Abstract
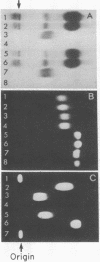
A pilot trial of enzyme replacement with splenic and plasma alpha-galactosidase A (alpha-D-galactosidase; alpha-D-galactoside galactohydrolase, EC 3.2.1.22) isozymes was undertaken in two brothers with Fabry disease, an X-linked glycosphingolipid storage disease. Six unentrapped doses (2000 units/kg) of each isozyme were administered intravenously to the respective recipients during a 117-day period. The circulating half-life of the splenic isozyme was about 10 min, whereas that for the plasma isozyme was approximately 70 min. No immune response was detected by skin and immunodiffusion tests or by alterations in the maximal activity or clearance kinetics for either isozyme after successive administrations. After each dose of the splenic isozyme, the concentration of the accumulated circulating substrate, trihexosylceramide (globotriaosylceramide), decreased maximally (approximately 50% of initial values) in 15 min and returned to preinfusion levels by 2-3 hr. In marked contrast, injection of the plasma isozyme decreased the circulating substrate levels 50-70% by 2-6 hr; the concentrations gradually returned to preinfusion values by 36-72 hr.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Barkal A., Di Cesare J. L. Influence of sialic acid groups on the retention of glycosphingolipids in blood plasma. Biochim Biophys Acta. 1975 Aug 25;398(2):287–293. doi: 10.1016/0005-2760(75)90144-7. [DOI] [PubMed] [Google Scholar]
- Bearpark T., Stirling J. L. Clearance of human N-acetyl-beta-hexosaminidases from rat circulation. Biochem J. 1977 Dec 15;168(3):435–439. doi: 10.1042/bj1680435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke J. N., Dagher S. M., Massey T. H., Deal W. C., Jr Rapid, multisample isoelectric focusing in sucrose density gradients using conventional polyacrylamide electrophoresis equpiment: a two-peak transient in the approach-to-equilibrium. Anal Biochem. 1975 Nov;69(1):1–9. doi: 10.1016/0003-2697(75)90558-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Sweeley C. C. Plasma alpha-galactosidase A:properties and comparisons with tissue alpha-galactosidases. Biochim Biophys Acta. 1978 Aug 7;525(2):399–409. doi: 10.1016/0005-2744(78)90235-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Wampler D. E., Sgouris J. T., Bonefeld R. J., Anderson D. K., Hawley M. C., Sweeley C. C. Pilot scale purification of alpha-galactosidase A from Cohn fraction IV-1 of human plasma. Biochim Biophys Acta. 1978 May 11;524(1):109–120. doi: 10.1016/0005-2744(78)90109-2. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Gal A. E., Bradley R. M., Martensson E., Warshaw A. L., Laster L. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967 May 25;276(21):1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Pentchev P. G., Gal A. E., Hibbert S. R., Dekaban A. S. Replacement therapy for inherited enzyme deficiency. Use of purified glucocerebrosidase in Gaucher's disease. N Engl J Med. 1974 Nov 7;291(19):989–993. doi: 10.1056/NEJM197411072911901. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Tallman J. F., Johnson W. G., Gal A. E., Leahy W. R., Quirk J. M., Dekaban A. S. Replacement therapy for inherited enzyme deficiency. Use of purified ceramidetrihexosidase in Fabry's disease. N Engl J Med. 1973 Jul 5;289(1):9–14. doi: 10.1056/NEJM197307052890103. [DOI] [PubMed] [Google Scholar]
- Clarke J. T., Stoltz J. M. Uptake of radiolabeled galactosyl-(alpha1 goes to 4)-galactosyl-(beta1 goes to 4)-glucosylceramide by human serum lipoproteins in vitro. Biochim Biophys Acta. 1976 Jul 20;441(1):165–169. doi: 10.1016/0005-2760(76)90291-5. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Allen K. Y., Desnick S. J., Raman M. K., Bernlohr R. W., Krivit W. Fabry's disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med. 1973 Feb;81(2):157–171. [PubMed] [Google Scholar]
