason to abide by the moderate drinking guideline |
Although 2 earlier alcohol self-administration paradigms were developed to model “loss of control” over drinking, neither involved any type of limit placed on alcohol consumption (Ludwig, Wikler, & Stark, 1974; Marlatt., Demming, & Reid, 1973). This is a key omission, as only alcohol use that exceeds limits is indicative of impaired control (Heather et al., 1993). Older paradigms were designed to model negative consequences of alcohol use, but the consequences were neither probabilistic, nor distal and were delivered in a manner not feasible given current human subjects’ standards (e.g., social isolation; Wilson, Leaf, & Nathan, 1975).
Excessive alcohol consumption in this paradigm is indicative of impaired control over alcohol use because it supersedes a limit placed on drinking and occurs despite possible negative consequences. As such, excessive drinking in this paradigm may reflect current problem drinking and subsequent risk for more serious problems. If the impaired control laboratory paradigm models a key aspect of problem drinking that is relevant to young adult drinkers, this paradigm would be useful for multiple research purposes in future studies. These include testing the effects of experimental manipulations (e.g., stress-induction), on impaired control over alcohol use; testing preliminary efficacy of alcohol reduction interventions targeting young adults; and predicting risk of future problem drinking prospectively. The main goal of the present study was to establish the internal validity of the key elements of the impaired control laboratory paradigm.
In this initial between-subjects study, participants were randomized to an experimental condition including provision of moderate drinking guidelines and possible pay reductions or a free-drinking condition without these elements. Compared to participants in the free-drinking condition, we hypothesized that participants in the experimental condition would self-administer fewer standard drinks of alcohol, drink to lower peak estimated blood alcohol concentrations (eBAC) during a 3-hour ad-libitum drinking period and exhibit lower actual peak breath alcohol concentrations (BrAC) following ad-libitum drinking.
At the same time, we predicted a broad range of responses in the experimental condition. The ability to elicit a broad range of responses is a key feature of prior self-administration models (McKee et al., 2006; O’Malley, Krishnan-Sarin, Farren, Sinha, & Kreek, 2002), as such variability is necessary to observe effects of experimental manipulations or interventions on self-administration behavior in future studies. To enhance ecological validity, all self-administration sessions were conducted in an actual bar (Davidson, Swift, & Fritz, 1996; Davidson, Palfai, Bird, & Swift, 1999). Consistent with prior alcohol administration studies involving young adults (e.g., Corbin, Gearhardt, & Fromme, 2008; Sayette et al., 2012), sessions were conducted in groups of 2–4 participants to account for social factors that are critical to young adult drinking behavior (Sayette et al., 2012; Wood, Read, Palfai, & Stevenson, 2001). No assessments were conducted during ad-libitum drinking to allow alcohol consumption to occur as naturally as possible.
Variability in drinking behavior in the experimental condition would suggest that the impaired control laboratory paradigm may capture individual differences in drinking behavior occurring despite possible pay reductions and provision of moderate drinking guidelines. To the extent that these individual differences are meaningful, significant correlations should be observed between alcohol self-administration and established risk factors for problem drinking. For example, we predicted significant positive relationships between alcohol self-administration in the experimental condition and self-reported impaired control over alcohol use. While there is precedent for low correspondence between self-report and behavioral indices of self-control-related constructs (e.g., Krishnan-Sarin, Reynolds et al., 2007), we nonetheless predicted a moderate, significant correlation given that the laboratory paradigm was developed to be an alternate measure of impaired control. We also predicted a significant correlation with low initial response to alcohol because those with a low response may receive insufficient subjective cues to slow down or stop drinking (Schuckit, 1985), perhaps leading to impaired control over alcohol use. We posited that a positive family history would also have a significant relationship to alcohol self-administration in the experimental condition. Those with a family history of alcohol problems are more likely than those without to have a low response to alcohol (Quinn & Fromme, 2011), and positive family history is an independent risk factor for alcohol dependence (Grant, 1998). Finally, we predicted that there would be a significant negative correlation between drinking in the experimental condition and age of alcohol use onset. Earlier age of onset predicted heavier drinking and alcohol-related problems prospectively in undergraduates (Morean, Corbin, & Fromme, 2012). While non-daily smoking has been related to heavier alcohol use in young adults (Harrison et al., 2008), we did not have a prediction as to whether such relationships would apply to the current sample of frequent heavy drinkers.
Method
Participants
Young adults (ages 21–25 years) were recruited using web-based advertising and flyer postings on and around college campuses and other public areas. Advertisements stated that we were seeking 21–25 year-old individuals who drink alcohol at least twice per week (though the inclusion criterion was slightly higher than this) and meet other inclusion requirements. It was stated clearly that the study involved no treatment or medications. The maximum possible compensation of up to $200 was also stated clearly in these advertisements.
Regarding inclusion criteria, over the prior 30 days, participants were required to report at least 4 heavy drinking days (≥ 5 drinks for men, ≥ 4 for women), 12 any-drinking days and 1 day with an estimated blood alcohol concentration (eBAC) ≥ 0.10%. Thus, this sample was composed of frequent heavy drinkers, as opposed to lighter, social-drinking samples in many alcohol administration studies involving young adults (e.g., Weafer & Fillmore, 2012; Sayette et al., 2012). Exclusion criteria were current treatment-seeking or past 12-month history of substance abuse treatment; current dependence on addictive substances other than alcohol, including nicotine and cannabis; a past history of alcohol withdrawal or current withdrawal; two breath alcohol readings > 0.00% at the outset of study appointments; positive urine drug screening for opiates, cocaine, phencyclidine, amphetamines, methamphetamine, barbiturates, methadone and benzodiazepines; current use of or a recent prescription for psychotropic drugs; disliking beer or no recent beer consumption; severe medical or psychiatric conditions; a body mass index < 18.5 or > 35; and for women, pregnancy, nursing or failure to use reliable birth control. Participants were also required to perform normatively on 4 cognitive/psychomotor tasks (described below) that formed the basis for the possible pay reductions discussed above. Treatment of participants in this study was in accordance with APA ethical standards and the study was approved by the Human Investigation Committee at the Yale School of Medicine.
