Abstract
Purpose:
To determine the roles of interleukin (IL)-1β and IL-10 in the vitreous of proliferative diabetic retinopathy (PDR).
Materials and Methods:
Vitreous samples were obtained from 26 eyes of 26 patients with PDR and from eight eyes of eight cases without PDR. The IL-1β and IL-10 concentration in the vitreous was measured by using an enzyme-linked immunosorbent assay (ELISA).
Results:
Levels of IL-1β and IL-10 in vitreous were higher in PDR patients compared with control group. And there was significantly negative correlation between IL-1β and IL-10 in control group (r = −0.795; P = 0.032), whereas there was no significant correlation in PDR group (r = 0.176; P = 0.391).
Conclusion:
Levels of IL-1β and IL-10 were upregulated in vitreous of PDR patients, and these two cytokines play roles in regulating the development and progression of PDR.
Keywords: Diabetic retinopathy, enzyme-linked immunosorbent assay, immunology, interleukin-1β, interleukin-10, proliferative
Diabetic retinopathy (DR) is caused by complications of diabetes mellitus (DM) and characterized by progressive damage of the retinal microvascular endothelial cells. Resulted from retinal capillary nonperfusion and pathologic intraocular proliferation, advanced DR, especially proliferative DR (PDR), is among the leading causes of blindness in elderly people.[1] In recent years, some researchers have studied the importance of DR. The pathogenesis of DR was quite similar to the changes of chronic inflammatory diseases.[2] A number of metabolic abnormalities of inflammation have been found in retina of diabetic animals or patients.[3] Levels of inflammatory, adhesion, and proinflammatory cytokines were upregulated in the pathology of DR. And these cytokines may play critical roles in the pathogenesis of DR.[4] Elevated vitreous levels of certain proinflammatory cytokines have been described.[5] During the inflammatory reaction, anti-inflammatory cytokines are also produced and tend to modulate the inflammatory process. However, the function of anti-inflammatory cytokines in PDR is not very clear.[6]
Here, we supposed that there is an imbalance between pro-and anti-inflammatory factors in PDR. We tested levels of vitreous interleukin (IL)-1β and IL-10 in patients with PDR and nondiabetic controls.
Materials and Methods
Samples
This study followed the tenets of the Declaration of Helsinki. The protocol for sample collection was approved by the hospital ethics committee, and informed consent was obtained from patients. A total of 26 patients (26 eyes) with PDR were recruited into this study. Patients with former vitrectomy, neovascularization of nondiabetic etiology, recent vitreous hemorrhage (<2 months), or a history of ocular inflammation and photocoagulation in recent 3 months were excluded from the study. All cases were diagnosed and were for surgery in the ophthalmology department from March 2011 to January 2012. The cases with PDR included 12 males and 14 females. Mean age of PDR group was 55.1 ± 13.2 (38-73) years old. The course of DM ranged from 2 to 30 years, and the mean was 13.8 ± 2.9 years. Eight controls (eight eyes) that accidently died and were without any history of eye disease were selected, including four males and four females. Mean age of control group was 13.8 ± 2.9 (2-30) years old.
Undiluted vitreous samples were obtained immediately at the start of vitrectomy prior to the start of infusion and were immediately placed on ice, centrifuged, and the supernatant was stored at −20°C until laboratory analysis.
ELISA
Enzyme-linked immunosorbent assay (ELISA) was used to evaluate the concentrations of IL-1β and IL-10 in the vitreous samples according to the manufacturer's instructions. IL-1β levels were determined using sandwich ELISA kits (R and D Systems, Minneapolis, Minnesota (MN)), with detection range of 0-240 pg/ml and sensitivity of 0.1 pg/ml. And IL-10 levels were determined using sandwich ELISA kits (R and D Systems, Minneapolis, MN), with detection range of 10-300 pg/ml and sensitivity of 0.1 pg/ml. The values were read at 450 nm in an ELISA reader, and IL-1β and IL-10 concentrations were calculated from specific calibration curves prepared with known standard solutions.
Statistical analysis
All data were analyzed with SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL). All experimental results are summarized as the mean-SEM and are reported with a 95% confidence interval (CI). The Student's t-test was used to analyze the difference of the concentrations of IL-1β and IL-10 between two groups. Linear regression analysis was applied to analyze the relevance among the concentrations of IL-1β and IL-10. P values less than 0.05 were considered significant.
