Abstract
The immune system, best known as the first line of defense against invading pathogens, is integral to tissue development, homeostasis and wound repair. In recent years, there has been a growing appreciation that cellular and humoral components of the immune system also contribute to regeneration of damaged tissues, including limbs, skeletal muscle, heart and the nervous system. Here, we discuss key findings that implicate inflammatory cells and their secreted factors in tissue replacement following injury via stem cells and other reparative mechanisms. We highlight clinical conditions that are amenable to immune-mediated regeneration and suggest immune targeting strategies for tissue regeneration.
Keywords: regeneration, inflammation, macrophage, regulatory T cell
Introduction
For centuries, biologists have marveled at the ability of organisms such as salamanders to re-grow near perfect copies of amputated body parts through a precisely orchestrated process called epimorphic regeneration. Epimorphic regeneration occurs via the formation of a blastema, a mass of undifferentiated and differentiated cells containing a heterogeneous pool of progenitor cells. Instead of forming a blastema, the few mammalian tissues that are capable of regenerating, such as blood, skeletal muscle and epithelium, renew predominantly through stem cells. However, stem cell based regeneration has not proven broadly effective for most tissues plagued by degenerative processes such as the heart and nervous system. Here, we suggest that immune-mediated mechanisms of regeneration and repair may complement existing stem cell therapies or may be a viable alternative to using stem cells as a way to promote functional regrowth of vital tissues.
Regenerative capacity and the development of a mammalian immune system: an inverse relationship
Following injury, immune cell activation is among the first responses detectable at the site of damage (Figure 1A). Whether immune activation results in tissue regeneration or scarring is determined by a variety of factors including age, species, and the availability of a stem or progenitor cell pool. Evolutionary and developmental advances in the immune system have been inversely correlated with the capacity to fully regenerate damaged tissues (Figure 1B) (Fukazawa et al., 2009; Mescher and Neff, 2005; Mescher et al., 2013). The more phylogenetically primitive urodele amphibians (salamanders), the only vertebrates with the ability to completely regenerate limbs as adults, have a “weak” immune response in terms of specificity, speed of onset, and memory compared to their anuran amphibian (frog) relatives. Selective pressure to seal wounds rapidly with impermeable scars may have increased as vertebrates left aquatic environments and became homeothermic, driving evolution of a robust inflammatory response and refined adaptive immune system at the expense of epimorphic regeneration. Likewise, Xenopus larvae, which start with an ancestral-like immune system, can regenerate hindlimbs and tails. After the peak of metamorphosis, when the immune system matures into a highly evolved, mammalian-like system, regenerative capacity is lost (King et al., 2012). In mammals, wounds penetrating the dermis undergo scarring postnally. During fetal development, however, dermal injuries regenerate in a distinct process of scarless wound healing (Table 1). A number of factors likely enable scarless healing (Larson et al., 2010), including varied composition of the extracellular matrix (ECM), intrinsic differences in fibroblasts, and a transient inflammatory response characterized by significant decreases in platelet aggregation, leukocyte infiltration, and cytokine production. In depth understanding of the unique immune systems of fully regenerating organisms or developmental stages may provide clues to therapeutic avenues to restore damaged tissues in mammals.
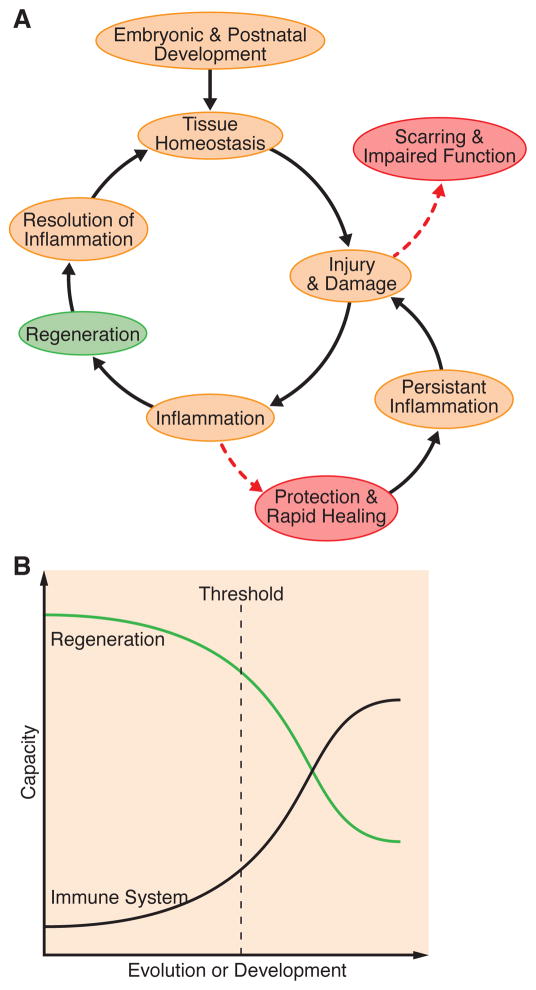
Figure 1. The influence of the immune system on development, homeostasis and disease.
(A) During embryonic and postnatal development, the immune system regulates processes such as branching morphogenesis, ductal formation and angiogenesis. Similar functions are maintained in some adult tissues to maintain normal homeostasis. Injury or disease elicits an inflammatory response that can either promote functional restoration of the tissue (regeneration) or a rapid healing response that may protect the organism at the expense of preserving structure and function. Inflammation usually resolves in the regenerative response while inflammation often persists in wound healing and scar formation, ultimately impairing the normal function of the tissue. (B) Inverse relationship between the capacity to regenerate and the strength and intricacy of the immune system during development or evolution. The threshold indicated on the graph conceptualizes the balance point at which the pro-regenerative components of the immune response are maintained within the context of a more advanced immune system. Identifying this threshold will be a key step towards the development of regenerative therapies targeted at immunity.
Table 1. Summary of the regenerative capacity, mechanisms, and disease states of representative mammalian tissues.