Procedures
Prospective participants were given an overview of the study via a web page or telephone script read by a research assistant. Interested individuals were screened initially either via telephone or a secure, web survey. Those who appeared eligible following preliminary screening were invited to an in-person screening appointment.
The in-person screening appointment began with a breathalyzer reading and informed consent. Breathalyzer readings, including those during the self-administration sessions, were conducted using a hand-held Alcohol-Sensor III breathalyzer (Intoximeter Inc., St. Louis, MO). Participants were required to have a BrAC of 0.00% to give informed consent. Participants could reschedule once due to a positive BrAC. In the consent process, participants were given details about study participation, including the pay structure.
After obtaining informed consent, we conducted urine drug testing, pregnancy testing for women and weight measurement using a calibrated scale. A timeline followback (TLFB) interview (Sobell & Sobell, 2003) was conducted by a trained research assistant or the principal investigator (PI) to obtain self-reports of alcohol use and cigarette smoking during the prior 30 days. Data from the TLFB were used to establish eligibility vis-à-vis alcohol and cigarette-smoking-related inclusion criteria and to assess relationships between recent self-reported alcohol use and smoking and subsequent alcohol self-administration during the session. The Structured Clinical Interview for DSM-IV-TR Axis-1 Disorders (First, Spitzer, Gibbon, & Williams, 2002), also conducted by a trained research assistant or the PI, was used to diagnose alcohol and drug dependence. The study nurse documented participants’ medical history, including medication use; symptom inventory; menstrual cycle data for women and history of psychiatric diagnosis and treatment. The nurse also administered the Clinical Institute Withdrawal Assessment for Alcohol Revised (CIWA-AR; Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989). A research assistant or the PI administered a battery of 4 cognitive/psychomotor tasks (see below). Participants were told that they must perform the tasks normatively to be eligible for the study. Participants then completed questionnaires on-line.
Eligible, enrolled participants were scheduled in groups of 2–4 for an alcohol self-administration session at the earliest possible date. Sessions were held in the late afternoon/night at a local bar on Tuesdays, Wednesdays or Thursdays. Efforts were made to include members of both sexes in each session. We avoided scheduling participants from the same university or those who lived in close geographic proximity as our goal was for participants to be unacquainted. Randomization to the experimental or free-drinking condition was by session, meaning that all participants in a given session were randomized to the same condition. The study design was between-subjects (i.e., participants completed only the experimental or free-drinking condition).
On the day of the session, a brief appointment was held in the morning or early afternoon at our research office. Breathalyzer readings and urine drug and pregnancy tests for women were repeated. Participants were reminded of the session rules and arrangements were made for study-provided taxi transportation to the bar, which participants were required to take. They were instructed not to consume alcohol, and to eat lunch, but not to eat after 1pm. After arrival, participants completed baseline self-reports and repeated the 4 cognitive/psychomotor tasks to provide pre-drinking data and to become acclimated to completing the tasks in a bar. Participants were then informed of their condition assignment (Figure 1). Study activities took place off to one side of the bar, which was open for business to other customers. Participants were informed of this during the consent process. Interaction between participants and bar patrons was minimal.
Figure 1.
Timeline of participation on the day of an alcohol-self-administration session. BrAC, breath alcohol concentration; eBAC, estimated blood alcohol concentration during the ad-libitum-drinking period; RA, research assistant; “tasks,” the battery of 4 cognitive/psychomotor tasks administered initially at the in-person screening appointment
Regardless of condition assignment, participants were informed that they could consume as many beers as they liked during the 3-hour ad-libitum drinking period unless they reached the maximum allowable eBAC. All participants were told they would complete the 4 cognitive/psychomotor tasks again after the ad-libitum drinking period and that their completion of the tasks allowed us to observe task performance before and after alcohol consumption in a bar setting. Participants were asked to complete the tasks to the best of their ability and that they would receive feedback on their performance at the follow-up appointment.
Experimental condition participants were also told that for each of the 4 tasks they did not perform comparably to initial screening, they would draw from a hat for a possible pay reduction ($0, $6 or $12) at a follow-up appointment 1–3 days after the session. Thus, total possible pay reduction varied from $0 to $48. Here, comparable performance referred to their prior performance, plus or minus ½ of 1 standard deviation based on normative data, though participants were not informed of this precise standard. Experimental condition participants were informed of the following verbally and in writing: “To avoid having to draw for possible pay reductions, we offer a guideline that you consume no more than 3 beers (2 for women) in the course of the 3-hour alcohol drinking period.” Participants were advised they were not required to abide by the guideline but doing so would improve their chances of completing the tasks successfully and avoiding possible pay reductions. Participants in the free-drinking condition were not given drinking guidelines, and payment was not linked to task performance.
The ad-libitum-drinking period began at 5pm. For 3 hours, participants could self-administer 12-ounce (oz) beers or non-alcoholic beverages (i.e., soda or water) ad-libitum by ordering from a research assistant, who obtained the drinks from the bartender. Just before the beginning of the ad-libitum period, study staff asked participants if they would like a drink. All subsequent ordering was initiated by participants only. There were 3 beer options: Budweiser, Molson Canadian and Smithwick’s Ale. All are 5% alcohol by volume with approximately 150 calories per 12-oz. Participants could switch brands but had to complete one drink (alcoholic or non-alcoholic) before ordering another. A supervisor standing nearby monitored each drink ordered including the beer brand or type of non-alcoholic beverage and the time at which consumption of each drink began and ended. Beer consumption was monitored in comparison with an eBAC chart made for each participant, based on sex and weight (http://depts.washington.edu/mcsurvey/bal/index.php). For safety reasons, no participant was allowed to order a beer that, if consumed, would lead to an eBAC ≥ 0.10%. Given that participants were required to consume one drink before ordering another, partial drinks were only possible on the final drink. Drinks partially consumed were measured with a measuring cup and converted to standard drink units, however only one participant opted not to finish a beer (6 oz = 0.5 standard drink). Another beer was spilled accidentally and replaced immediately. It was impossible to ascertain how much of it had been consumed; thus, the spilled beer was not counted in drink estimates or drinking topography calculations (see data analysis section).