Results
The clinical characteristics of the study subjects are summarized in Table 1. The concentration of IL-1β in vitreous from PDR cases was 14.916 ± 3.125 pg/ml (CI: 8.480-21.352) and from control cases was 12.505 ± 0.560 pg/ml (CI: 11.181-13.829). There was significant difference between groups (t = 1.204; P = 0.018) [Fig. 1]. The concentration of IL-10 in vitreous from PDR cases was 224.789 ± 43.801 pg/ml (CI: 134.579-314.999) and from control cases was 160.143 ± 11.833 pg/ml (CI: 132.162-188.124). There was significant difference between groups (t = 15.098; P = 0.000) [Fig. 1]. And there was negative correlation between IL-1β and IL-10 in control group (r = −0.795; P = 0.032) [Fig. 2], whereas there was no significant correlation in PDR group (r = 0.176; P = 0.391).
Table 1.
Clinical characteristics of study population

Figure 1.

The concentration of IL-1β an IL-10 between PDR group and control group. PDR = Proliferative diabetic retinopathy
Figure 2.
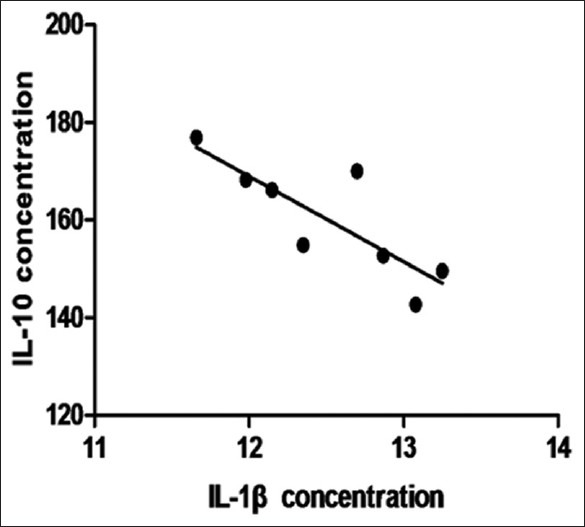
The correlation between IL-1β and IL-10 in control group
Discussion
The role of cytokines in the pathogenesis of DR is not completely understood. Previous studies on PDR reported the elevated levels of several inflammatory factors in the vitreous, which associated with the progression of PDR.[7,8] In the present study, to determine the roles of IL-1β and IL-10 in progress of PDR, we tested levels of them in the vitreous samples from PDR and nondiabetic control patients.
Diabetes is known to display a strong inflammatory component.[4] The secretion of some inflammatory cytokines by peripheral blood monocytes can be stimulated by hyperglycemia.[9] That is the reason why some inflammatory cytokines increased in serum of PDR patients.[10] And disturbances of the blood-retinal barrier in PDR patients facilitate the transition of these inflammatory cytokines and proteins. Moreover, monocytes from peripheral blood could enter into the vitreous body, and inflammatory cytokines were upregulated in the vitreous body.[9] On the other hand, macrophages, monocytes, retinal pigment epithelial cells, and glial cells are found in the vitreous of patients with PDR, and the majority of these cells are capable of producing cytokines.[11] Our results are consistent of these previous studies.
IL-1β was known as a proinflammatory cytokine, levels of which increased in retinas from diabetic rats[12,13,14] and in serum or vitreous of PDR patients.[9,15] Previous studies have demonstrated that the angiogenic IL-1β is active in vivo, which can regulate the development of ocular neovascularization.[15] However, studies of vitreous IL-1β concentrations in patients with PDR have come to different conclusions.[4] In the present study, the median IL-1β concentration was statistically higher in the vitreous of the PDR patients (14.916 pg/ml) than in the control group (12.505 pg/ml), which was similar to Zhou's study.[15] It was reported that increased IL-1β in vitro could directly cause degeneration of retinal capillary endothelial cells and induce retinal ischemia.[2] IL-1β can also damage retinal capillary endothelial cells by stimulating increased NF-κB and inducing oxidative stress reaction, which can promote the DR progression.[2]
IL-10 is an anti-inflammatory cytokine with potent deactivating properties on macrophages.[6] In addition, antitumoral effects of IL-10 have been associated with its ability to prevent angiogenesis associated with tumor growth. Furthermore, it has been recently demonstrated that its effect of antiangiogenicity is associated with the downregulation of vascular endothelial growth factor (VEGF) expression. However, there were few studies to investigate the relationship between IL-10 and PDR, besides most researches were in serum of PDR. Paine SK showed that IL-10 allele was a potent risk factor for the pathogenesis of PDR.[16] Hernández C et al., reported that serum IL-10 levels were lower in diabetic patients with PDR than control subjects.[17] Lee reported that higher IL-10 levels were related to lower risk of DR in DM patients.[10] In our study, the median IL-10 concentration was also statistically significantly higher in the vitreous of the PDR patients (224.789 pg/ml) than in the control group (160.143 pg/ml), which was different from Hernández C study. Our findings have suggested that levels of IL-10 in vitreous may reflect the state of retina better. IL-10 was thought to be a protective cytokine for its anti-inflammatory effect. When proinflammatory cytokines, such as IL-1β, elevated in PDR, as we discussed before, higher secreting activity of IL-10 would be a protective factor against the development of PDR.[18] Moreover, a predominance of macrophages (50%) in the PDR samples by cytological examination of the vitreous specimens was revealed.[19] Increased macrophages might produce more IL-10 resisting other proinflammatory cytokines.
