Abstract
Aim:
To investigate whether, intraocular pressure (IOP) is affected when there is a second fetus in the uterus during pregnancy.
Materials and Methods:
Eighty eyes of 40 twin pregnancies (TwPs), 80 eyes of 40 singleton pregnancies (SiPs) and 80 eyes of 40 non-pregnant females (NoPs) were included in the study.
Statistical Analysis:
Repeated measurements analysis of variance with two factors, one-way analysis of variance (ANOVA) and theTukey's multiple comparison test were used.
Results:
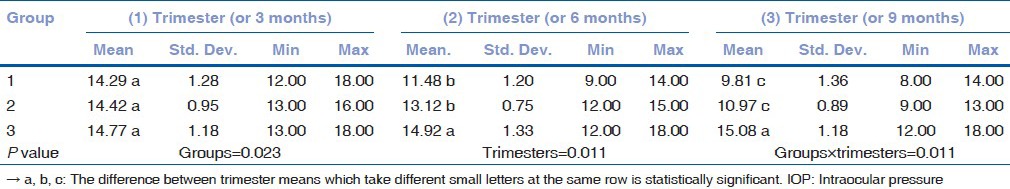
The mean IOP (MIOP) values in TwPs were 14.29 ± 1.28, 11.48 ± 1.20, and 9.81 ± 1.36 mmHg and the MIOP values in SiPs were 14.42 ± 0.95, 13.12 ± 0.75, and 10.97 ± 0.89 mmHg in subsequent trimesters. The MIOP values in NoPs were 14.77 ± 1.18, 14.92 ± 1.33, and 15.08 ± 0.89 mmHg in subsequent 3-month measurements. The results show that the MIOP values for the TwPs group were significantly lower than the SiPs in all trimesters.
Conclusions:
During pregnancy, the number of fetuses in the uterus is an indirectly important factor that influences the decrease in IOP. We hypothesize that the increased ocular hypotensive effect of TwPs is most likely related to the presence of higher levels of hormones, particularly estrogen, progesterone and relaxin compared with SiPs.
Keywords: Intraocular pressure, singleton pregnancy, the number of fetuses, twin pregnancy
Pregnancy is a complex physiological process that is managed by the endocrine system.[1] Pregnancy results in many hormonal changes in the body and the eyes are also affected from these changes.[2,3] A number of hormones affect the intraocular pressure (IOP). Of these hormones, female sex hormones are the predominant hormones that cause variations in the IOP.[4] Many important studies have examined IOP reduction in pregnancy.[4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] However, these studies were all related to IOP changes during singleton pregnancies (SiPs). Multiple pregnancy (MuP) occurs when more than one fetus develops in the womb. It is generally recognized that 97% of MuPs are twin pregnancies (TwPs).[21] To the best of our knowledge, any study related to IOP changes during TwPs has not been previously described. The objective of this study is to determine whether, IOP is affected when there is a second fetus in the uterus during pregnancy.
Materials and Methods
The subjects were selected from patients attending ‘routine antenatal outpatient’ clinics at the Obstetrics and Gynecology department of a Special Hospital over a 3-year period. Eighty eyes of 40 TwPs, 80 eyes of 40 SiPs and 80 eyes of 40 NoPs were included in the study. All groups were age matched (aged 25-33 years). Along with the antenatal examinations, a local examination of each eye was performed. Any subject with current or previous systemic and ocular diseases was excluded from the study. Pregnant women who developed complications, such as preeclampsia, eclampsia, and premature delivery during pregnancy and NoPs who were menstruating, used oral contraceptives, or became pregnant during the follow-up were also excluded from the study.
We compared trimester-related IOP changes in the TwPs (group-1), the SiPs (group-2,) and the NoPs (group-3) at the same time intervals. The IOP was measured using a Goldmann applanation tonometer. The IOP measurements were recorded at 12, 24, and 36 weeks of pregnancy and at 3-month intervals in the NoPs. The IOP was measured between 8 a.m. and 10 a.m. to avoid diurnal variation. In each session, three consecutive IOP measurements were recorded and the average of these readings were recorded as the mean IOP (MIOP) of each trimester or 3-month period. Prior to the study, each subject was informed in detail about the objective of the study, the research protocol and the method for consent.
Statistical analysis
Descriptive statistics for the studied variables (characteristics) were presented as the mean, standard deviation (Std. Dev.), and minimum and maximum values. Repeated measurements analysis of variance with two factors (the trimester is a repeated factor) and one-way analysis of variance (ANOVA) were used to compare the groups and trimesters. The Tukey's multiple comparison test was performed to compare the different groups and trimesters or 3-month periods.