Participants sat at one table or two adjacent tables off to one side of the bar. They conversed among themselves and with the research assistant who acted as the server. The bar had televisions, a pool table, jukebox and video games. Playing cards and table games were provided. No cigarette smoking was allowed during this period. The ad-libitum-drinking period ended promptly at 8pm, at which point participants completed self-reports, a breathalyzer reading and the same 4 cognitive/psychomotor tasks in that order. No feedback on BAC or task performance was given. After the 8pm study activities were completed, participants were fed and cigarette smoking was allowed just outside the bar. Participants continued to engage in the same activities (e.g., card games) and were allowed the same non-alcoholic beverages. Breathalyzer readings and self-reports were repeated hourly until midnight, the earliest possible dismissal time. Participants were brought home by taxi when their BAC levels were ≤ 0.02%.
Follow-up interviews were scheduled for 1–3 business days after the session. In the experimental condition, pay reduction drawings took place for participants whose task performance following drinking was not equivalent to their screening appointment. Feedback regarding estimated and actual BACs was given. Participants were debriefed regarding the purpose of the study and a motivational interview focused on alcohol use was provided by a clinical psychology pre-doctoral intern or post-doctoral fellow.
Measures
Alcohol and cigarette use
The TLFB (Sobell & Sobell, 2003) involves a calendar with memory prompts to facilitate recall of substance use on each day in a specified period (past 30 days in this study). The reliability and validity of past 30-day estimates from the TLFB have been established (Carey, 1997). Alcohol data from the TLFB were used to determine eligibility and for comparing recent self-reported alcohol use to alcohol self-administration in the study.
Impaired control
Part 2 of the Impaired Control Scale (ICS; Heather et al., 1993) is a reliable (α =.85) and valid measure of how often participants have experienced difficulty controlling alcohol consumption, including unsuccessful attempts to limit, cut down and stop drinking. A 3-month time frame was used. The 10 items were rated on a 0 (never) to 4 (always) scale and summed with higher scores indicating greater difficulty controlling alcohol use.
Negative alcohol consequences
The Young Adult Alcohol Consequences Questionnaire (YAACQ; Read, Kahler, Strong, & Colder, 2006) is a reliable, valid measure. Participants reported whether 48 consequences happened to them in the prior 3 months. Items were rated dichotomously and summed (0 = no, 1 = yes) to yield a total score out of 48 (α = .90).
Early subjective response
The original form of the Self-Rating of the Effects of Alcohol (SRE; Schuckit, Smith, & Tipp, 1997) captures 4 effects of alcohol (slurred speech, feeling intoxicated, passing out, and stumbling gait). Morean and Corbin (2008) added 2 items capturing stimulation/arousal and relaxation/calmness/sedation. Higher scores on each item reflected the number of drinks needed to experience each effect. Each effect was measured for the individual’s first 5 drinking experiences (with higher scores indicative of low initial subjective response to alcohol [α =.80]); his/her heaviest period of drinking; and, the most recent 3-month period of regular drinking. Only the initial response items are included in this report.
Alcohol history
Participants reported the age at which they started drinking, excluding small tastes or sips of alcohol. For family history of alcohol problems, items were based on the Addiction Severity Index (McLellan et al., 1992). Participants were asked whether any relatives ever “had a significant problem with alcohol or drugs, one that either led to treatment or should have led to treatment.” Participants reported on alcohol and drug problems of family members separately and only alcohol problems are reported here. Those reporting an alcohol-use-problem history for at least one biological parent were considered family-history-positive.
Cognitive/psychomotor tasks
Four tasks found to be sensitive to effects of alcohol (Brumback, Cao, & King, 2007; Chait & Perry, 1994) were administered at screening and during the session before and after ad-libitum drinking. The Digit Symbol Substitution Test (DSST) from the WAIS-R (Wechsler, 1981) is a pencil-and-paper perceptual-motor processing task. Participants completed as many items as possible in 90 seconds with scores comprising the number of correct responses. The Grooved Pegboard (Lafayette Instruments, Lafayette, IN) is a test of fine motor coordination and speed in which participants retrieve, rotate as needed and insert 25 small pegs into 25 slotted holes in random orientations on a board as fast as possible, using their non-dominant hand only. In the Time Production task, participants reported when they believed 30, 60 and 120 seconds had passed. Lastly, participants completed an eyes-closed, One-Leg Stand with arms outstretched for up to 30 seconds on each foot. At screening, participants’ score was their longest time out of three chances on each foot. At the ad-lib session, their score was their best time out of two chances on each foot. Participants always completed the tasks in this order. Norms for study inclusion and performance evaluation for the former two tasks were based on Brumback et al. (2007) and for the latter two, on Chait and Perry (1994).
Analyses
Prior to conducting the primary analyses, distributions and normal probability plots were examined for continuous variables. We then determined whether there were differences in self-reported alcohol consumption or any other self-report variables included in this report by study condition. All analyses were conducted using SPSS, version 19. Four types of analyses were conducted: 1) planned primary and secondary analyses to compare alcohol self-administration between the two study conditions; 2) a post-hoc analysis to compare study conditions on an alternate alcohol self-administration outcome (i.e., whether or not participants met heavy drinking criteria); 3) mechanistic analyses to ascertain approaches that participants who drank moderately may have utilized to control their consumption; and 4) exploratory analyses.