The process of regeneration is summarized for six different tissues, each with varying regenerative capacities: skin, heart, skeletal muscle, CNS, liver and bone. For each, steps that have the potential to be influenced by immunity are listed as well as the disease state and summary of immune targeted therapeutics.
| TISSUE | DEBRIS CLEARNACE MECHANISM |
PROGENITOR CELL POOL |
IMMUNE-DERIVED STIMULI |
POLARIZATION & HETEROGENITY |
DISEASE STATE DUE TO FAILED REGENERATION |
DEFECT | DRUGS TARGETING IMMUNITY |
|---|---|---|---|---|---|---|---|
| Skin (dermis) | Macrophages, fibroblasts, keratinocytes | Fibroblasts, endothelial cells | TGFβ, EGF, VEGF, IGF | Early and late macrophages (similar to M1/M2) | Full-thickness wound scarring in adults | Excessive inflammation Decreased migration Collagen accumulation |
IFN-α, IFN-β, IFN-γ; steroids |
| Heart | M1 or Ly-6Chi monocytes/macrophages | Resident cardiomyocytes | n/a | Four pools of cardiac macrophages; sequential Ly6Chi, Ly6Clo | Myocardial infarction Heart failure | Lack of progenitors; Insufficient immune polarization | ACE inhibitors |
| Skeletal muscle | Macrophages and FAPs | Satellite cells | Treg amphiregulin, IL-6, IL-1β, TNFα, IGF1, TGFβ | Muscle Treg; M1/M2 | Muscular dystrophy | Myofiber defect; satellite cell exhaustion; chronic inflammation | Glucocorticoids |
| CNS (myelination) | Macrophages and microglia | Oligodendrocyte progenitor cells | IGF1 | M1/M2 | Multiple sclerosis | Impaired clearance of myelin debris | anti-GM-CSF; Pioglitazone (PPARg agonist); Glatiramer acetate |
| Liver | Kupffer cell and liver macrophages | Hepatocytes and Hepatic Progenitor Cells | Eosinophil IL-4; Kupffer IL-6, TNFα | Inflammatory and restorative macrophages; Th1, Th2, Th17 lymphocytes | Chronic liver disease (viral, toxic, autoimmune, metabolic) | Unresolved fibrosis | TLR4, CCR2 inhibitors; PPAR-α/β agonists |
| Bone | Osteoclast | Monocyte progenitor | Csf1 | n/a | Osteopetrosis or osteoperosis | Lack of or excessive bone resorption | IFN-γ1b, corticosteroid; RANKL inhibitor (mAb) |
Developmental and homeostatic functions of the immune system
The immune system is integral to the initial development of an organism as well as the continuous replacement of differentiated cell types to maintain homeostasis (Figure 1A) (reviewed in (Pollard, 2009; Wynn et al., 2013)). Branching morphogenesis and remodeling in the kidney, pancreas, mammary gland, retina and vascular system are regulated by leukocytes and soluble inflammatory factors. Myeloid cells, for example, modulate vascularity (Nucera et al., 2011) by mediating angiogenic branching (Kubota et al., 2009) and anastomosis (Fantin et al., 2010). Mice deficient in the primary regulator of mononuclear phagocyte production, colony-stimulating factor 1 (Csf1), or its receptor Csf1R lack the majority of functional myeloid cells and display numerous developmental abnormalities due to disrupted ECM remodeling (Banaei-Bouchareb et al., 2004; Dai et al., 2002; Pollard JW, 1996; Rae et al., 2007). Branching morphogenesis in the mammary gland depends on eosinophils and mast cells (Gouon-Evans et al., 2000; Lilla and Werb, 2010), illustrating that numerous immune cells coordinate development. Immune cells also influence morphogenesis by acting directly on mammary stem cells (Gyorki et al., 2009) and phagocytizing both apoptotic and senescent cell debris (Dai et al., 2002; Munoz-Espin et al., 2013). Microglia phagocytize synaptic debris and are essential for the pruning of synapses during normal postnatal brain development (Paolicelli et al., 2011). Synapse pruning in the developing retina relies on complement proteins C1q and C3 to tag CNS synapses for destruction (Stevens et al., 2007). Conversely, C1q and C3 are up-regulated in retinal synapses during glaucoma, suggesting aberrant reactivation of this developmental pathway promotes CNS degenerative disease.
Gene expression profiling, lineage tracing, and genetic models have been increasingly used to identify novel tissue-specific, subsets of immune cells that shape development and regeneration (King et al., 2012; Wynn et al., 2013). The recent discoveries discussed below begin to shed light on how these specialized or polarized populations of immune cells may be integral to promoting regeneration in mammalian tissues. The nature and efficiency of the reactivation of developmental functions during injury may be critical to the ability of an organism to completely regenerate injured tissue or not (Figure 1A). In this review, we focus on recent advances showing a proactive role of the immune system and its response to injury as a central mediator of tissue regeneration. By drawing from different systems, common mechanisms and themes occurring in injury and disease that may be relevant to new therapeutic avenues are highlighted.
Immune mechanisms of tissue regeneration
Mammals respond to organ damage through either compensatory growth of the remaining tissue, by activating resident precursor cell proliferation, or by the formation of a scar. Successful mammalian regeneration requires precise coordination of multiple processes, which include scavenging cellular debris, proliferation and activation of progenitor cells, immune modulation, angiogenesis and innervation of the newly forming tissue. While the involvement of immune cells in tissue repair has been appreciated since Metchnikoff observed that macrophages play a role in tissue repair in the late 1800’s, recent advances highlight new mechanisms that the immune system employs to regulate regeneration.
Debris clearance
Efficient clearance of cellular debris prevents the persistence of potentially toxic or immunogenic material in the tissue environment and also activates phagocytes to secrete immunomodulatory factors that perform downstream effector functions (Figure 2). Recent findings indicate that defects in debris clearance can prevent effective regeneration. Macrophages, the professional phagocytes of the immune system, mount a polarized, biphasic response to tissue injury. Macrophages and the monocytes from which they are derived exhibit considerable heterogeneity that is not yet fully understood. Following conditioning by the inflammatory milieu including local growth factors and cytokines, macrophages polarize into classically activated (M1) or alternatively activated (M2) subtypes based on their markers, function, and cytokine profiles (Gordon and Martinez, 2010). Typically, M1 cells produce high levels of pro-inflammatory cytokines and nitric oxides that aid in host defense but can also damage healthy tissue while M2 macrophages mediate wound healing, tissue repair and the resolution of inflammation. However, M1 cells can also play a positive role in tissue regeneration. In the heart and skeletal muscle, early infiltration by M1 macrophages facilitates clearance of necrotic tissue (Arnold et al., 2007; Nahrendorf et al., 2007), and disrupting macrophage polarization impairs healing and regeneration, respectively (Perdiguero et al., 2011). While macrophages influence multiple facets of muscle regeneration, it appears that in some instances, debris clearance may supersede their roles in satellite cell proliferation, myofiber growth, and endothelial cell activation. For example, Hif-1a, the master transcriptional regulator of the hypoxia response, is dispensable in satellite cells during skeletal muscle regeneration. Surprisingly, myeloid-specific deletion of Hif-1a leads to decreased activation of Cox-2, decreased macrophage phagocytosis, and a subsequent delay in skeletal muscle regeneration (Scheerer et al., 2013).
