Abstract
Background/Aims:
Minimal hepatic encephalopathy (MHE) implies subtle impairment of cognitive functions in the absence of features of overt encephalopathy. We aimed to determine the prevalence of MHE in patients with liver cirrhosis and to find out the effect of rifaximin, probiotics, and l-ornithine l-aspartate (LOLA) individually in reversal of MHE by comparing it with placebo group.
Patients and Methods:
This study was carried out in two phases. Phase I included the recruitment of 250 apparently healthy controls and extraction of normative data utilizing three neuropsychometric tests (NPTs) and critical flicker frequency (CFF) test. Phase II consisted of screening and recruitment of patients of MHE followed by drugs trial. A total of 317 cirrhotics were screened; 111 were excluded and the remaining 206 cirrhotics were screened for MHE using NPTs and/or CFF test. Of these, 124 patients with MHE were randomized to receive LOLA (n = 31), rifaximin (n = 31), probiotics (n = 32), for 2 months and were compared with patients who were given placebo (n = 30).
Results:
Out of 206 cirrhotics, 124 (60.19%) had MHE. Among these 124 MHE patients, 87 (70.16%) patients had CFF <39Hz, 112 (90.32%) patients with MHE had two or more abnormal NPTs, and 75 (60.48%) patients had abnormality on both the CFF values and more than two abnormal NPTs. Intention-to-treat analysis showed the number of patients who improved after giving treatment were 67.7% (21/31), 70.9% (22/31), 50% (16/32), and 30% (9/30) for LOLA, rifaximin, probiotics, and placebo, respectively. CFF scores and improvement in psychometric tests after treatment were significantly higher (P < 0.05) for LOLA, rifaximin, and probiotics as compared with placebo group.
Conclusions:
Prevalence of MHE is high in patients with cirrhosis of liver. Rifaximin, LOLA, and probiotics are better than giving placebo in patients with MHE.
Keywords: L-ornithine l-aspartate, minimal hepatic encephalopathy, rifaximin
Minimal hepatic encephalopathy (MHE) implies subtle impairment of cognitive functions in the absence of features of overt encephalopathy. MHE is a complex neuropsychological complication of cirrhosis, which is characterized by delayed reaction time and abnormal response inhibition. MHE is widely recognized in liver cirrhosis patients, significantly interferes with the normal functioning of daily routine activity, and also impairs health-related quality of life (HRQOL).[1] It also predisposes to motor vehicle accidents in the affected individuals because of their impaired attention skills and delayed reaction time. It has also been reported in patients with extrahepatic portal vein obstruction (EHPVO).[2] Because patients with MHE have no recognizable clinical symptoms of HE, namely, impaired intellectual functioning, personality changes, altered consciousness levels, and so on, but have mild cognitive and psychomotor deficits, a clinical diagnosis of it cannot be made.
Various tools have been evaluated for diagnosing MHE, including neuropsychological tests, computerized tests, short neuropsychological and computerized test batteries, and neurophysiological tests.[3,4,5,6]
Hyperammonemia is considered pivotal in the pathogenesis of MHE. In liver cirrhosis, hyperammonemia is secondary to portal hypertension leading to portosystemic shunting of blood, bypassing the hepatic filter, and exposing the systemic vasculature to high ammonia, hepatic parenchymal functional impairment leading to inadequate ammonia removal from portal venous blood. Thus, hepatic parenchymal dysfunction in liver cirrhosis is instrumental in the pathogenesis of MHE. Because the pathogenesis of MHE is believed to be similar to that of HE, the therapeutic measures that aim to reduce ammonia levels have been shown to be beneficial in this setting too.[7,8,9,10] Most studies have shown improvement in psychomotor functions of the patients with decrease in ammonia levels.
MHE is a subclinical form of hepatic encephalopathy (HE) with no recognizable clinical symptoms of HE but with mild cognitive and psychomotor deficits. The diagnostic criteria for MHE have not yet been standardized. It still rests on careful patient history and physical examination, normal mental status examination, demonstration of abnormalities in cognition and/or neurophysiological function, and exclusion of concomitant neurological disorders. Therefore, we conducted a study to help diagnosing MHE.
PATIENTS AND METHODS
This study was carried out in the Department of Gastroenterology, Swaroop Rani Nehru Hospital, M. L. N. Medical College, Allahabad, from August 2009 to August 2010. It was broadly divided into two phases: (1) recruitment of healthy controls and extraction of normative data to define cutoffs for three NPTs to detect MHE, and (2) screening and recruitment of patients of MHE for drug trial.