The primary outcome variable was number of beers self-administered in standard drink units. Secondary outcomes were peak estimated BAC (eBAC) during the ad-libitum-drinking period and peak actual breath alcohol concentration (BrAC) after the ad-libitum-drinking period. Peak eBAC was calculated because no BrAC readings were taken during the ad-libitum-drinking period. An eBAC was calculated based on the time at which each beer was completed using the following formula: (((number of drinks/2) * (a constant of 9 for women and 7.5 for men/weight)) – (number of hours × .016)) (Matthews & Miller, 1979). These two BAC variables were complementary. Peak eBAC captures alcohol self-administration at its heaviest point vis-à-vis the amount of time elapsed in the ad-libitum drinking period and participants’ sex and weight. BrAC after the end of the ad-libitum period was thought to reflect the possible impact of alcohol on performance on the cognitive/psychomotor tasks, which occurred soon after the first post-drinking BrAC reading. The cognitive/psychomotor tasks were of importance because performance on these tasks had a potential effect on pay reductions for participants in the experimental condition. Separate multiple regressions were planned for each of the three main outcomes. Study condition and sex were included as predictors in these and all other regression analyses. Regression analyses were followed by mixed-model analyses with session group (i.e., the 2–4 participants who completed each session together) entered as a random variable along with study condition and sex as fixed variables. The latter analyses were conducted to account for the nested structure of the data within these sessions. A similar approach to analyzing alcohol self-administration data collected within nested session groups (i.e., regressions then mixed models) was taken by Leeman, Corbin and Fromme (2009). Our hypothesis was that experimental condition participants would self-administer fewer beers and drink to lower BACs than those in the free drinking condition, due to some participants moderating their drinking due to the drinking guideline and possible pay reductions in the experimental condition. At the same time, we expected a great deal of variability on all outcomes within the experimental condition.
Using logistic regression, we also compared incidences of participants meeting heavy drinking criteria between study conditions. We defined heavy drinking as either having been “cut off” from further alcohol consumption for safety reasons or meeting the common benchmarks of ≥ 5 drinks for men or ≥ 4 for women during the ad-libitum period. We included this analysis because of the clinical relevance of this outcome. Our hypothesis was that experimental condition participants would be less likely to meet heavy drinking criteria, but we also postulated there would be considerable variability among experimental condition participants.
We conducted a series of analyses to better understand mechanisms that may have contributed to more moderate consumption in the experimental condition as compared with the free-drinking condition. Using logistic regression, we examined differences between the two conditions in likelihood of choosing to order at least one non-alcoholic beverage during the ad-libitum drinking period before being “cut off” for safety reasons. This corresponds to the moderate drinking technique of alternating alcoholic with non-alcoholic beverages (also known as “spacing”) that is taught as part of motivational interviewing-based interventions for young adults, such as Brief Alcohol Screening and Intervention for College Students (BASICS; Dimeff, Baer, Kivlahan, & Marlatt, 1999). Using multiple regression, we also examined differences between conditions in three measures of drinking topography: mean drink duration for participants’ first three beers and duration of inter-drink intervals between the first and second and between the second and third beers. Longer drink durations and inter-drink intervals correspond to “pacing” techniques that are also taught as part of interventions such as BASICS. Our hypotheses were that participants in the experimental condition would be more likely to order at least one non-alcoholic beverage, drink significantly more slowly, and have longer inter-drink intervals than participants in the free drinking condition. Again, we expected variability within the experimental condition and accordingly, expected that a considerable proportion of participants would not drink moderately, despite the risk of pay reductions.
Lastly, on an exploratory basis within the experimental condition, we examined correlations between the three main alcohol self-administration variables and self-reported alcohol use, as well as other self-report variables pertaining to problem-drinking risk. We hypothesized that alcohol self-administration in the experimental condition would relate significantly and positively to self-report measures of alcohol consumption at initial screening. We also predicted significant positive correlations with self-reported impaired control, low initial response to alcohol and family history of alcohol problems. We posited significant, negative correlations with age of onset of alcohol use. No prediction was made regarding relationships to current cigarette smoking status (i.e., any smoking or not in the last 30 days based on the TLFB).
We also compared performance on the cognitive/psychomotor tasks following alcohol self-administration by study condition on an exploratory basis. We predicted that experimental condition participants would perform these tasks more effectively.
Results
Preliminary analyses
Forty-six participants were deemed eligible. Seven subsequently decided they were no longer interested, leaving 39 participants, 20 of whom were randomized to the experimental condition (14 men) and 19 to the free drinking condition (13 men). Sample descriptives are provided in Table 2. Due to skewed distributions, the peak eBAC variable and inter-drink interval variables were log transformed. The second inter-drink interval variable (between the second and third beer) was skewed regardless of whether or not two participants who consumed only two beers were included in the analysis (see below). Outliers on the low initial response to alcohol variable were winsorized to a value 3 SD greater than the mean. There were no significant differences between participants randomized to the two conditions on self-reported alcohol consumption or any other variables assessed at screening (all p values >.15).
Table 2.