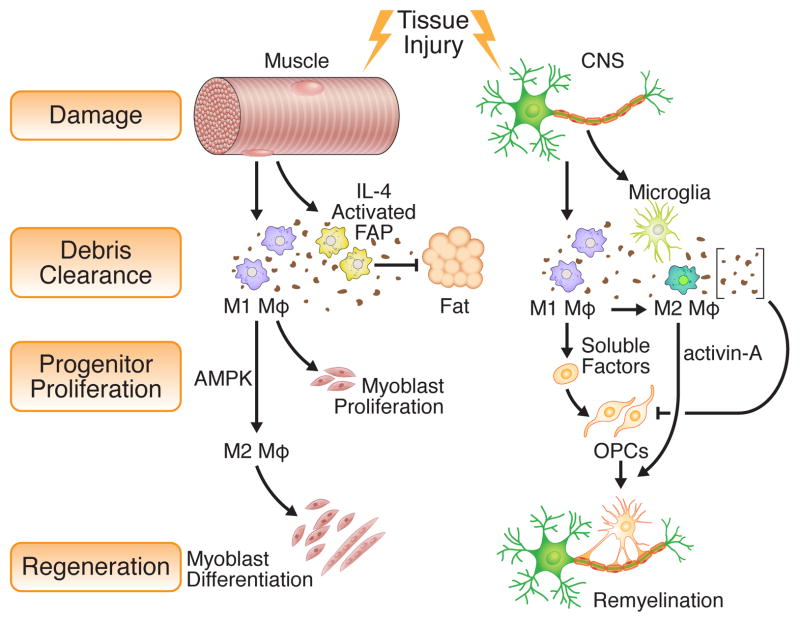
Figure 2. Debris clearance as a coordinator of regeneration.
Debris clearance orchestrated by the immune system is a key activator of subsequent steps in regeneration, including progenitor cell activation, differentiation, and immune polarization. The comparison shown between skeletal muscle and the CNS highlights the key cell types and soluble factors involved. Following skeletal muscle injury, both M1 macrophages and fibro/adipocyte progenitors (FAPs) clear cellular debris. FAP phagocytic activity depends on eosinophil derived IL-4; in its absence, the progenitors differentiate into fat, causing muscle dysfunction. Phagocytic M1 macrophages promote myoblast proliferation and polarize to an M2 phenotype via AMPK-mediated signaling. M2 polarization is required for appropriate myoblast differentiation. In the CNS, mature neurons lack robust regenerative potential. However, remyelination occurs in young, healthy adults by activation of oligodendrocyte progenitor cells (OPCs). Activation of OPC proliferation depends on efficient clearance of myelin debris, which contains inhibitory factors, and also macrophage -derived soluble factors. Similar to myoblasts, the proliferation and recruitment of OPCs depends on M1 macrophages while differentiation of OPCs and remyelination relies on M2 macrophage-secreted activin-A.
Perhaps the strongest link between immune-mediated debris clearance and regenerative capacity has been documented in the CNS and demyelinating diseases (Figure 2). The sensitivity of the CNS to debris clearance may be attributed to the numerous inhibitory properties of myelin, the membrane sheath that insulates axons, when deposited in damaged tissue. While remyelination is robust in the CNS of young, healthy mice, the ability to restore the myelin sheath declines with age or in disease. Clearance of myelin debris depends on macrophages (Kotter et al., 2006) and recent data suggest that the decline in efficient CNS regeneration is linked to the immune system. Specifically, parabiosis experiments indicate that young macrophages recruited as monocytes from the blood have a greater capacity to efficiently clear myelin debris than old macrophages (Ruckh et al., 2012). Furthermore, chronic degenerative disease occurs when phagocytosis is compromised by loss-of-function mutations in microglial Triggering Receptor Expressed On Myeloid Cells 2 (TREM2) or DAP12 (also known as TYRO protein tyrosine kinase-binding protein), its transmembrane adaptor and signaling molecule. (Neumann and Takahashi, 2007). Conversely, transplantation of myeloid cells that over-express TREM2 into experimental autoimmune encephalomyelitis (EAE) mice, a model of multiple sclerosis, improved myelin removal and facilitated regeneration of the spinal cord (Takahashi et al., 2007). Given the inhibitory effects of myelin on oligodendrocyte differentiation, these data suggest a model in which augmenting the clearance of debris by immune cells can enhance CNS regeneration through efficient remyelination. Elegant genetic studies such as those in skeletal muscle injury models, discussed below, are beginning to reveal the significance of non-macrophage cell types in debris clearance and regeneration (Figure 2). For example, debris clearing fibro/adipocyte progenitors have recently been shown to be pivotal mediators of skeletal muscle regeneration and are discussed in greater detail below (Heredia et al., 2013). Whether varied types of debris and their key phagocytic cell types can influence regeneration in other tissues will be an important topic for future investigation
Progenitor cell activation and stem cell function
Perhaps the most astonishing discoveries in immune-mediated regeneration have been made in skeletal muscle, a well-studied model for adult mammalian regeneration that employs activation of satellite cells, the resident progenitors of the muscle. While the importance of macrophages in skeletal muscle regeneration have long been appreciated (Arnold et al., 2007), both eosinophils and regulatory T cells (Tregs) have now been shown to be necessary for activation of satellite cells, which give rise to newly formed myofibers following injury (Burzyn et al., 2013; Heredia et al., 2013; Wynn et al., 2013) (Figure 3A). Using a number of genetic models to trace and delete soluble factors and their receptors in a cell-type specific fashion, Heredia et al. showed that IL-4-secreting eosinophils mediate skeletal muscle regeneration by activating fibro/adipocyte progenitors (FAPs) which, as mentioned previously, mediate necessary debris clearance. In the absence of IL-4, FAPs do not clear debris and instead differentiate into adipocytes, which contribute to muscle degeneration (Figure 2). Tregs modulate the activity of not only lymphocytes but also other immune cells such as macrophages, and thereby can indirectly influence the regenerative process. However, genetic ablation of Tregs has shown that muscle Tregs directly enhance satellite cell activation and differentiation by secreting amphiregulin (Burzyn et al., 2013) (Figure 3A).
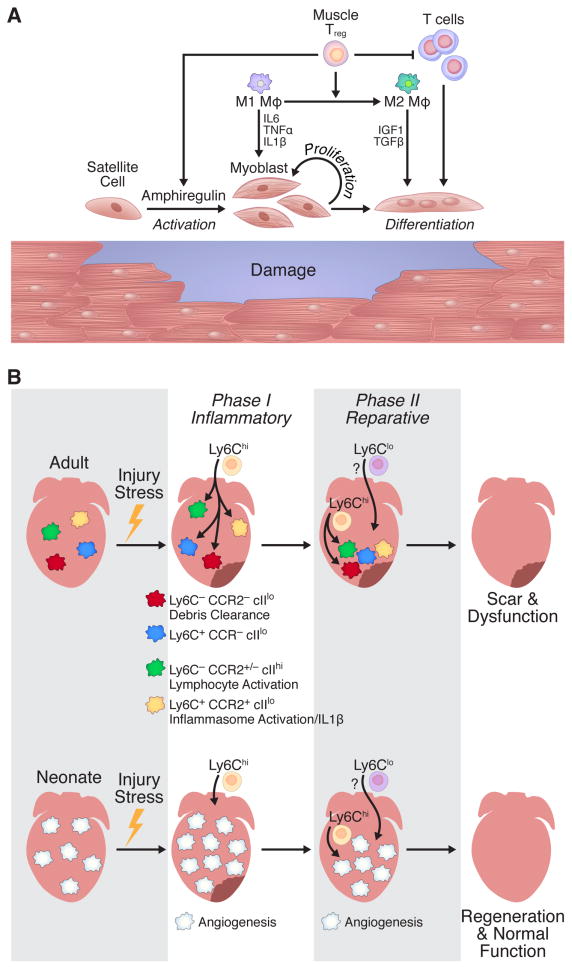
Figure 3. Immune cell polarization and heterogeneity are key components of tissue repair or regeneration.