Recruitment of healthy controls and extraction of normative data
In Phase I, 250 apparently healthy individuals were recruited after a brief interview (a prestructured questionnaire was used to guide the interview) regarding their knowledge of numbers, consumption of psychotropic drugs and alcohol amount, vision problems, and so on. The selected candidates had a fair knowledge of numbers, a minimum of 2 years of education in school, age more than 18 years, normal vision or corrected vision with the aid of specs/lens, no evidence of alcohol abuse/misuse, and were not using any psychotropic drug for the previous 6 months. After obtaining an informed written consent, they were asked to perform three psychometric [number connection test-A (NCT-A), Figure connection test-A (FCT-A), and Digit Symbol Test (DST)] tests. Each test was explained to them and they were asked to practice it once in the same format before their final performance. The first two tests measured the number of seconds the subjects took for completion of the tests, whereas in the third test a fixed time of 90 s was allocated to the subjects and the maximum number of correct squares they could fill was taken into consideration.
The measurements were done in quite surroundings without any distracting noises. Patients were examined with “corrected vision.” Patients needing spectacles were asked to wear them while taking the reading.
At the end of 250 recruitments, the mean number of seconds taken to complete the first two tests and mean numbers of squares filled correctly by the subjects for the third test were calculated, respectively. Scores above +2 standard deviation (SD) from the mean score in NCT-A and FCT-A and below −2 SD in DST were considered abnormal.
Screening and recruitment of cases of MHE for study
Patient's characteristics and eligibility criteria
The diagnosis of cirrhosis was based on clinical, biochemical, endoscopic evidence, ultrasonographic findings, and liver histology findings (if available). Biochemical tests included hemogram, liver function tests, renal function tests, serum electrolytes, coagulogram, HBsAg, and anti-HCV. All the patients underwent general physical examination and systemic examination, including complete neurological and mental state examination. Child-Pugh scoring and oesophagogastroduodenoscopy were done in each case.
A total of 317 cirrhotics were screened for the study of whom 111 were excluded due to various reasons. The exclusion criteria included: (1) patients with overt HE or a history of overt HE in the past 6 weeks; (2) history of alcohol intake during past 6 weeks; 3) history of antibiotic or lactulose or probiotics use within the past 3 weeks; (4) gastrointestinal bleed in the past 6 weeks; (5) history of recent use of drugs (<6 weeks) effecting psychometric performance, such as antidepressents, antiepileptic, sedatives, psychotropic drugs; (6) spontaneous bacterial peritonitis, or other infection in the past 7 days; (7) renal insufficiency with creatinine >1.5 mg/Ll; (8) electrolyte imbalance; (9) hepatocellular carcinoma; (10) significant comorbid illness, such as heart, respiratory or renal failure; and any neurological disease that could interfere with intellect or motor performance of the person such as Alzheimer's or Parkinson's disease, respectively, or nonhepatic metabolic encephalopathies; (11) previous transjuglar intrahepatic portosystemic shunt or shunt surgery; (12) patients who restarted alcohol consumption during follow up; (13) inability to do psychometric tests due to poor vision, or those having color blindness; (14) patients not having a fair knowledge of numbers and not having been to school for at least 2 years; (15) women who were pregnant.
The remaining patients underwent Clinical Hepatic Encephalopathy Staging Scale (CHESS) to exclude overt encephalopathy.[11] Patients who fulfilled our eligibility criteria were evaluated for MHE by psychometric tests (NCT-A, FCT-A, and DST), and Critical Flicker Frequency (CFF).
CFF measurement was performed using a HEPAtonorm analyzer (Acc-136 GmbH, D-72127, Kusterdingen, Germany). It was measured in a quiet and semi-darkened room. Patients were first instructed and trained about the procedure. Flicker frequencies were measured eight times, and then the mean value was calculated. Measurement of the CFF thresholds was performed by intrafoveal stimulation with a luminous diode. Decreasing the frequency of the light pulses from 60 Hz downward, the CFF threshold was determined as the frequency when the impression of fused light turned to a flickering one. CFF was considered abnormal when the value was less than 39 Hz. After evaluation of test scores, a patient with cirrhosis was diagnosed as MHE if any two of the three psychometric tests were abnormal and/or CFF was < 39 Hz. If none of the above conditions got fulfilled, the patient was labeled as not to be having MHE.