- Desnick R. J., Fiddler M. B., Douglas S. D., Hudson L. D. Enzyme therapy XI: immunologic considerations for replacement therapy with unentrapped, erythrocyte- and liposome-entrapped enzymes. Adv Exp Med Biol. 1978;101:753–764. doi: 10.1007/978-1-4615-9071-2_70. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Sweeley C. C., Krivit W. A method for the quantitative determination of neutral glycosphingolipids in urine sediment. J Lipid Res. 1970 Jan;11(1):31–37. [PubMed] [Google Scholar]
- Fiddler M. B., Desnick R. J. Enzyme therapy. Differential in vivo retention of bovine hepatic, renal, and splenic beta-glucuronidases and evidence for enzyme stabilization by intermolecular exchange. Arch Biochem Biophys. 1977 Mar;179(2):397–408. doi: 10.1016/0003-9861(77)90127-8. [DOI] [PubMed] [Google Scholar]
- Furbish F. S., Steer C. J., Barranger J. A., Jones E. A., Brady R. O. The uptake of native and desialylated glucocerebrosidase by rat hepatocytes and Kupffer cells. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1047–1053. doi: 10.1016/0006-291x(78)91456-0. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Johnson D. L., Del Monte M. A., Cotlier E., Desnick R. J. Fabry disease: diagnosis by alpha-galactosidase activities in tears. Clin Chim Acta. 1975 Aug 18;63(1):81–90. doi: 10.1016/0009-8981(75)90382-4. [DOI] [PubMed] [Google Scholar]
- Johnson D. L., Desnick R. J. Molecular pathology of Fabry's disease. Physical and kinetic properties of alpha-galactosidase A in cultured human endothelial cells. Biochim Biophys Acta. 1978 Jan 18;538(2):195–204. doi: 10.1016/0304-4165(78)90346-x. [DOI] [PubMed] [Google Scholar]
- Johnson W. G., Desnick R. J., Long D. M., Sharp H. L., Krivit W., Brady B., Brady R. O. Intravenous injection of purified hexosaminidase A into a patient with Tay-Sachs disease. Birth Defects Orig Artic Ser. 1973 Mar;9(2):120–124. [PubMed] [Google Scholar]
- Kint J. A. Fabry's disease: alpha-galactosidase deficiency. Science. 1970 Feb 27;167(3922):1268–1269. doi: 10.1126/science.167.3922.1268. [DOI] [PubMed] [Google Scholar]
- Mapes C. A., Anderson R. L., Sweeley C. C., Desnick R. J., Krivit W. Enzyme replacement in Fabry's disease, an inborn error of metabolism. Science. 1970 Sep 4;169(3949):987–989. doi: 10.1126/science.169.3949.987. [DOI] [PubMed] [Google Scholar]
- Mapes C. A., Anderson R. L., Sweeley C. C. Galactosylgalactosylglucosylceramide: Galactosyl hydrolase in normal human plasma and its absence in patients with fabry's disease. FEBS Lett. 1970 Apr 2;7(2):180–182. doi: 10.1016/0014-5793(70)80151-x. [DOI] [PubMed] [Google Scholar]
- Stahl P., Rodman J. S., Schlesinger P. Clearance of lysosomal hydrolases following intravenous infusion. Kinetic and competition experiments with beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):594–605. doi: 10.1016/0003-9861(76)90471-9. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki K. Specific radioactive labeling of terminal n-acetylgalactosamine of glycosphingolipids by the galactose oxidase-sodium borohydride method. J Lipid Res. 1972 Sep;13(5):687–690. [PubMed] [Google Scholar]
- Swallow D. M., Stokes D. C., Corney G., Harris H. Differences between the N-acetyl hexosaminidase isozymes in serum and tissues. Ann Hum Genet. 1974 Jan;37(3):287–302. doi: 10.1111/j.1469-1809.1974.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Krivit W., Sweeley C. C. Concentrations of glycosyl ceramides in plasma and red cells in Fabry's disease, a glycolipid lipidosis. J Lipid Res. 1969 Mar;10(2):188–192. [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yang H. J., Hakomori S. I. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971 Mar 10;246(5):1192–1200. [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegouwen H., Los W., Wurzer-Figurelli E., Doppert B., Vervloet M., Meera Khan P. Enzyme electrophoresis on cellulose acetate gel. II. Zymogram patterns in man-Chinese hamster somatic cell hybrids. Humangenetik. 1974;25(3):189–201. doi: 10.1007/BF00281426. [DOI] [PubMed] [Google Scholar]