(A) The regenerative capacity of skeletal muscle is driven by satellite cell activation, proliferation and differentiation. M1 macrophages are early responders that secrete cytokines with proliferative effects on myoblasts. The next phase of the response involves myotube differentiation driven by M2 macrophage-secreted IGF1 and TGFβ. Specialized muscle Treg cells influence all stages of regeneration: early secretion of amphiregulin activates myoblast proliferation, while in subsequent phases of regeneration muscle Treg are necessary for myotube differentiation, M1 to M2 polarization and attenuation of excessive T lymphocyte responses. (B) The adult mammalian heart, which lacks regenerative capacity, contains different cardiac macrophage subsets, with diverse functions, developmental origins, and mechanisms of homeostasis. The four populations, segregated by expression levels of CCR2, Ly-6C, and MHC class II, perform different functions as indicated. Upon injury such as a MI, a biphasic monocyte response occurs to promote an initial inflammatory phase followed by a reparative phase mediated by Ly-6Chi or Ly-6Clo splenic monocytes respectively. The two systems are linked by the ability of splenic Ly-6Chi monocytes to replenish all four subsets in Phase I. In the neonatal mouse heart, which can fully regenerate following MI, there is also a biphasic splenic monocyte response though the characterization of subsets of resident cardiac macrophages has yet to be investigated. Interestingly, neonatal cardiac macrophages differ from adult in their localization, abundance, and gene expression profile following injury and are required for regeneration by promoting angiogenesis. The regenerative subtype in the neonate has yet to be defined.
Neurons do not efficiently regenerate in mammals and several studies suggest that the inflammatory response to injury impedes neurogenesis (Carpentier and Palmer, 2009; Ekdahl et al., 2003; Monje et al., 2003). In the zebrafish brain, which has the capacity to regenerate and replace neurogenic activity, recent work shows that inflammation is necessary and sufficient to initiate neurogenesis via progenitor cell activation. Following injury, microglial cells and other leukocytes activate radial glial cell proliferation and neurogenesis by secreting Leukotriene C4 (LTC4)(Kyritsis et al., 2012). Even in the absence of injury, LTC4 alone increases proliferation of progenitor glial cells and the production of newborn neurons. Furthermore, inflammation alone initiated production of S100β, an EF-hand type Ca2+-binding protein, by radial glia and activation of the Gata3 transcription factor. These factors represent molecular switches that could be potentially targeted to promote neuronal differentiation and survival (Stella, 2012). Similarly, in rats, promoting inflammation in the eye through injury or pharmacological treatment can stimulate axon outgrowth in the normally non-regenerative retinal ganglion cell (RGC) axons of the primary visual pathway (Leon et al., 2000; Yin et al., 2003). Recent evidence suggests that in mice both neutrophils and macrophages are major sources of oncomodulin (Ocm), a key soluble factor in inflammation-induced regeneration, and are essential for neurite outgrowth in the CNS (Kurimoto et al., 2013; Kwon et al., 2013; Yin et al., 2006).
While the liver is a unique example of adult mammalian organ regeneration, the capacity for regeneration is compromised in chronic disease (Table 1). The presence of bipotent hepatic progenitor cells (HPCs) that can be activated to regenerate both cell types involved in bile synthesis, cholangiocytes and hepatocytes, leaves a window of therapeutic opportunity open. Recent studies in mice and humans have shown that macrophages control signaling pathways that regulate hepatic progenitor cell fate (Boulter et al., 2012). By studying divergent disease patterns in human samples and inhibiting Notch signaling in mouse models, the authors showed that Notch is required for adult biliary specification during chronic liver injury. During hepatocyte regeneration, the Notch signaling pathway is repressed though the ubiquitin ligase Numb, such that the loss of Notch signaling mediates exit from a biliary fate, and the acquisition of a hepatocyte phenotype in HPCs. Furthermore, phagocytosis of hepatic debris activates Wnt3a on macrophages, which, in turn, drives the Numb-activated hepatocyte program. In kidney regeneration, macrophage-derived Wnt7b is required for renal tubular epithelial regeneration (Lin et al., 2010). Cross-talk between the immune system and progenitor cell populations mediated by critical modulators of cell-to-cell signaling such as Notch and Wnt therefore seems to be a common regenerative signaling pathway in different tissues.
The immune system also affects progenitor and stem cells by creating the appropriate microenvironment for their development and function. The idea that macrophages create a niche for newly forming blood cells during erythropoiesis, one component of the robustly regenerating hematopoietic system, was suggested half a century ago with the identification of erythroblastic islands (a central macrophage surrounded by developing erythroblasts) in the bone marrow. Only recently, in vivo studies using genetic and chemical models of macrophage depletion confirmed a supportive role for macrophages during red blood cell development and diseases affecting erythropoiesis such as polycythemia vera (Chow et al., 2013(Ramos et al., 2013). As noted in the introduction, ductal morphogenesis in mammary gland development depends on immune cells. Mammary stem/progenitor cells also rely on the continued presence of macrophages evidenced by their diminished repopulating activity in macrophage deficient (Csf-1op/op) mice or following chemical ablation of macrophages (Gyorki et al., 2009), suggesting macrophages may constitute part of the normal mammary stem/progenitor cell niche. While macrophages are not required for intestinal development and normal crypt morphology, injury-activated macrophages in the colonic epithelial progenitor cell niche express a number of factors that promote proliferation and survival of epithelial progenitors. Furthermore, intestinal macrophages recruited to the site of injury and activated by the microbiota in a TLR-dependent manner support and promote proliferation of colonic epithelial progenitors (Pull et al., 2005). Therefore, the immune system is a crucial element for shaping the crypt progenitor cell niche in the injured intestinal epithelium.