After the diagnosis of MHE was made, the patients were randomized into four groups: DRUG-1 [l-ornithine l-aspartate (LOLA), 2 sachets 3 g each thrice a day] n = 31, DRUG-2 (Tab Rifaximin 400 mg thrice a day) n = 31, DRUG-3 (Cap Velgut one capsule twice a day) n = 32, and DRUG-4 (Placebo twice a day) n = 30.
The study was not blinded and the block randomization method was utilized for random allocation of drugs. The sequence remained concealed from the investigator and the generator of the random blocks did not participate in screening, enrolment, or drug delivery. Duration of the treatment was 2 months ± 3 days or unless the patient developed overt encephalopathy, expired, or was lost to follow-up. There were fortnightly follow-ups in the interim to keep a check on the compliance and development of complications. Patients bought medicines for 20 days once they came to the hospital. On follow-up after 15 ± 2 days, they were supposed to bring their empty packing of drugs and left over medicines. Those who were found to be noncompliant for 2 or more days in two follow-ups were considered drop-outs and were excluded from the analysis. Patients who did not return for follow-up after 17 days of previous visit were contacted on telephone and instructed to return for a regular check-up.
In the DRUG-1 group, LOLA [HepaMerz, Win-Medicare, New Delhi, India] was given to 31 patients in a dose of two sachets thrice a day (18 g of LOLA per day in three divided doses). Those in DRUG-2 group were the candidates for rifaximin [Rifagut, SUN Pharmaceutical Industries Limited, Andheri (E), Mumbai, India] given in dose of 400 mg thrice a day. They were also 31 in number. DRUG-3 had 32 members and they received probiotics (Velgut, ERIS Pharmaceuticals, Ahmadabad, India) as two capsules per day.
Velgut is composed of total 5 billion CFUs included Lactobacillus acidophilus 0.7 billion, Lactobacillus rhamnosus 0.6 billion, Lactobacillus plantarum 0.6 billion, Lactobacillus casei 0.6 billion, Bifidobacterium longum 0.6 billion, Bifidobacterium infantis 0.6 billion, Bifidobacterium breve 0.6 billion, Sacchromyces boulardi 0.1 billion, and Streptococcus thermophilus 0.6 billion.
Statistical Analysis
Proportions, mean, and standard deviation were utilized to present the descriptive statistics. Paired t test and Student's t test were used for univariate analysis of continuous variables. Fisher's Least Significant Difference (LSD) test, Analysis of Variance Analysis (ANOVA), and multiple comparisons analysis after ANOVA were utilized. Statistical Package for Social Sciences (SPSS) version 22.0 was used for data analysis.
Patient information sheet was given and was well explained to all the participants. Written consent on patient information and consent form were obtained from all the participants. Ethical approval was obtained from the Institutional Ethics Committee of MLN Medical College, Allahabad.
RESULTS
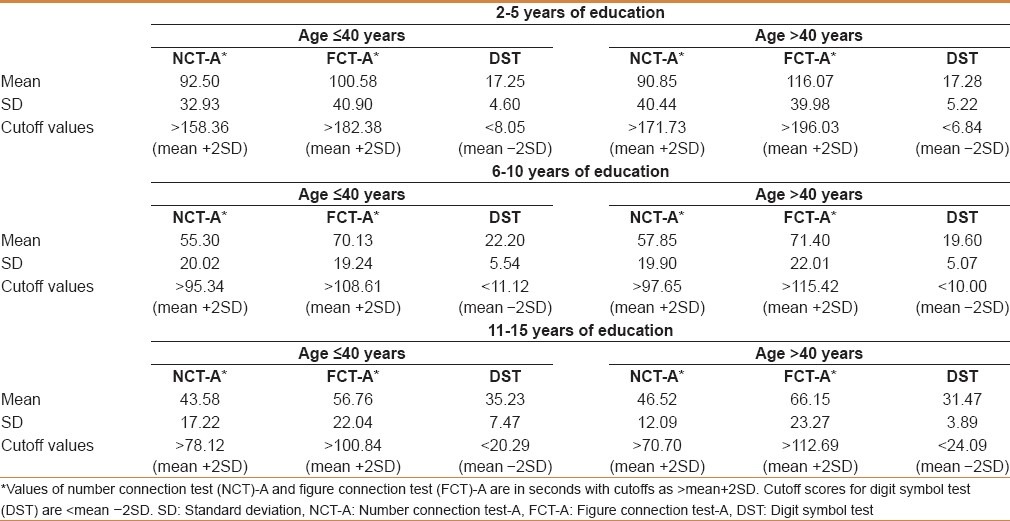
Between August 2009 and August 2010, 250 healthy individuals were selected to detect the cutoff values of three NPTs (NCT-A, FCT-A, and DST) to diagnose MHE as described in patients and methods under the section “recruitment of healthy controls and extraction of normative data” as Phase I. Different cutoffs were identified as per the age and education level of the individuals [Table 1].