Sample Characteristics Overall and by Study Condition
| Variable | Experimental condition (n = 20) |
Free Drinking condition (n = 19) |
Overall (N = 39) |
|---|---|---|---|
| Percent male | 70% | 68.4% | 69.2% |
| Race/ethnicity | |||
| White, non-Hispanic | 85% | 84.2% | 84.6% |
| White, Hispanic | 5% | 5.3% | 5.1% |
| African-American, non-Hispanic | 10% | 0% | 5.1% |
| American Indian | 0% | 5.3% | 2.6% |
| Other | 0% | 5.3% | 2.6% |
| Student status | |||
| Full-time | 55% | 42.1% | 48.7% |
| Part-time | 5% | 5.3% | 5.1% |
| Non-student | 40% | 52.6% | 46.2% |
| Family history positive | |||
| At least 1 first-order relative | 50% | 33% | 42.1% |
| At least 1 biological parent | 40% | 27.8% | 34.2% |
| Alcohol use disorder diagnoses | |||
| Alcohol abuse: lifetime | 60% | 57.9% | 59% |
| Alcohol abuse: current | 20% | 26.3% | 23.1% |
| Alcohol dependence: lifetime | 25% | 36.8% | 30.8% |
| Alcohol dependence: current | 10% | 26.3% | 17.9% |
| Current smoker | 40% | 31.6% | 35.9% |
| Past 30-day alcohol frequency/quantity reported at screening: mean (SD), range of responses | |||
| Frequency of any use | 18.35 (5.53), 12–30 | 17.79 (5.71), 12–28 | 18.08 (5.55), 12–30 |
| Frequency of heavy use | 9.27 (4.13), 4–21 | 11.37 (4.86), 4–21 | 10.29 (4.56), 4–21 |
| Drinks per drinking day | 5.54 (2.47), 2.41–12.73 | 6.71 (2.67), 2.67–11.36 | 6.11 (2.60), 2.41–12.73 |
| Peak quantity of alcohol use | 12 (4.08), 6–20 | 13.32 (4.89), 6–20 | 12.64 (4.48), 6–20 |
| Other self-report variables indicative of problem drinking risk assessed at screening | |||
| Scores on Part 2 of the ICS (out of a possible 40) | 11.40 (6.27), 1–22 | 9.47 (8.06), 2–35 | 10.74 (7.05), 1–35 |
| Age of onset of alcohol use | 16.85 (1.95), 14–20 | 16.16 (2.04), 12–19 | 16.61 (1.94), 12–20 |
| Number of drinks needed to experience subjective response during first 5 drinking experiences on the modified SRE | 5.10 (1.69), 2.67–9.57 | 4.9 (1.2), 3.17–7.33 | 5.01 (1.46), 2.67–9.57 |
Note: Heavy use: 5 or more drinks in a day for me, 4 for women; ICS: Impaired Control Scale, SRE: Self-Rating of the Effects of Alcohol
Main outcome analyses
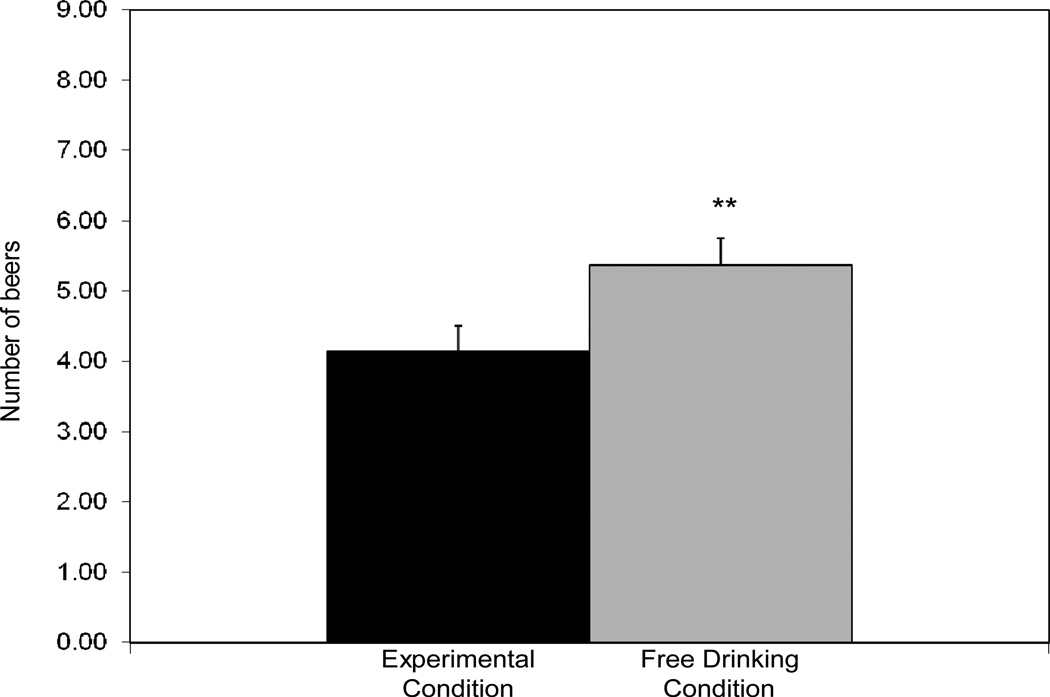
In separate regressions, study condition was a significant predictor of the number of beers self-administered, β = −0.37, p = 0.004, peak eBAC during the ad-libitum-drinking period, β = −0.47, p = 0.003, and peak BrAC after the ad-libitum period, β = −0.48, p = 0.002. Experimental condition participants self-administered fewer beers (Figure 2.1) and drank to lower eBACs and BrACs (Figure 2.2) than those in the free-drinking condition. While experimental condition participants drank less overall, they nonetheless consumed slightly more than 4 beers, on average, and reached an average eBAC of just over .06%. There was a significant effect of sex on number of beers consumed, β = 0.57, p < 0.001, with men (M = 5.37, SD = 1.60) self-administering more than women (M = 3.33, SD = 0.89).
Figure 2.1.
Number of beers self-administered during a 3-hour ad-libitum-drinking period in the experimental and free-drinking conditions. ** p<.01
Figure 2.2.
Blood alcohol concentration-related outcomes. On the left, peak estimated blood alcohol concentration (eBAC) during a 3-hour ad-libitum-drinking period in the experimental and free-drinking conditions. On the right, peak actual breath alcohol concentration (BrAC) obtained after the end of the ad-libitum-drinking period in the experimental and free-drinking conditions. ** p<.01
Mixed-model analyses including a random effect of session group yielded comparable results in models predicting number of beers self-administered and peak BrAC following the ad-libitum period. In a mixed-model analysis predicting peak eBAC during the ad-libitum period, the effect of study condition was no longer significant, though the effect approached statistical significance, t = 2.07, p = .057.
Alternate outcome analysis
Based on a logistic regression, study condition was a significant predictor of heavy drinking, odds ratio (OR) = 0.045, p=.006, with participants in the experimental condition less likely to meet criteria for heavy drinking (45%) than those in the free drinking condition (95%). Sex was not a significant predictor. This result further demonstrates that participants in the experimental condition were more likely to drink moderately than those in the free-drinking condition overall, though many participants in the experimental condition also drank excessively.
Possible mechanisms underlying moderate drinking in the experimental condition
Based on logistic regression, experimental condition participants were significantly more likely to self-administer at least 1 non-alcoholic drink, OR = 13.31, p = .004 (Figure 3.1). Based on multiple regression, experimental condition participants had significantly longer drink durations, β = 0.37, p = 0.014. The second inter-drink interval was extended out to the end of the ad-libitum-drinking period for 2 participants who self-administered only 2 beers each (their estimated intervals were 40 and 63 minutes). Experimental condition participants had significantly longer intervals between their second and third beers, β = 0.34, p = 0.024 (Figure 3.2). There was no significant difference in the interval between the first and second beer, β = 0.24, p = 0.147. Sex was a significant predictor of the same two topography variables, due to longer drinking duration and longer intervals between the second and third beer among women.