Immunomodulation and immune cell heterogeneity
Appropriate spatial and temporal regulation of the immune response to injury or disease determines the soluble factor milieu and therefore the future fate of the tissue. Resolution of the inflammatory response leads to regeneration or chronic inflammatory cell activation and soluble factor production perpetuates tissue damage and hampers repair (Figure 1A). Commonly, a temporal shift or polarization occurs in the immune response that is typically driven by M1, or pro-inflammatory macrophages, and M2, or anti-inflammatory and reparative macrophages. Both arms of the immune response are required for repair in many systems such as heart, skeletal muscle, and the CNS. If initial, pro-inflammatory signals are not controlled, for example, excessive tissue damage can occur and block repair. Conversely, premature initiation of the anti-inflammatory program can also disrupt efficient tissue healing; for example, skeletal muscle regeneration is impaired when macrophages are prematurely skewed by treatment with IL-10 or genetic loss of MKP-1 (Perdiguero et al., 2011). Also, in both skeletal muscle regeneration and remyelination, M1 macrophages recruit and stimulate progenitor proliferation while M2 macrophages mediate differentiation, dispelling the common view that M1 macrophage responses are overall bad while M2 are good.
A host of M1 or M2 soluble factors are implicated in skeletal muscle regeneration. While M1 macrophages activate the proliferative stage of myogenesis and satellite cell proliferation through production of IL6, TNFα, IL1β, and G-CSF; IGF1 and TGFβ production by M2 macrophages supports myogenic differentiation and growth (Arnold et al., 2007; Lu et al., 2011; Saclier et al., 2013) (Figure 3A). Furthermore, the impact of M1/M2 macrophage skewing on modulating the inflammatory response and skeletal muscle regeneration has recently been highlighted with the identification of a new regulator of M1/M2 balance (Figure 3A). AMP-activated protein kinase (AMPK), which regulates energy homeostasis by sensing ADP:ATP and AMP:ATP ratios, mediates the switch in macrophage polarization from M1 to M2 and is necessary for regeneration following skeletal muscle injury (Mounier et al., 2013). In wild-type mice, the phagocytosis of muscle debris triggers M1 macrophages to skew towards M2 (Figure 2). Mice with macrophages deficient in AMPK have impaired skeletal muscle regeneration due to the inability of AMPK-deficient macrophages to skew towards M2 following phagocytosis. While the lack of AMPK does not impede myoblast proliferation in vivo or in vitro, myogenesis is impaired by a defect in differentiation and myotube formation. The potential importance of AMPK signaling in other cell types and the signal from M2 macrophages that mediates myogenic differentiation and tissue regeneration remain to be identified and may hold promise as therapeutic targets for degenerative muscle diseases.
Dual roles of immune cells in regenerating tissue are an emerging theme. In addition to their direct role in satellite cell activation described above, Tregs are central immunomodulators of skeletal muscle regeneration by controlling T cell numbers and the bi-phasic, sequential recruitment of pro- and anti- inflammatory macrophages (Burzyn et al., 2013) (Figure 3A). Oligodendrocyte differentiation efficiently forms new myelin sheets around axons in young, healthy mammals. Peripherally derived macrophages and resident microglia are important for clearing debris but also have been recently shown to directly drive remyelination by promoting oligodendrocyte differentiation through secretion of activin-A (Figure 2). Importantly, a switch from M1 to M2 polarization was critical for effective remyelination (Miron et al., 2013).
Another emerging theme is that tissue-specific varieties of leukocytes and lymphocytes perform multiple functions beyond their roles in theimmune system that are instrumental for tissue homeostasis and disease. In addition to the muscle Treg described previously, recent genetic fate-mapping and deletion studies reveal four different cardiac macrophage populations, the origins from which they arise, and the mechanisms that maintain macrophage homeostasis and expansion in response to cardiac stress (Epelman et al., 2014) (Figure 3B). Not only do their developmental origins differ, but also transcriptional profiling and functional assays show that each of the four subsets, tracked by expression of CCR2, Ly-6C, and MHC class II, have specialized functions. The resident cardiac macrophages that were MHC-IIlo contribute to homeostasis by phagocytosis of cardiomyocyte debris. Furthermore, cardiac stress led to upregulation of inflammasome and IL-1β related genes from monocyte-derived macrophage subsets that were CCR2+. Finally, the macrophages that expressed high levels of MHC-II efficiently processed and presented antigen to T cells, suggesting a role in immunosurveillance. These findings shed light on the paradox raised by previous data that upon injury, blocking CCR2 can be cardioprotective while depleting macrophages by other strategies further increases injury and hampers cardiac function (Kaikita et al., 2004; van Amerongen et al., 2007). Together, these data suggest that preserving resident cardiac macrophage expansion via proliferation, while targeting peripheral monocyte recruitment, might lead to improved myocardial recovery after injury. This landmark study (Epelman et al., 2014) highlights the need to further delineate phenotypic and functional differences among immune cells within specific tissues during homeostasis and following injury so that therapies can be developed to preserve subsets that are cytoprotective while targeting the activation or recruitment of immune cells, in a subset-specific way, that contribute to damage. Furthermore, gene expression profiling comparing cardiac macrophages to splenic and brain macrophages illustrates that in addition to heterogeneity within the same organ, significant variation in resident macrophages from tissue to tissue warrants consideration (Pinto et al., 2012).
Angiogenesis
The re-establishment of adequate blood flow to injured and newly forming tissue is a key aspect of regeneration. Immune cells support developmental angiogenesis by secreting soluble factors, remodeling matrix and physically pruning and supporting the vasculature during the maturation process. In the developing mouse retina, vessel remodeling is under the control of macrophages (Stefater et al., 2011) via expression of the Wnt ligands Wnt5a and Wnt11, which enhance the expression of the VEGF inhibitory receptor Flt1. Interestingly, genetic disruption of the myeloid non-canonical Wnt pathway can enhance wound angiogenesis and repair, suggesting the same signaling pathway found in the retinal vasculature could have therapeutic applications for modulating myeloid cell signaling to treat wounds (Stefater et al., 2013).
The wound healing response in adult mice and humans relies on immune cells for secreting pro-angiogenic factors. Interestingly, skin wounds in macrophage-deficient PU.1−/− mice heal normally and show minimal scar formation (Martin et al., 2003). However, temporal deletion of the myeloid lineage through diphtheria toxin-mediated cell death reveals that macrophages have distinct functions in different phases of skin wound repair. When deleted early, the loss of pro-inflammatory macrophages minimized scar formation due to reduced keratinocyte cell death and other damage while later deletion resulted in hemorrhage and lack of closure due to defects in angiogenesis, vascular maturation, and stabilization (Lucas et al., 2010). Similar conditional depletion of myeloid cells in a model of sciatic nerve injury resulted in a decrease in vascular density and delayed neural cell proliferation, implicating the immune system in the regenerative angiogenesis of neural tissue (Barrette et al., 2008).