Table 1.
Normative data for age groups less than and more than 40 years
In Phase II, a total of 317 cirrhotics were screened, of whom 206 (64.98%), who met our inclusion criteria, were enrolled for the study. A total of 111 (35.01%) patients were excluded from the study due to various reasons.
A total of 124 (60.19%) cirrhotics who had MHE based on CFF and/or abnormal neuropsychiatric tests (NCT-A, FCT-A, and DST), using age- and education-matched controls from the normative data [Table 1], were the final inclusions of the study on whom randomization and follow-ups were performed. The prevalence of MHE in our study was 60.19%. One hundred and twelve (90.32%) patients with MHE had 2 or more abnormal psychometric tests. In 75 (60.48%) patients, both the CFF values and more than two NPTs were abnormal.
Demographic variants
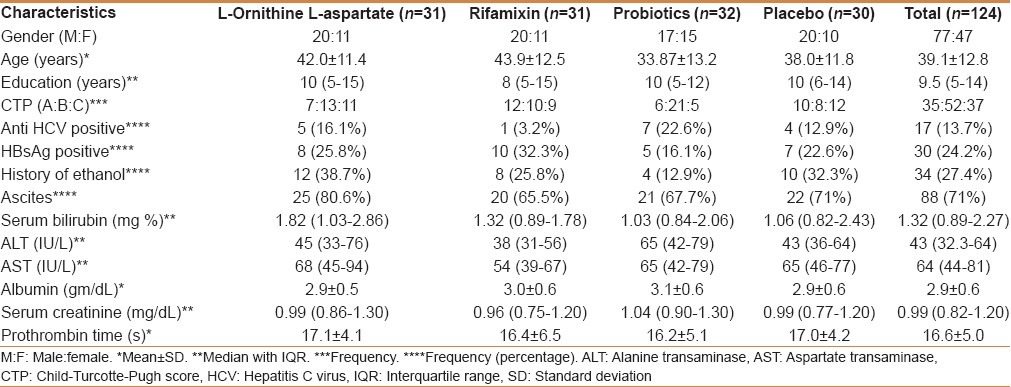
Thirty-five (28.22%) MHE patients were Child's A, 52 (41.93%) were Child's B, and 37 (29.83%) were Child's C cirrhotics. The mean age of the patients was 39.1 (±12.8) years with 77 males and 47 females. Seventy-two patients were ≤40 years, whereas 52 patients were older than 40 years. Median duration of education of the recruited population was 9.5 years. Details of baseline characteristics are given in [Table 2].
Table 2.
Baseline characteristics of patients with MHE in different interventional groups
Cirrhotic patients with MHE (n = 124) were randomized into four groups and were given LOLA (n = 31), rifaximin (n = 31), probiotics (n = 32), and placebo (n = 30), respectively, and were followed up fortnightly up to 2 months. A total of 20 patients could not be followed up to the end of the study: 10 lost to follow up, 6 went into overt HE, and 4 expired.
Of the total 10 patients who lost to follow-up, maximum numbers were for LOLA (4 cases) group followed by placebo (3 cases) group. Maximal number of deteriorations in clinical state, that is, development of overt HE among patients occurred in the placebo (3 cases) group. Of the total 4 deaths, 2 were in placebo group and one each in rifaximin and Velgut groups. However, there was no death in the LOLA group.
Because our test results were based on intention-to-treat analysis, 20 of the 124 patients who were lost to follow up, deteriorated, or expired were also included in the analysis. Patients who expired, deteriorated to overt HE, or lost to follow up were assigned a CFF value of 38.9 Hz and were given a value for all the 3 tests as abnormal (based on median values). CFF values showed a significant change before and after treatment with various drugs, but the change was not significant in the placebo group [Table 3]. Number of patients who improved after giving treatment were 67.7% (21/31), 70.9% (22/31), 50% (16/32), and 30% (9/30) for LOLA, rifaximin, probiotics, and placebo groups, respectively.