Figure 3.1.
Percentage of participants in each condition who self-administered at least 1 non-alcoholic beverage during a 3-hour ad-libitum-drinking period. ** p<.01
Figure 3.2.
Depicts, from left to right, the mean duration taken to consume up to the first three beers during a 3-hour ad-libitum-drinking period by study condition; the mean interval between the end of the first and the beginning of the second beer by condition; and the mean interval between the end of the second and the beginning of the third beer by condition, * p<.05
Exploratory analyses
We also conducted a series of correlations between self-report variables assessed at screening and the three main alcohol self-administration outcomes (i.e., number of beers self-administered, peak eBAC and post-drinking BrAC). Number of beers self-administered correlated with self-reported frequency of alcohol consumption in the prior 30 days (r = .46, p = .043). Other correlations between self-administration and self-reported alcohol use were in the expected direction but not significant (r values ranging from .14 to .34). Correlations between alcohol self-administration and negative consequences measured at screening with the YAACQ were not significant (r values ranging from −.03 to .13). Correlations with cigarette smoking status (smoker versus non-smoker) were also not significant (r values ranging from −.08 to .19).
Contrary to predictions, self-reported impaired control over alcohol use did not correlate significantly with any of the three main alcohol self-administration outcomes in the experimental condition (r values ranging from −.07 to .14). However, initial low response to alcohol on the SRE correlated significantly with number of beers self-administered (r = .54, p = .013), but not with the two BAC-related outcomes (r = .16 and .19). Those who reported needing a higher number of drinks to experience subjective effects of alcohol during their initial drinking experiences tended to self-administer more beer. Age of drinking onset was significantly correlated with peak eBAC during the ad-libitum-drinking period (r = −.57, p = .008), with those who began at an earlier age drinking to higher eBACs. Correlations with the other two self-administration variables were in the same direction but not significant (number of beers: r = −.23; peak BrAC: r = −.39). There was a statistical trend relating family history positive status to peak BrAC (r = .42, p = .063); however, correlations with number of beers consumed (r = .30) and peak eBAC (r = .24) did not approach significance.
Performance on the cognitive/psychomotor tasks was compared between study conditions on an exploratory basis. While no differences in task performance were statistically significant, all comparisons favored the experimental condition, with the exception of change in grooved pegboard performance between the screening appointment and post-drinking, which favored the free drinking condition. Full results may be obtained from the authors.
Discussion
Participants in the experimental condition, which introduced moderate drinking guidelines and possible payment reductions for excessive drinking, self-administered significantly fewer beers, drank to lower BACs and were less likely to meet heavy drinking criteria than those in a free-drinking condition without these key elements. At the same time, there was a range of drinking behaviors, and several participants in the experimental group drank excessively. These findings support the internal validity of the elements of the impaired control laboratory paradigm. Excessive alcohol consumption in this paradigm is indicative of impaired control because it exceeds a limit placed on drinking and occurs despite possible consequences.
Experimental condition participants were more likely than free drinking participants to intersperse non-alcoholic drinks with beers, to drink beer at a slower pace and to allow longer periods of time between their second and third beers. These behaviors parallel moderate drinking strategies included in motivational interviewing-based brief interventions for alcohol use reduction in young adults (Dimeff et al., 1999) and suggest that the impaired control paradigm might offer a means of assessing protective strategy use in the laboratory. Unfortunately, no self-report measure or interview was included to ascertain whether participants engaged in these behaviors with the intention of moderating their drinking, though this seems likely. Future studies will include such items to assess moderate drinking strategies within this paradigm.
Participants in the experimental condition, however, did not uniformly moderate their drinking. For instance, almost half of participants in the experimental condition met heavy-drinking criteria. Prior laboratory paradigms developed by members of our group have been utilized to test the impact of pharmacotherapy (McKee et al., 2009; O’Malley et al., 2002); risk factors such as family history (Krishnan-Sarin, Krystal, Shi, Pittman, & O’Malley, 2007) and experimental manipulations such as food deprivation (Leeman, O’Malley, White, & McKee, 2010) on self-administration behavior. A key feature that contributes to the utility of these paradigms is their ability to elicit a range of responses from participants (McKee et al, 2006; O’Malley et al., 2002). Initial findings suggest that the impaired control laboratory paradigm also yields a range of alcohol self-administration behavior and may therefore have similar utility.
Conclusions that can be derived from exploratory analyses within the experimental condition are necessarily limited due to small sample size and the correlational nature of the analyses. However, some findings suggested that individual differences observed in alcohol self-administration behavior in the experimental condition may be meaningful. Significant relationships were found between alcohol self-administration variables and more frequent baseline drinking, earlier age of drinking onset and low initial subjective response to alcohol. These variables have been tied to problem drinking in young adults and risk for greater problems in the future (Morean et al., 2012; Trim et al., 2009) suggesting that heavier drinking in the impaired control laboratory paradigm may be indicative of problem drinking. Multiple negative findings were notable, including non-significant correlations with self-reported impaired control over alcohol use and negative consequences of alcohol use. Small sample size may have contributed, as effect sizes were considerable (r values > .20) for many of the non-significant correlations. However, correlations between measures derived from the impaired control paradigm and self-reported impaired control over alcohol were small and inconsistent in direction. Impaired control over alcohol may follow other self-control-related constructs (e.g., impulsivity) in demonstrating weak relationships between behavioral and self-report measures (Krishnan-Sarin, Reynolds et al., 2007; Reynolds et al., 2006). Nonetheless, relationships between alcohol self-administration in this paradigm and other measures of difficulty with self-control (both self-report and behavioral tasks) will continue to be addressed in future studies. Further, all correlational findings in this study should be replicated in future studies.