The adult mammalian heart is notoriously resistant to regeneration following injury, due primarily to the irreversible withdrawal of postnatal cardiomyocytes from the cell cycle. Following injury of the adult heart, the inflammatory response and specifically the monocyte/macrophage response has a dual function in scar formation. During the later phase, Ly6Clo and M2 macrophages are necessary for mediating angiogenesis concomitantly with fibrosis to form a scar (Nahrendorf et al., 2007). In contrast, before postnatal day 7, neonatal mice efficiently regenerate up to 20% of the mass of the heart following surgical ablation or myocardial infarction (Porrello et al., 2011; Porrello et al., 2013). Recent studies showed that the immune response to cardiac injury differs quantitatively and qualitatively during regeneration in comparison to the pro-fibrotic response mentioned (Aurora et al., 2014). Also, depletion of macrophages in P1 mice subjected to myocardial infarction impaired heart regeneration, at least in part, due to a lack of neoangiogenesis (Figure 3B). Given that the neonatal mouse does not mount a robust fibrotic response following ischemic cardiac injury, the data suggests that in mammalian heart regeneration, macrophages have the potential to promote angiogenesis without activating fibroblasts. The relative distribution in the neonate of the four cardiac macrophage populations found in the adult and the potential contribution of each population to the process of neonatal heart regeneration and angiogenesis remain to be defined (Figure 3B). The unique gene expression profile of early neonatal macrophages is of future interest for developing therapies that target the immune system to promote angiogenesis and tissue regeneration.
Adult teleost fish, such as zebrafish, are highly regenerative and equipped to regrow fins, retinae, spinal cord, and heart muscle following amputation or injury. Zebrafish cardiomyocytes are small, mononucleated and have underdeveloped sarcomeric structure, similar to embryonic and early postnatal cardiomyocytes, and their proliferative capacity is integral to the heart’s regenerative capacity. In both zebrafish and neonatal mice, the regenerated myocardium is derived from pre-existing cardiomyocytes (Jopling et al., 2010; Kikuchi et al., 2010). The epicardium provides progenitors important for angiogenesis during embryonic development and injury-induced heart repair or regeneration in both organisms (Bock-Marquette et al., 2009; Kikuchi et al., 2011; Smart et al., 2010) and recently has also been shown to regulate the inflammatory response and neutrophil infiltration after injury (Huang et al., 2012). Thymosin β4 (Tβ4) is a key activator of the epicardial progenitors that participate in neovascularization and its natural derivative β4-sulfoxide (Tβ4-SO) functions to prevent chronic inflammation and promote wound healing (Evans et al., 2013), further suggesting a link between regulation of the immune response and regenerative angiogenesis.
Regenerating skeletal muscle also relies on monocytes/macrophages for neoangiogenesis. Sterile injury models using transgenic lineage tracing of endothelial progenitors have shown that macrophage depletion compromises the differentiation of endothelial progenitors through the secretion of growth factors (Zordan et al., 2014). In place of compromised progenitor differentiation and angiogenesis, collagen accumulates and the injured muscle becomes fibrotic.
Finally, ischemic disorders of the CNS, such as retinopathies, are strongly associated with deficient or aberrant angiogenesis. Recent studies in an ischemic retinopathy model show that ER stress in ischemic neurons leads to down-regulation of netrin-1, which suppresses vascular regeneration in the hypoxic CNS. Neuronal netrin-1 triggers an angiogenic switch in macrophages. Depleting retinal macrophages or antibody-mediated blockade of VEGF hinders vascular regeneration, suggesting that neuronal-derived netrin-1 is a potent mediator of myeloid-cell-induced CNS vascular regeneration (Binet et al., 2013).
The significance of immune regulation of angiogenesis, progenitor cell activity, debris removal, and appropriate polarization of subsequent immune responses and soluble factor secretion suggests that a closer look at pro-regenerative therapeutics targeted at immunity is warranted. Below, we discuss some of the diseases that lend themselves to such therapies and examine potential ways to harness the immune system to promote regeneration.
Clinical Perspectives and challenges
Disorders susceptible to regenerative immunity
Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease caused by inflammatory damage to the myelin sheath that protects the nerve cells, leading to progressive degeneration of the demyelinated neurons (Table 1). Research has largely focused on therapies that could replace the myelinating cells, called oligodendrocytes, which derive from neural stem cells or further restricted cells called oligodendrocyte progenitor cells. Inflammation plays a complex role in the disease course of MS. While inflammation had been generally thought to inhibit regeneration in the CNS, several recent studies suggest that promoting inflammation acutely, for example with zymosan injection, can stimulate oligodendrocyte production (Foote and Blakemore, 2005; Setzu et al., 2006). The positive influence of acute inflammation on oligodendrocytes has been confirmed by zebrafish studies showing that inflammation was sufficient for regeneration (Kyritsis et al., 2012). How these effects manifest during the chronic and oligodendrocyte-specific inflammatory response of MS remains to be investigated further. It appears that shifting or changing the polarization of the inflammatory response may represent an alternative approach to simply blocking or inducing inflammation in the context of demyelinating disease. As an example, the recent findings showing that activin-A, solely expressed from M2 macrophages, can support remyelination (Miron et al., 2013) suggests that immunomodulatory therapies that induce a M2 macrophage shift may be candidates for clinical use in MS (Weber et al., 2007).
Muscular dystrophy
Muscular dystrophies refer a group of muscle wasting diseases characterized by progressive skeletal muscle weakness, defects in muscle proteins, and the death of muscle cells and tissues. Despite the ability of skeletal muscle to regenerate from satellite cells, necrosis of myofibers persists in the context of inflammation and changes in the muscle environment that eventually weaken the muscle system and hamper function (Table 1).
The immune system plays a pivotal but complex role in the pathogenesis of muscular dystrophies. Through their synthesis of TGFβ, macrophages in MDX mice, the mouse model for Duchenne’s muscular dystrophy, directly induce collagen production in fibroblasts and further amplify collagen accumulation through activation of profibrotic alternatively activated macrophages (Vidal et al., 2008). Furthermore, treating MDX mice with a peptide that blocks the interaction between leukocyte expressed integrin αMβ2 and fibrinogen dampens muscle inflammation and ameliorates disease. Myeloid cell infiltration and its temporal shift from M1 to M2 are essential to regeneration but recent discoveries highlight the significance of other cell types like Tregs and eosinophils (Burzyn et al., 2013; Heredia et al., 2013). Not only do they modulate polarization of the immune response but they also have autonomous effects on satellite cell and progenitor cell differentiation, respectively. Given that glucocorticoids are currently the only therapy for muscular dystrophy, this disease may benefit from development of specific immunomodulatory therapies directed at Tregs and/or eosinophils or the soluble factors they produce.