Table 3.
Comparison of critical flicker frequency (Hz) between different treatment and placebo groups
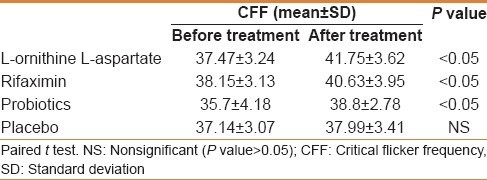
Paired sample t test is applied to pre- and posttreatment CFF scores for each of the four groups. The test is one tailed since the alternative hypothesis states that the effect should be positive (Posttest CFF minus Pretest CFF >0). It can be seen that P values for drugs 1, 2, and 3 are <0.05 indicating that the effects due to these groups are statistically significant.
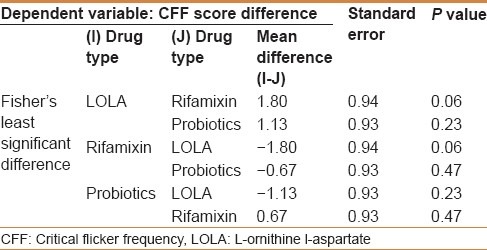
To find out which of the drugs among 1, 2, and 3 has the most significant effect, we did a one-way analysis of variance (ANOVA) with drug type as a factor and CFF as dependent variable. The post hoc test (LSD) indicated which drug has the best effect. The differences between the effect means were not statistically significant among the groups, however, it indicates that the mean effect (Posttest CFF minus Pretest CFF) was better for LOLA [Table 4].
Table 4.
Analysis of variance analysis with drug type and critical flicker frequency
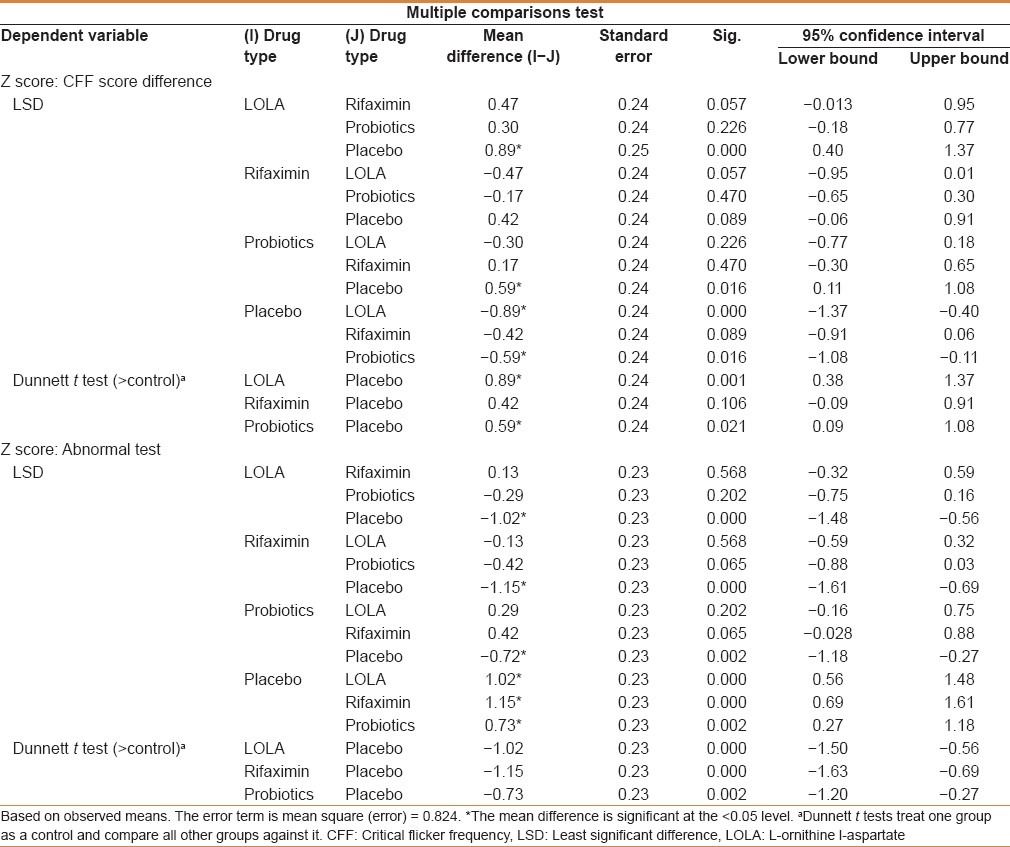
To see which of the four groups result in maximum improvement in scores for CFF and number of abnormal neuropsychiatric tests, the results of the post hoc test were investigated for multiple comparisons. Multiple comparisons indicate that the effect of LOLA and rifaximin are significantly different from that of placebo group. Comparisons between Group 1, 2, and 3 indicate that although the difference between pair groups is not significant, LOLA has a better effect as indicated from mean difference values. The mean differences are interpreted as follows: For CFF, the mean differences should be positive for the drug to be effective because posttest-pretest CFF values should be higher; For the drug to be effective, the posttest CFF value should increase; and for abnormal NPTs, negative mean differences indicate that the number of abnormal tests have decreased on an average after giving the particular drug. LOLA, rifamixin, and probiotics were found significantly better than placebo (P < 0.05) [Table 5].