Results
The mean and standard deviation of the IOP values and ages according to the groups were compared. There was no statistically significant difference between the ages of the groups [Table 1]. The MIOP values in the TwPs were 14.29 ± 1.28, 11.48 ± 1.20, and 9.81 ± 1.36 mmHg in subsequent trimesters. The MIOP values in the SiPs were 14.42 ± 0.95, 13.12 ± 0.75, and 10.97 ± 0.89 mmHg in subsequent trimesters. The MIOP values in the NoPs were 14.77 ± 1.18, 14.92 ± 1.33, and 15.08 ± 0.89 mmHg in subsequent 3-month measurements [Tables 2a and b]. Statistically significant decreases in the IOP were observed in all trimesters for the TwPs, whereas for the SiPs, these decreases were observed during the second and third trimesters. Interestingly, the IOP decreases in the TwPs were more pronounced than the SiPs for all trimesters.
Table 1.
Descriptive statistics and comparative results of age for each group
Table 2a.
Descriptive statistics and comparative results of IOP for each groups and trimesters (comparison of groups)
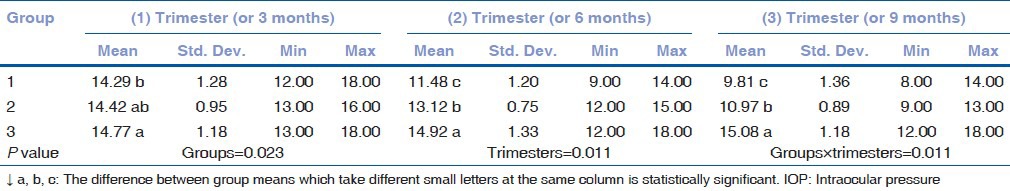
Table 2b.
Descriptive statistics and comparative results of IOP for each group and trimesters (comparison of trimesters)
Discussion
Pregnancy-induced ocular changes may be physiological and examples of these ocular changes include changes in the refractive state, visual fields, cornea sensitivity, IOP reduction, and dry eye. Pathologic changes include central serous chorioretinopathy. The most significant preexisting condition is diabetic retinopathy, which worsens during pregnancy. However, pregnancy-induced ocular changes can have beneficial effects on one preexisting condition, that is, glaucoma.[2,3] Brauner et al.[5] reported that target IOP values have been achieved without the use of drugs in glaucoma patients during pregnancy.
Although many authors[4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] have similar hypotheses regarding the reduction of IOP during pregnancy, there have been different and conflicting theories regarding the main factor(s) that cause(s) IOP reduction, as well as the primary physiological mechanisms that play a role in the reduction of IOP. Aqueous humor (AH) producing ciliary processes and AH drainage channels are sensitive to hormones.[7] Many studies,[7,14,15,16] show that reduced IOP is due to elevated hormonal levels, which cause an increase in fluid outflow conductance without altering the rate of fluid entry. However, it is well documented that the increased levels of progesterone and estrogen that occur during pregnancy cause the dilation of the circulatory system vessels, leading to decreased arterial pressure and reduced AH production.[17]
Relaxin may also reduce the IOP.[9] Relaxin increases the liquid passage between collagen in soft tissues. The same effect makes AH outflow easier.[7,9,19] During pregnancy, the release of relaxin, causes the relaxation of the mother's pelvic ligaments such that the sacroiliac joints become relatively limber and the symphysis pubis becomes elastic. It is postulated that this softening of the ligaments during late pregnancy may extend to the ligament of the corneoscleral envelope to produce decreased ocular rigidity and therefore cause a decrease in the IOP.[9] Improved uveoscleral outflow, which results from the hormonal changes during late pregnancy, may also be a likely explanation for the decrease in pressure.[14]
Wilke et al.[20] reported a decrease in the episcleral venous pressure because of general decrease in peripheral vascular resistance during pregnancy. This decrease also facilitates AH outflow.
There have also been conflicting reports of the timing of IOP reductions during pregnancy. In several of these studies,[4,12] the decrease in IOP is continuous during pregnancy, whereas other studies,[10,15] report that the reduction in IOP occurs during the second half of pregnancy or is limited to the third trimester.[14] Ebeigbe et al.[22] reported that this ocular hypotensive effect continues with a gradual decrease until 6 weeks postpartum.