Heart failure
A myocardial infarction (MI) occurs every 25 seconds in the United States and often leads to heart failure, the leading cause of death in the developed world (Minino et al., 2011). Given that the adult mammalian heart lacks the inherent ability to regenerate, ischemic loss of tissue is accompanied by replacement of myocytes with a fibrous, noncontractile scar tissue which compromises cardiac function and ultimately leads to heart failure. Although some studies suggest that mammalian cardiomyocytes have measurable capacity for turnover (reviewed in (Porrello and Olson, 2010)), the presence of a true cardiac stem cell is controversial and the response of the heart is not sufficient to recover functional myocardium following significant cellular loss. Failure of several stem cell therapies suggests that providing progenitors may not be adequate and that approaches aimed at the immune response may be required (Santini and Rosenthal, 2012). Furthermore, while a general wound healing response occurs, complete with an inflammatory reaction, no therapeutic measures have been developed to modulate the immune system to prevent heart failure. Starting more than 30 years ago, clinical trials aimed at general blocking inflammation to more target approaches such as anti- CD18 integrin and complement inhibition have had detrimental or little effect respectively on outcome following MI (reviewed in (Frangogiannis, 2012)).
Experimental models of MI and data from patients suggests that while an inflammatory response is required for infarct healing, defects in resolution, and containment of the response result in adverse remodeling of the infarcted heart (Table 1). A number of recent advances in understanding the immune response to MI, in particular monocytes and macrophages, suggest that the heart harbors a unique spectrum of myeloid cells and elicits specialized immune responses in response to injury (Figure 3B). Greater understanding of the monocyte subsets and the kinetics by which they are recruited to the injured heart (Nahrendorf et al., 2007) has led to a number of additional studies that are increasing our understanding of how current therapies might affect cardiac immunity. For example, angiotensin-converting-enzyme (ACE) inhibitors, a standard treatment for MI and heart failure, may be beneficial to infarct healing by having anti-inflammatory properties and influencing specific monocyte migration to the heart (Leuschner et al., 2010). Furthermore, Ly-6Chi monocytes recruited in the initial inflammatory phase of the response to myocardial infarction also dictate the reparative phase by differentiating into Ly-6Clo macrophages (Figure 3B) (Hilgendorf et al., 2014).
In spite of these advancements, the focus of therapeutic strategies has been to stop uncontrolled or prolonged inflammation in order to prevent adverse remodeling and progression to heart failure. Studies have yet to shift to promoting specific components of immunity to stimulate regeneration of the myocardium, instead of modulating scar formation. Until recently, the concept of mammalian heart regeneration was not tractable due to lack of in vivo models. The capacity for neonatal mice to regenerate their hearts depends on a unique population of macrophages, suggesting a therapeutic opportunity may exist to promote heart regeneration by modulating the immune response either alone or in combination with therapies that stimulate cardiomyocyte proliferation. Lessons from skeletal muscle and remyelination suggest efficient debris clearance is key to successful regeneration, by both eliminating inhibitory factors from the tissue milieu and by triggering signaling cascades in phagocytes that are necessary for downstream soluble factor release or effector functions that promote regeneration. Careful genetic dissection of the key regulators of debris clearance and the consequences following cardiac injury may provide molecular targets for new therapies
Liver fibrosis
The liver continues to be a unique example of adult mammalian solid organ regeneration. However, chronic liver disease, caused by viral infection, autoimmunity, toxic injury or steatosis, remains a major cause of morbidity and mortality worldwide (Table 1). Recent efforts have uncovered opposing roles for the immune system in controlling regeneration during chronic liver disease. Pro-inflammatory Kupffer cells and infiltrating macrophages initiate and promote fibrosis by stimulation of stellate cells. In contrast, M2-like restorative macrophages and NK cells drive the resolution of fibrosis by inducing stellate apoptosis and senescence and also provide Wnt signals to drive hepatocyte regeneration (Boulter et al., 2012; Krizhanovsky et al., 2008). In addition, Arg1-expressing M2 macrophages protect the liver during schistosomiasis, not by battling the infection, but by suppressing liver fibrosis and chronic inflammation (Pesce et al., 2009). Current therapies focus on deterring fibrosis by dampening inflammation and fibroblast activation but have yet to enhance the positive, M2-like cells.
The underlying mechanisms of degenerating diseases in the heart, CNS, skeletal muscle, or other organs clearly differ by the presence or absence of a functional progenitor cell population that has the capacity to repopulate the damaged tissue. However, whether the regenerative strategy is to stimulate endogenous progenitors, reprogram other cell types to replenish the tissue, or to transplant exogenous cells, the native inhibition of these processes that is present within the diseased tissue will have to be appropriately modulated. The research highlighted here suggests that understanding and having the tools to fine-tune inflammation will be key to promoting a permissive environment for regeneration.
Inflammation: Harnessing the good and halting the bad
Within minutes of injury, infiltrating neutrophils and other inflammatory cells release reactive molecules that can further damage the tissue. Furthermore, chronic inflammation perpetuates tissue remodeling and functional impairment in a number of diseases. As a consequence, drug development and therapy has historically emphasized anti-inflammatories by way of steroids, NSAIDS, and even more targeted approaches such as anti-TNF monoclonal antibodies. The studies we highlight suggest that new developments require a more fine-tuned approach that allows specific blockade of the negative effects of inflammation in an environment that is permissive for the positive effects of the immune response. Systematic dissection of these components will aid in defining the threshold for which immunity is strong but regeneration is permitted (Figure 1B) and allow for precise therapeutic modulation to promote regeneration.
One emerging theme is to modulate the polarization of the immune response and recent data suggests modulating the port of entry for inflammatory cells may be one strategy. Similar to repair of the heart or skeletal muscle (Arnold et al., 2007; Nahrendorf et al., 2007), recovery from spinal cord injury requires a biphasic monocyte/macrophage response, in which M1 and M2 cells enter the injured tissue through distinct routes (Shechter et al., 2013). M1 macrophages penetrate the injured spinal cord through the leptomeninges while the cerebrospinal-fluid filled choroid plexus provides a permissive environment for M2 macrophages to repair the spinal cord. These results suggest that therapeutic modulation at distinct ports of entry to the injured CNS could be a novel approach to promote repair and regeneration.
Scrutiny of the immune responses in animal models of efficient regeneration will be essential for clues to therapeutically modulate the disease environment. Recent studies showed direct evidence for immunological control of complete regeneration in the adult vertebrate and neonatal mammal. Axolotls, aquatic salamanders, deploy a rapid and robust inflammatory response in the amputated limb that includes nearly immediate and early up-regulation of anti-inflammatory cytokines and macrophage dynamics. Systemic macrophage depletion of macrophages blocks axolotl limb regeneration, which can be restored upon re-amputation and replenishment of the macrophage population (Godwin et al., 2013). This dynamic and simultaneous induction of inflammatory processes in regenerating axolotl limbs is reminiscent of the rapid and concurrent fibroblast activity and cytokine secretion noted during scarless wound healing in the mammalian fetus (Larson et al., 2010). In addition, macrophages are necessary for heart regeneration in the neonatal mouse (Aurora et al., 2014).