Table 5.
Multiple comparisons of drugs for critical flicker frequency and neuropsychometric tests
DISCUSSION
The prevention of episodes of hepatic encephalopathy is an important goal in the treatment of patients with liver disease.[12] MHE is now a well-established entity which has been proved to be a harbinger of HE and if recognized and treated in time, an attack of HE can be aborted. Symptoms of overt encephalopathy are debilitating and decrease the ability for self-care, leading to improper nutrition and nonadherence to a therapeutic regimen, which in turn leads to severe symptoms, frequent hospitalizations, and a poor quality of life. There is no gold standard for the diagnosis of MHE. Various tests used to diagnose MHE include the neuropsychological or psychometric tests, neurophysiologic tests, regional cerebral blood flow changes, magnetic resonance imaging, and magnetic resonance spectroscopy.
We used CFF and/or neuropsychiatric tests (NPTs) to diagnose MHE. Correlation of mean CFF with psychometric tests and CFF together with the Child-Pugh class predicts the development of overt HE on follow-up. CFF is a reliable, simple, easy to apply, and is not influenced by age, education level, day time, or interobserver variability. CFF is a well-established neurophysiological technique that measures the ability of the central nervous system to detect flickering light, and which is directly influenced by the cortical activity.
The appropriate cutoff to identify abnormal CFF is still not defined. In the study by Kircheis et al., the threshold was 39 Hz on comparing healthy subjects with the cirrhotic patients and patients with encephalopathy.[4] However, Gomez et al. found a better sensitivity and specificity for diagnosis of MHE using a threshold of 38 Hz.[13] In our study, the number of cirrhotics diagnosed as having MHE from the CFF results (irrespective of the number of abnormal NCTs) was 87 when a cutoff of 39 Hz was used, which decreased to 74 when we used a cutoff of 38 Hz. Hence we had 13 patients whose CFF values fell in the range of 38-39 Hz. Of these 13 patients, three were MHE only on CFF values (their abnormal NPTs were <2).
Sharma et al. showed that upon taking a cutoff of 39 Hz, 80% patients with MHE had abnormal CFF, whereas in our case it is 70.16%. This difference might be because of the lower mean age of the patients in our study (39.12 ± 12.75 years) as compared with the study by Sharma et al. (mean age of the cohort = 41.03 ± 12.5 years).[14] Although earlier it was thought to be independent of age, a recent study by Dhiman et al. showed that CFF decreases as age advances, and therefore age-adjusted values may be required.[15]
The prevalence of MHE has ranged from 22% to 74% in cirrhotics in various studies based on different diagnostic cutoffs and patient population studied.[15] The range of diagnostic criteria used in these studies included neuropsychological tests in different combinations with different cutoffs (abnormal scores >2 SD or >1 SD below mean), short neuropsychological batteries (Psychometric Hepatic Encephalopathy Score [PHES]), various computerized tests including critical clicker frequency (CFF), and inhibitory control test (ICT), neurophysiological tests (NP) (electroencephalography [EEG], P300 evoked responses) with different cutoff or their combinations. In our study using CFF and/or two abnormal neuropsychological tests (NCT A, FCT A, DST), the prevalence of MHE was found to be 60.19%. This value is in accordance with the study done by Liu et al. who estimated a prevalence of 60% using NCT and measurement of brainstem auditory evoked potentials.[10] Prasad et al. found the prevalence of MHE to be 67.7% based on combination of quantitative NP tests, including 2 number connection tests (NCT-A and NCT-B), figure connection tests (FCT-A and FCT-B), picture completion, and block design tests.[16]
Neuropsychological tests are influenced by variables such as age, educational status, and learning effects. To avoid learning effects, different variations of NP tests were used at the time of diagnosis and after 2 months (upon the completion of intervention). We derived normative data of age- and education-matched controls to compare the test results with cirrhotics. Our baseline data of NPTs is representative of the population of eastern Uttar Pradesh. The prevalence of MHE has been reported to be higher in patients with cirrhosis with CTP classes B and C, advanced age, alcoholic etiology, a previous episode of overt HE, and portosystemic shunts.[17] Our study also showed a higher prevalence of MHE in Child B (52.42%) and C (37.30%) when compared with Child A (35.28%).