The significant random effect of group in a mixed model analysis to predict eBAC during the ad-libitum-drinking period was notable. Given the impact of social factors on alcohol use (Sayette et al., 2012; Wood et al., 2001), it is not surprising that participants appeared to be influenced by other participants in their self-administration behavior. Nonetheless, there were significant differences between study conditions on two main outcomes with a near significant trend for the third. The fact that the impaired control paradigm incorporates social factors influencing young adult drinking could be considered a potential strength of this approach.
A number of limitations should be considered. While we strove for ecological validity, laboratory studies necessarily involve artificial contingencies, which represent departures from “real world” behaviors. The need to limit the range of eBAC during self-administration, while necessary for safety, represents a departure from the way young adults typically consume alcohol. Other limitations included the small sample size and racial homogeneity of the sample.
The key elements of the impaired control laboratory paradigm have face validity as models of limits placed on alcohol consumption and negative consequences of alcohol use; however, it is crucial that the paradigm be validated further. Future validations might include stress induction (Fox, Bergquist, Hong, & Sinha, 2007) which would be hypothesized to impair self-control and result in greater drinking in the impaired control paradigm compared to a neutral mood manipulation. Conversely, participants randomized to receive interventions with established efficacy – either pharmacotherapies (e.g., naltrexone; O’Malley et al., 2002) or behavioral/counseling (e.g., BASICS; Dimeff et al., 1999) – should display less impaired control and more moderate alcohol self-administration in this paradigm. Prospective research is underway to establish the predictive validity of alcohol self-administration behavior in this paradigm (specifically relationships to subsequent heavy drinking and alcohol-related problems). Future studies could also use the paradigm to screen novel interventions.
In conclusion, this experimental laboratory paradigm models individual differences in impaired control over alcohol use, which prior evidence suggests is an early indicator of problem drinking. A laboratory model of this phenomenon for use with young adults is a potentially valuable research tool. Our findings suggest that the impaired control laboratory paradigm introduces disincentives for excessive drinking and reveals variability in participants’ abilities or willingness to moderate their drinking. The range of drinking behavior observed suggests that the paradigm may hold promise for testing novel interventions and assessing the impact of experimental manipulations and other individual differences on drinking behavior.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health grants K01 AA 019694, K05 AA014715, P20 DA027844, RL1 DA017539, the VA VISN1 MIRECC, ABMRF/the Foundation for Alcohol Research, the Connecticut Department of Mental Health and Addiction Services and the Connecticut Mental Health Center. The funding sources had no role other than financial support. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
All authors contributed substantively to the manuscript and all others read and approved the final manuscript.
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Psyadon, Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices, the federal public defender’s office and gambling organizations and businesses on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. Dr. O’Malley is a member of the American College of Neuropsychopharmacology workgroup, the Alcohol Clinical Trial Initiative which is sponsored by Alkermes, Abbott Laboratories, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck, Pfizer and Schering Plough; is a partner in Applied Behavioral Research; has received medication supplies from Pfizer and a contract with Nabi Biopharmaceuticals. She consulted to Pfizer and serves on the Scientific Panel of Advisors of the Hazelden Foundation. All other authors have no disclosures.
The authors would like to thank Jane Taylor, Ph.D. for helpful discussions regarding the study’s methods; Dena Davidson, Ph.D., for advice on conducting alcohol self-administration in bar settings; Ty Brumback and Andrea King, Ph.D. for providing further information about their study and Alana Rojewski, Ph.D. for helpful comments on a prior version of the manuscript. The authors would also like to thank Rosa Cohen and Elisa Gagliardi for assistance with data collection; Kelly DeMartini, Ph.D. and LaTrice Montgomery, Ph.D., for conducting the alcohol-focused motivational interviews during the follow-up appointments; study nurse Denise Romano, APRN, for conducting the medical screens; and Elaine LaVelle, M.S. for assistance with data management.
Footnotes
DISCLOSURES
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript.
References
- Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug and Alcohol Dependence. 2007;91:10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB. Reliability and validity of the Timeline Follow-Back Interview among psychiatric outpatients: A preliminary report. Psychology of Addictive Behaviors. 1997;11:26–33. [Google Scholar]
- Carey KB, Scott-Sheldon LAJ, Carey MP, DeMartini KS. Individual-level interventions to reduce college student drinking: a meta-analytic review. Addictive Behaviors. 2007;32:2469–2494. doi: 10.1016/j.addbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115:340–349. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- Corbin WR, Gearhardt A, Fromme K. Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology. 2008;197:327–337. doi: 10.1007/s00213-007-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcoholism: Clinical and Experimental Research. 1999;23:195–203. [PubMed] [Google Scholar]
- Davidson D, Swift R, Fitz E. Naltrexone increases the latency to drink alcohol in social drinkers. Alcoholism: Clinical and Experimental Research. 1996;20:732–739. doi: 10.1111/j.1530-0277.1996.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Dimeff LA, Baer JS, Kivlahan DR, Marlatt G. Brief alcohol screening and intervention for college students (BASICS): A harm reduction approach. New York: Guilford Press; 1999. [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Moeller FG, Swann AC. Suicidal behaviors and drug abuse: Impulsivity and its assessment. Drug and Alcohol Dependence. 2004;76S:S93–S105. doi: 10.1016/j.drugalcdep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Falk D, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and drug use disorders. Findings from the National Epidemiologic Survey of Alcohol Use and Related Conditions (NESARC) Alcohol Research & Health. 2008;31:100–110. [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: Current approaches and findings. Behavioral and Cognitive Neuroscience Reviews. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- First B, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationships between age at onset of alcohol use and DSM-IV dependence. Alcohol Health and Research World. 1998;22:144–148. [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Desai RA, McKee SA. Non-daily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: Findings from the NESARC. Alcoholism: Clinical and Experimental Research. 2008;32:2081–2087. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Booth PG, Luce A. Impaired control scale: cross-validation and relationships treatment outcome. Addiction. 1998;93:761–771. doi: 10.1046/j.1360-0443.1998.93576112.x. [DOI] [PubMed] [Google Scholar]