We hypothesize that the additional decrease in IOP in the TwPs can be revealed by detailed analysis and by understanding the maternal physiological adaptations to MuPs. Important information related to the physiologic and endocrine status of MuPs is available in the literature. The maternal physiologic adaptation to SiPs is exaggerated in MuPs and involves every organ system.[23] These physiological changes in MuPs are very important and may become so intense that they border on pathological. These changes are primarily associated with the presence of more than one fetus in the uterine cavity.[21] The serum levels of progesterone, estrogen, b-human chorionic gonadotropin (B-HCG), cortisol, and alpha-fetoprotein are increased in women with MuPs compared with SiPs.[24] TwPs are associated with an approximate doubling of maternal estrogen levels, compared with SiPs.[25,26] It has been hypothesized that a high level of progesterone during TwPs reduces the subsequent risk of endometrial cancer because progesterone inhibits endometrial cell division.[27] Normally, the peak level of relaxin is reached during the 14 weeks of the first trimester and again at delivery. In normal pregnancies, relaxin concentrations in the third trimester are lower than during early and mid-pregnancy; however, relaxin levels in the TwPs during the third trimester are higher than in the SiPs. MuPs have significantly increased serum relaxin concentrations.[28,29]
Based on these data and the results of our study, although they are not conclusive and because many other explanations may exist, we can make logical deductions.
First, all types of pregnancy, including SiPs and MuPs, show an increasing tendency for the IOP to decrease, which is consistent with increasing hormonal (estrogen and progesterone) levels with advancing pregnancy. This finding positively confirms the relationship between pregnancy hormones and decreased IOP. This reduction is more pronounced in TwPs than in SiPs because of the presence of higher levels of hormones that affect the IOP in TwPs.
Similar opinions to ours are that progesterone appears to be the most likely effector, as suggested by Becker and Friendwald,[6] but that the effect of relaxin among a background of estrogen and progesterone is also a strong possibility.[19]
Second, all of the mechanisms involved in reducing IOP in SiPs operate at a higher level and therefore give rise to a further decrease in the IOP in TwPs. We postulate that both AH production and AH outflow rates may be affected in any type of pregnancy. The third trimester rise in relaxin, progesterone and estrogen may affect the AH outflow to a higher degree and/or cause a greater decrease in ocular rigidity and the IOP of TwPs. Additionally, the episcleral venous pressure may be more affected by higher levels of prostagens in TwPs.
Although our study was performed in a small cohort, we conclude that this study is important, because it is the first study related to baseline IOP changes in TwPs. In glaucoma screening, particular attention should be paid to the effects of TwPs on the IOP.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Avasthi P, Sethi P, Mithal S. Effect of pregnancy and labor on intraocular pressure. Int Surg. 1976;61:82–4. [PubMed] [Google Scholar]
- 2.Coll MR. Comparism of intraocular pressure between normal and ocular hypertensive women during various phases of the menstrual cycle. Pakmedinet. 2000;4:39–43. [Google Scholar]
- 3.Cho EY, Moon JI. Intraocular pressure change in the pregnant glaucoma or ocular hypertension patients and normal pregnant women. Korean J Ophthalmol. 2004;45:1880–4. [Google Scholar]
- 4.Horven I, Gjonnaess H. Cornea indentation pulse and intraocular pressure in pregnancy. Arch Ophthalmol. 1974;91:92–8. doi: 10.1001/archopht.1974.03900060098002. [DOI] [PubMed] [Google Scholar]
- 5.Brauner SC, Chen TC, Hutchinson BT, Chang MA, Pasquale LR, Grosskreutz CL. The course of glaucoma during pregnancy: A retrospective case series. Arch Ophthalmol. 2006;124:1089–94. doi: 10.1001/archopht.124.8.1089. [DOI] [PubMed] [Google Scholar]
- 6.Becker B, Friendwald JS. Clinical aqueous outflow. AMA Arch Ophthalmol. 1953;50:557–71. doi: 10.1001/archopht.1953.00920030567002. [DOI] [PubMed] [Google Scholar]