Different studies have shown that pathogenesis of MHE is similar to that of overt HE.[18,19] Several therapeutic agents including branched chain amino acids, lactulose, LOLA, probiotics, and dietary protein manipulation have been tried to treat this condition on the premise that they reduce blood ammonia levels. Lockwood et al. showed that both the cerebral metabolic rate for ammonia and the permeability-surface area product for ammonia were significantly higher in patients with MHE than in controls.[18] The increased permeability-surface area product of the blood–brain barrier permits ammonia to diffuse across the blood–brain barrier into the brain more freely than normal. This may cause ammonia-induced encephalopathy even though arterial ammonia levels are normal or near normal. Prasad et al. observed concomitant improvement in cognitive functions and HRQOL in MHE patients following lactulose therapy.[16]
Our study compared LOLA, rifaximin, and probiotics in patients with MHE to placebo group after 2 months of therapy. When the response of three drugs was compared with that of the placebo group, pre- and posttreatment CFF scores were statistically significant (P < 0.05) for LOLA, rifaximin, and probiotics. One-way analysis of variance with drug type as factor and mean difference in CFF score as variable was conducted followed by post hoc test analysis [Table 4]. Although the mean differences between effects were not statistically significant, the mean difference values indicate that the mean effect (Posttest CFF-Pretest CFF) was best for Drug 1 (LOLA) [Table 4].
LOLA, rifaximin, and probiotics were better than placebo in improving the results of abnormal NPTs [Table 5].
Although surplus scientific literature is available on the effect of the above-mentioned drugs on overt HE, paucity exists as far as MHE is concerned. Lactulose decreases blood ammonia levels and improves psychometric performance and HRQOL. A recent study that compared lactulose, a probiotic, and LOLA with placebo, confirmed that significantly greater improvement in blood ammonia levels, psychometry scores, and HRQOL occurred in the treated patients as compared with the nontreated ones.[20]
Studies have shown that LOLA results in the reduction of plasma ammonia levels and improvement in abnormal psychometric test scores, but recent observations suggest that the reduction in the levels of plasma ammonia with administration of LOLA may be associated with a later increase in ammonia once LOLA is discontinued.[21] This has been attributed to a significant rise in glutamine levels, which eventually become a source of ammoniagenesis by the kidney and gut through the effects of glutaminase. Because our patients were not followed up after 2 months of treatment, studies are needed where patients with MHE once treated with LOLA are followed up after the drug stoppage to look for any deterioration in cognitive function. We did not quantify blood ammonia levels as most of the studies previously did. We assumed that improvement in MHE in our patients was possibly because of the reduction of blood ammonia.
Prebiotics, probiotics, or synbiotics (probiotics and fermentable fiber) are effective in treating patients with MHE and can also be used as long-term therapy.[20] Liu et al. showed that modulation of gut microecology and acidification of gut lumen in patients with liver cirrhosis and MHE by treatment with synbiotics resulted in increased fecal content of non–urease-producing Lactobacillus species, whereas the number of urease-producing pathogenic Escherichia coli and Staphylococcal species decreased.[10] This effect persisted for 14 days after cessation of supplementation. It was associated with a significant reduction in blood ammonia and endotoxin levels and reversal of MHE in nearly 50% of the patients.