Moreover, in our study, there was significantly negative correlation between IL-1β and IL-10 in control group, whereas there was no significantly negative correlation in PDR group. Based on our results, we proposed there was a relative balance between IL-1β as proinflammatory cytokines and IL-10 as anti-inflammatory cytokines in vitreous. When diabetes occurred, the balance was broken and IL-1β/IL-10 was abnormal.
Taken together, we studied the immune factors in the mechanism of DR by testing vitreous IL-1β and IL-10 levels in PDR patients. And these cytokines may be pathogenically important in PDR.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Abu El-Asar AM, Al-Mezaine HS, Ola MS. Pathophysiology and management of diabetic retinopathy. Exp Rev Opthalmol. 2009;4:627–47. [Google Scholar]
- 2.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–7. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007. 2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koskela UE, Kuusisto SM, Nissinen AE, Savolainen MJ, Liinamaa MJ. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013;49:108–14. doi: 10.1159/000342977. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim S, Park J, Lee HK, Park KS, Yu HG, et al. Differential expression of vitreous proteins in proliferative diabetic retinopathy. Curr Eye Res. 2006;31:231–40. doi: 10.1080/02713680600557030. [DOI] [PubMed] [Google Scholar]
- 6.Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabetic Med. 2005;22:719–22. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 7.Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina. 2008;28:817–24. doi: 10.1097/IAE.0b013e31816576d5. [DOI] [PubMed] [Google Scholar]
- 8.Adamiec-Mroczek J, Oficjalska-Mlynczak J, Misiuk-Hojlo M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: Analysis of vitreous samples. Cytokine. 2010;49:269–74. doi: 10.1016/j.cyto.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Koleva-Georgieva DN, Sivkova NP, Terzieva D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 2011;53:44–50. doi: 10.2478/v10153-010-0036-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Lee W, Kwon OH, Kim JH, Kwon OW, Kim KH, et al. Cytokine profile of peripheral blood in type 2 diabetes mellitus patients with diabetic retinopathy. Ann Clin Lab Sci. 2008;38:361–7. [PubMed] [Google Scholar]
- 11.El-Ghrably IA, Dua HS, Orr GM, Fisher D, Tighe PJ. Intravitreal invading cells contribute to vitreal cytokine milieu in proliferative vitreoretinopathy. Br J Ophthalmol. 2001;85:461–70. doi: 10.1136/bjo.85.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–30. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 13.Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: Effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–6. doi: 10.1167/iovs.04-0633. [DOI] [PubMed] [Google Scholar]
- 14.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–65. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416–20. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- 16.Paine SK, Sen A, Choudhuri S, Mondal LK, Chowdhury IH, Basu A, et al. Association of tumor necrosis factor α, interleukin 6, and interleukin 10 promoter polymorphism with proliferative diabetic retinopathy in type 2 diabetic subjects. Retina. 2012;32:1197–203. doi: 10.1097/IAE.0b013e31822f55f3. [DOI] [PubMed] [Google Scholar]
- 17.Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–22. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 18.Myśliwiec M, Zorena K, Balcerska A, Myśliwska J, Lipowski P, Raczyńska K. The activity of N-acetyl-beta-D-glucosaminidase and tumor necrosis factor-alpha at early stage of diabetic retinopathy development in type 1 diabetes mellitus children. Clin Biochem. 2006;39:851–6. doi: 10.1016/j.clinbiochem.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Canataroglu H, Varinli I, Ozcan AA, Canataroglu A, Doran F, Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005;13:375–81. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]