The development of treatments targeting the immune system is currently hindered by the lack of markers to discriminate amongst subpopulations of immune cells, creating a gap in our understanding of how various subsets behave in normal and diseased tissues. An additional strategy to identify novel immunomodulatory targets for regenerative therapies is to thoroughly dissect the diversity and function of resident tissue-specific immune cells under conditions of homeostasis and injury, similar to the recent discovery of four cardiac macrophage subsets discussed above (Figure 3B) (Epelman et al., 2014). Therapies targeted at individual immune cell populations or soluble factors are being tested, though they primarily aim to curtail inflammation and affiliated fibrosis. Small molecules or monoclonal antibody inhibitors to CSF1R target macrophages, by blocking its ligand, ligand binding, or activation signaling (Patel and Player, 2009). While targeting inflammation alone could be sufficient to promote regeneration, a more plausible scenario will be the need to create an immunologically permissive environment in the context of other regenerative therapies. As an example, a recent study showed that acinar cell to beta-like cell conversion occurs in response to treatment with cytokines (Baeyens et al., 2014). In addition, recent findings suggest that efficient iPS formation depends on chromatin remodeling changes that are mediated by TLR signaling (Lee et al., 2012).
Evolution clearly selected fast healing and containment of injury or infection at the expense of the ability to reform a completely functional tissue. In order to selectively undo the loss of regenerative capacity without compromising the specificity and strength of the mammalian immune system, much remains to be learned about the functions of immunity, good and bad, in development, homeostasis and injury. The recent advances highlighted here improve our understanding of the cells and signals involved and underscore the potential of immunotherapies for tissue regeneration.
Acknowledgments
We are grateful to Jose Cabrera for graphics. We apologize to authors whose papers we could not cite due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stange G, Shemer R, Nord C, Scheel DW, Pan FC, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nature biotechnology. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S, Cerani A, Leboeuf D, Tremblay S, Rezende F, et al. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell metabolism. 2013;17:353–371. doi: 10.1016/j.cmet.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Bock-Marquette I, Shrivastava S, Pipes GC, Thatcher JE, Blystone A, Shelton JM, Galindo CL, Melegh B, Srivastava D, Olson EN, et al. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol. 2009;46:728–738. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nature medicine. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MA, Smart N, Dube KN, Bollini S, Clark JE, Evans HG, Taams LS, Richardson R, Levesque M, Martin P, et al. Thymosin beta4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nature communications. 2013;4:2081. doi: 10.1038/ncomms3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain : a journal of neurology. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. 2009;136:2323–2327. doi: 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast cancer research : BCR. 2009;11:R62. doi: 10.1186/bcr2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf I, Gerhardt L, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherrer-Crosbie M, et al. Ly-6Chigh Monocytes Depend on Nr4a1 to Balance both Inflammatory and Reparative Phases in the Infarcted Myocardium. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338:1599–1603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, Poss KD. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138:2895–2902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MW, Neff AW, Mescher AL. The developing Xenopus limb as a model for studies on the balance between inflammation and regeneration. Anat Rec (Hoboken) 2012;295:1552–1561. doi: 10.1002/ar.22443. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI. Neutrophils express oncomodulin and promote optic nerve regeneration. J Neurosci. 2013;33:14816–14824. doi: 10.1523/JNEUROSCI.5511-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33:15095–15108. doi: 10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plastic and reconstructive surgery. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla JN, Werb Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Developmental biology. 2010;337:124–133. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:358–369. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Current biology : CB. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Advances in biochemical engineering/biotechnology. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW, King MW. Changes in the Inflammatory Response to Injury and Its Resolution during the Loss of Regenerative Capacity in Developing Xenopus Limbs. PLoS One. 2013;8:e80477. doi: 10.1371/journal.pone.0080477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature neuroscience. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell metabolism. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. Journal of neuroimmunology. 2007;184:92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Patel S, Player MR. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Current topics in medicinal chemistry. 2009;9:599–610. doi: 10.2174/156802609789007327. [DOI] [PubMed] [Google Scholar]
- Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardi M, Caelles C, Serrano AL, Munoz-Canoves P. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. The Journal of cell biology. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS pathogens. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JWSE. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic. Adv Dev Biochem. 1996;4:153–193. [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Olson EN. Building a new heart from old parts: stem cell turnover in the aging heart. Circ Res. 2010;107:1292–1294. doi: 10.1161/CIRCRESAHA.110.235168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, Little MH. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Developmental biology. 2007;308:232–246. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, Marongiu MF, Gupta R, Levine RL, Abdel-Wahab O, et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nature medicine. 2013;19:437–445. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Santini MP, Rosenthal N. Myocardial regenerative properties of macrophage populations and stem cells. Journal of cardiovascular translational research. 2012;5:700–712. doi: 10.1007/s12265-012-9383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer N, Dehne N, Stockmann C, Swoboda S, Baba HA, Neugebauer A, Johnson RS, Fandrey J. Myeloid hypoxia-inducible factor-1alpha is essential for skeletal muscle regeneration in mice. J Immunol. 2013;191:407–414. doi: 10.4049/jimmunol.1103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, Franklin RJ. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Clark JE, Ehler E, Miquerol L, Rossdeutsch A, Marber MS, Riley PR. Thymosin beta4 facilitates epicardial neovascularization of the injured adult heart. Ann N Y Acad Sci. 2010;1194:97–104. doi: 10.1111/j.1749-6632.2010.05478.x. [DOI] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard JW, Ferrara N, Lang RA. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood. 2013;121:2574–2578. doi: 10.1182/blood-2012-06-434621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: macrophages in development, homeostasis and regeneration. Trends in molecular medicine. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N. Neuroscience. Inflammation to rebuild a brain. Science. 2012;338:1303–1304. doi: 10.1126/science.1232331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS medicine. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal B, Serrano AL, Tjwa M, Suelves M, Ardite E, De Mori R, Baeza-Raja B, Martinez de Lagran M, Lafuste P, Ruiz-Bonilla V, et al. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway. Genes Dev. 2008;22:1747–1752. doi: 10.1101/gad.465908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nature medicine. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nature neuroscience. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Zordan P, Rigamonti E, Freudenberg K, Conti V, Azzoni E, Rovere-Querini P, Brunelli S. Macrophages commit postnatal endothelium-derived progenitors to angiogenesis and restrict endothelial to mesenchymal transition during muscle regeneration. Cell death & disease. 2014;5:e1031. doi: 10.1038/cddis.2013.558. [DOI] [PMC free article] [PubMed] [Google Scholar]