Thus, this study concluded that the prevalence of MHE is high in patients with cirrhosis of liver. Rifaximin, LOLA, and probiotics are better than giving none of these in patients with MHE. However, a large population is needed to further validate the results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45–9. doi: 10.1002/hep.510280108. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Sharma BC, Puri V, Sarin SK. Minimal hepatic encephalopathy in patients with extra hepatic portal vein obstruction. Am J Gastroenterol. 2008;103:1406–12. doi: 10.1111/j.1572-0241.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman RK, Saraswat VA, Verma M, Naik SR. Figure Connection Test: A universal test for assessment of mental state. J Gastroenterol Hepatol. 1995;10:14–23. doi: 10.1111/j.1440-1746.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 4.Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Haussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357–66. doi: 10.1053/jhep.2002.30957. [DOI] [PubMed] [Google Scholar]
- 5.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neurophysiological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–73. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 6.Saxena N, Bhatia M, Joshi YK, Garg PK, Dwivedi SN, Tandon RK. Electrophysiological and neuropsychological tests for the diagnosis of subclinical hepatic encephalopathy and prediction of overt encephalopathy. Liver. 2002;22:190–7. doi: 10.1034/j.1600-0676.2002.01431.x. [DOI] [PubMed] [Google Scholar]
- 7.De Bruijn KM, Blendis LM, Zilm DH, Carlen PL, Anderson GH. Effect of dietary protein manipulation in subclinical portal-systemic encephalopathy. Gut. 1983;24:53–60. doi: 10.1136/gut.24.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kircheis G, Nilis R, Held C, Berndt H, Buchner M, Görtelmeyer R, et al. Therapeutic efficacy of l-ornithine-l-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: Results of placebo-controlled, double-blind study. Hepatology. 1997;25:1351–60. doi: 10.1002/hep.510250609. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, et al. Clinical efficacy of lactlulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26:1410–4. doi: 10.1053/jhep.1997.v26.pm0009397979. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: Effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–9. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz M, Cordoba J, Doval E, Jacas C, Pujadas F, Esteban R, et al. Development of a clinical hepatic encephalopathy staging scale. Aliment Pharmacol Ther. 2007;26:857–67. doi: 10.1111/j.1365-2036.2007.03394.x. [DOI] [PubMed] [Google Scholar]
- 12.Munoz SJ. Hepatic Encephalopathy. Med Clin North Am. 2008;92:795–812. doi: 10.1016/j.mcna.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Gomez M, Boza F, Garcia-Valdecasas MS, Garcia E, Agilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–23. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Sharma BC, Puri V, Sarin SK. Critical flicker frequency: Diagnostic tool for minimal hepatic encephalopathy. J Hepatol. 2007;47:67–73. doi: 10.1016/j.jhep.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Dhiman RK, Saraswat VA, Sharma BK, Sarin SK, Chawla YK, Butterworth R, et al. Indian National Association for Study of the Liver. Minimal hepatic encephalopathy: Consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029–41. doi: 10.1111/j.1440-1746.2010.06318.x. [DOI] [PubMed] [Google Scholar]
- 16.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–59. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz M, Jacas C, Cordoba J. Minimal hepatic encephalopathy: Diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(Suppl 1):S45–53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood AH, Yap EW, Wong WH. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cerb Blood Flow Metab. 1991;11:337–41. doi: 10.1038/jcbfm.1991.67. [DOI] [PubMed] [Google Scholar]
- 19.Cordoba J, Alonso J, Rovira A, Jacas C, Sanpedro F, Castells L, et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1) H-magnetic resonance abnormalities after liver transplantation. J Hepatol. 2001;35:598–604. doi: 10.1016/s0168-8278(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 20.Mittal VV, Sharma P, Sharma BC, Sarin S. Treatment of minimal hepatic encephalopathy: A randomised controlled trial comparing lactulose, probiotics and l-ornithine l-aspartate with placebo. Hepatology. 2009;50(Suppl):471A. doi: 10.1097/MEG.0b013e32834696f5. [DOI] [PubMed] [Google Scholar]
- 21.Davies NA, Wright G, Ytrebø LM, Stadlbauer V, Fuskevåg OM, Zwingmann C, et al. l-Ornithine and phenylacetate synergestically produce sustained reduction in ammonia and brain water in cirrhotic rats. Hepatology. 2009;50:155–64. doi: 10.1002/hep.22897. [DOI] [PubMed] [Google Scholar]