- 7.Kass MA, Sears ML. Hormonal regulation of intraocular pressure. Surv Ophthalmol. 1977;22:153–76. doi: 10.1016/0039-6257(77)90053-4. [DOI] [PubMed] [Google Scholar]
- 8.Sunness JS. The pregnant woman's eye. Surv Ophthalmol. 1988;32:219–38. doi: 10.1016/0039-6257(88)90172-5. [DOI] [PubMed] [Google Scholar]
- 9.Phillips CI, Gore SM. Ocular hypotensive effect of late pregnancy with and without high blood pressure. Br J Ophthalmol. 1985;69:117–9. doi: 10.1136/bjo.69.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi IA, Xi XR, Yaqob T. The ocular hypotensive effect of late pregnancy is higher in multigravidae than primigravidae. Graefes Arch Clin Exp Ophthalmol. 2000;238:64–7. doi: 10.1007/s004170050011. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi IA. Intraocular pressure and pregnancy: A comparison between normal and ocular hypertensive subjects. Arch Med Res. 1997;28:397–400. [PubMed] [Google Scholar]
- 12.Weinreb RN, Lu A, Key T. Maternal ocular adaptations during pregnancy. Obstet Gynecol Surv. 1987;42:471–83. doi: 10.1097/00006254-198708000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Kubicka-Trzaska A, Karska-Basta I, Kobylarz J, Romanowska-Dixon B. Pregnancy and the eye. Klin Oczna. 2008;110:401–4. [PubMed] [Google Scholar]
- 14.Qureshi IA, Xi XR, Wu XD. Intraocular pressure trends in pregnancy and in the third trimester hypertensive patients. Acta Obstet Gynecol Scand. 1996;75:816–9. doi: 10.3109/00016349609054709. [DOI] [PubMed] [Google Scholar]
- 15.Ziai N, Ory SJ, Khan AR, Brubaker RF. Beta-human chorionic gonadotropin, progesterone and aqueous dynamics during pregnancy. Arch Ophthalmol. 1994;112:801–6. doi: 10.1001/archopht.1994.01090180099043. [DOI] [PubMed] [Google Scholar]
- 16.Posthumus RG. The use and the possibilities of progesterone in the treatment of glaucoma. Ophthalmologica. 1952;124:17–25. doi: 10.1159/000301245. [DOI] [PubMed] [Google Scholar]
- 17.Pilas-Pomykalska M, Luczak M, Czajkowski J, Woźniak P, Oszukowski P. Changes in intraocular pressure during pregnancy. Klin Oczna. 2004;106:238–9. [PubMed] [Google Scholar]
- 18.Treumer K. Glaucoma therapy by corpus luteum hormone. Klin Monbl Augenheilkd Augenartzl Fortbild. 1952;120:523–34. [PubMed] [Google Scholar]
- 19.Paterson GD, Miller SJ. Hormonal influence in simple glaucoma: A preliminary report. Br J Ophthalmol. 1963;47:129–37. doi: 10.1136/bjo.47.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilke K. Episcleral venous pressure and pregnancy. Acta Ophthalmol Suppl. 1975;125:40–1. doi: 10.1111/j.1755-3768.1975.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 21.Dera A, Bręborowicz GH, Keith L. Twin pregnancy: Physiology, complications and the mode of delivery. Arch Perinat Med. 2007;13:7–16. [Google Scholar]
- 22.Ebeigbe JA, Ebeigbe PN, Ighoroje ADA. Intraocular Pressure in Pregnant and Non-Pregnant Nigerian Women. Afr J Reprod Health. 2011;15:20–3. [PubMed] [Google Scholar]
- 23.Yeast JD. Maternal physiologic adaptation to twin gestation. Clin Obstet Gynecol. 1990;33:10–7. doi: 10.1097/00003081-199003000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Cleary-Goldman J, D’Alton M E. Physiologic effects of multiple pregnancy on mother and fetus. In: Creasy RK, Resnik R, editors. Maternal and fetal medicine. 4th ed. Philadelphia: WB Saunders Company; 1999. pp. 197–210. [Google Scholar]
- 25.Duff DB, Brown JB. Urinary oestriol excretion in twin pregnancies. J Obstet Gynaecol Br Commonw. 1974;81:695–700. doi: 10.1111/j.1471-0528.1974.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 26.Wald N, Cuckle H, Wu TS, George L. Maternal serum unconjugated oestriol and human chorionic gonadotrophin levels in twin pregnancies: Implications for screening for Down's syndrome. Br J Obstet Gynaecol. 1991;98:905–8. doi: 10.1111/j.1471-0528.1991.tb13513.x. [DOI] [PubMed] [Google Scholar]
- 27.Key TJ. Hormones and cancer in humans. Mutat Res. 1995;333:59–67. doi: 10.1016/0027-5107(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan AH, Nicolson R, Green RC. Serum relaxin in pregnancy. Lancet. 1986;2:241–3. doi: 10.1016/s0140-6736(86)92068-4. [DOI] [PubMed] [Google Scholar]
- 29.Haning RV, Jr, Steinetz B, Weiss G. Elevated serum relaxin levels in multiple pregnancy after menotropin treatment. Obstet Gynecol. 1985;66:42–5. [PubMed] [Google Scholar]