- Heather N, Dawe S. Level of impaired control predicts outcome of moderation-oriented treatment for alcohol problems. Addiction. 2005;100:945–952. doi: 10.1111/j.1360-0443.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, Zamir R. Development of a scale for measuring impaired control over alcohol consumption: a preliminary report. Journal of Studies on Alcohol. 1993;54:700–709. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, Weitzman ER. Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18–24, 1998–2005. Journal of Studies on Alcohol and Drugs Supplement. 2009;16:12–20. doi: 10.15288/jsads.2009.s16.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Gotham HJ, Wood PK. Transitioning into and out of large-effect drinking in young adulthood. Journal of Abnormal Psychology. 2001;110:378–391. doi: 10.1037//0021-843x.110.3.378. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Epstein EE, McCrady BA. Loss of control and inability to abstain: The measurement of and the relationship between two constructs in male alcoholics. Addiction. 1995;90:1025–1036. doi: 10.1046/j.1360-0443.1995.90810252.x. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Leggio L, Swift RM. A safety and tolerability laboratory study of the combination of aripiprazole and topiramate in volunteers who drink alcohol. Human Psychopharmacology. 2009;24:465–472. doi: 10.1002/hup.1042. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biological Psychiatry. 2007;62:694–7. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Corbin WR, Fromme K. Craving predicts within session drinking behavior following placebo. Personality and Individual Differences. 2009;46:693–698. doi: 10.1016/j.paid.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Fenton M, Volpicelli JR. Impaired control and undergraduate problem drinking. Alcohol & Alcoholism. 2007;42:42–48. doi: 10.1093/alcalc/agl095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, O’Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology. 2010;212:25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Patock-Peckham JA, Potenza MN. Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Experimental and Clinical Psychopharmacology. 2012;20:92–106. doi: 10.1037/a0026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Toll BA, Taylor LA, Volpicelli JR. Alcohol-induced disinhibition expectancies and impaired control as prospective predictors of problem drinking in undergraduates. Psychology of Addictive Behaviors. 2009;23:553–563. doi: 10.1037/a0017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine HG. The discovery of addiction: Changing conceptions of habitual drunkenness in America. Journal of Studies on Alcohol. 1978;39:143–174. doi: 10.15288/jsa.1978.39.143. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T. Acute alcohol impairs conditioning of a behavioral reward-seeking response and inhibitory control processes—implications for addictive disorders. Addiction. 2009;104:2013–2022. doi: 10.1111/j.1360-0443.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- Lozano BE, Stephens RS. Comparison of Participatively-set and Assigned Goals in the Reduction of Alcohol Use. Psychology of Addictive Behaviors. 2010;24(4):581–591. doi: 10.1037/a0021444. [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A, Stark LH. The first drink: Psychobiological aspects of craving. Archives of General Psychiatry. 1974;30:539–547. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: An experimental analogue. Journal of Abnormal Psychology. 1973;81:233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- Marsh A, Smith L, Saunders B, Piek J. The impaired control scale: Confirmation of factor structure and psychometric properties for social drinkers and drinkers in alcohol treatment. Addiction. 2002;97:1339–1346. doi: 10.1046/j.1360-0443.2002.00190.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addictive Behaviors. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective alcohol effects and drinking behavior: the relative influence of early response and acquired tolerance. Addictive Behaviors. 2008;33:1306–1313. doi: 10.1016/j.addbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Fromme K. Age of first use and delay to first intoxication in relation to trajectories of heavy drinking and alcohol-related problems during emerging adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:1991–1999. doi: 10.1111/j.1530-0277.2012.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol dependent subjects and activates the hypothalamo-pituitaryadrenocorticol axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Read JP, Kahler CW, Strong D, Colder CR. Development and preliminary validation of the Young Adult Alcohol Consequences Questionnaire. Journal of Studies on Alcohol. 2006;67:169–178. doi: 10.15288/jsa.2006.67.169. [DOI] [PubMed] [Google Scholar]
- Patock-Peckham JA, Morgan-Lopez AA. College drinking behaviors: Mediational links between parenting styles, impulse control, and alcohol-related outcomes. Psychology of Addictive Behaviors. 2006;20:117–125. doi: 10.1037/0893-164X.20.2.117. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcoholism: Clinical and Experimental Research. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. Alcohol increases impulsivity and abuse liability in heavy drinking women. Experimental and Clinical Psychopharmacology. 2012;20:454–465. doi: 10.1037/a0029087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40:305–315. [Google Scholar]
- Rutherford HJV, Mayes LC, Potenza MN. Neurobiology of Adolescent Substance Use Disorders: Implications for Prevention and Treatment. Child and Adolescent Psychiatric Clinics of North America. 2010;19:479–492. doi: 10.1016/j.chc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Moreland RL. Alcohol and group formation: A multimodal investigation of the effects of alcohol on emotion and social bonding. Psychological Science. 2012;23:869–878. doi: 10.1177/0956797611435134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Archives of General Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of Alcohol (SRE) as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- Sobell L, Sobell M. Alcohol consumption measures. In: Allen P, Wilson VB, editors. Assessing alcohol problems: a guide for clinicians & researchers. 2nd edn. Bethesda: National Institute on Alcohol Abuse and Alcohol; 2003. pp. 75–99. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) British Journal of Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationship of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcoholism: Clinical and Experimental Research. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Acute tolerance to alcohol impairment of behavioral and cognitive mechanisms related to driving: Drinking and driving on the descending limb. Psychopharmacology. 2012;220:697–706. doi: 10.1007/s00213-011-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R manual: Wechsler Adult Intelligence Scale-Revised. New York: Harcourt, Brace, & Jovanovich; 1981. [Google Scholar]
- Wilson GT, Leaf RC, Nathan PE. The aversive control of excessive alcohol consumption by chronic alcoholics in the laboratory setting. Journal of Applied Behavior Analysis. 1975;8:13–26. doi: 10.1901/jaba.1975.8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD, Read JP, Palfai TP, Stevenson JF. Social influence processes and college drinking: The mediational role of alcohol outcome expectancies. Journal of Studies on Alcohol. 2001;62:32–43. doi: 10.15288/jsa.2001.62.32. [DOI] [PubMed] [Google Scholar]