Abstract
Background:
Stimuli-sensitive hydrogels are hydrophilic, three-dimensional, polymeric network structure capable of imbibing large amounts of water or biological fluids on stimulation, such as pH, temperature, and ionic change. Owing to the drawback of conventional therapy for ocular delivery, and to provide additive effect on intraocular pressure (IOP) reduction, stimuli sensitive hydrogel membranes containing a combination of timolol maleate and brimonidine tartrate were formulated for the treatment of glaucoma.
Materials and Methods:
Stimuli-sensitive hydrogel were formulated by timolol maleate and brimonidine tartrate. Poly acrylic acid (carbopol C 934p) is used as a gelling agent, hydroxylpropyl methylcellulose as viscolizer, sodium chloride as tonicity agent. Bezalkonium chloride as preservative. White rabbits of both sexes, weighing between 2 and 3 kg were used for the study. Stirring of ingredients in pH 4 phosphate buffers at high speed was carried out.
Result:
Viscosity of the prepared hydrogels lies in the optimum range that is, 25-55 cps. Infrared spectroscopy studies show that there is no interaction between the drug and polymer. Drug released up to 90% at the end of 8 h. The hydrogel membranes were found to be sterile, nonirritant to the eye. Marketed formulation showed a decrease in IOP up to 14 mmHg at the end of 5 h and then elimination of drug, F2 and F6 maintain the sustained effect up to 12 h.
Conclusion:
Stimuli-sensitive hydrogels was successfully formulated and evaluated for rheological studies, drug release studies, drug interaction studies, sterility studies, ocular irritation studies, and in vivo studies. IOP lowering activity of the combination of timolol maleate and brimonidine tartrate in stimuli-sensitive hydrogel was better when compared with alone medication, which shows the additive effect of combination medication.
Keywords: Brimonidine tartrate, carbopol, intraocular pressure, stimuli-sensitive hydrogel, timolol maleate
INTRODUCTION
The main objective of drug delivery system to the eye is to improve existing ocular dosage forms and exploit newer drug delivery system for improving the therapeutic efficiency. Topical application of eye drops is the most common method of administering drugs to the eye in the treatment of ocular diseases. Topical and localized applications are still an acceptable and preferred route, such dosage forms are no longer sufficient to overcome the various ocular diseases such as glaucoma due to poor bioavailability, due to the efficient mechanism protecting the eye from harmful materials and agents. This includes reflex, blinking, lachrymation, tear turnover, and drainage of tear results in the rapid removal of the drug from eye surface. Similarly, frequent instillation of concentrated medication is required at the site of action which is patient incompliance.[1] Stimuli-sensitive hydrogels are hydrophilic, three-dimensional networks, which are able to imbibe large amount of water or biological fluids and undergoes a phase transition after receiving a specific stimulus. This approach can be used for the treatment of glaucoma in ophthalmic drug delivery. Glaucoma comprises a group of chronic conditions that is characterized by progressive deformation of the optic nerve head and elevated intraocular pressure (IOP), a risk factor. It affects primarily the middle aged and elderly, the glaucoma currently constitute second most common cause of treatable blindness worldwide.[2]
Ophthalmic drug delivery is one of the most challenging and the most interesting endeavors facing the pharmaceutical scientist. The conventional ocular delivery systems like solutions, suspensions and ointments show drawbacks such as increased precorneal elimination, high variability in efficiency and blurred vision, respectively.[3,4] The major problem encountered with solution is the rapid and extensive elimination of drugs from the precorneal lachrymal fluid by solution drainage, lachrymation, and nonproductive absorption by the conjunctiva, which may lead to undesirable side-effects. It must be noted that this high drainage rate is due to the tendency of the eye to maintain its residence volume at 7-10 μl permanently, whereas volumes topically instilled range from 20 to 50 μl. Ointments increase the contact time, minimize the dilution by tears and resist nasolachrymal drainage, however are responsible for blurring of vision. Suspensions show high variability due to inadequate dosing, mainly due to lack of patient compliance inadequate shaking before use. Ophthalmic inserts constitutes a psychological and physiological barrier to user acceptance and compliance.[5]
Due to the above disadvantages of the conventional drug delivery systems, attention has been focused on developing controlled and sustained drug delivery system in order to reduce the frequency of dosing or to increase the effectiveness of the drug by localization at its site of action, decreasing the dose required or providing uniform drug delivery.
This problem can be overcome by using stimuli-sensitive hydrogels prepared from the polymers that exhibit reversible phase transition. Stimuli-sensitive hydrogels can be formulated in the liquid phase suitable to be administrated by instillation into the eye cavity and upon exposure to the stimuli such as pH, temperature, ion activated, etc., changes to the gel phase of high viscosity and thus improves the corneal residence time and bioavailability of the drug. There are various methods used to cause reversible phase transition on the ocular surface such as temp dependent concept (pluronics), pH triggered systems (including cellulose acetate hydrogen phthalate latex, carbopols, ion activated systems, including gelrite, gellan, and carbopol/pluronics.[6]
Timolol maleate is a beta blocker, which acts by reducing the synthesis of aqueous humor production through the blockade of β receptors on ciliary epithelium has a half-life of 2.5-5 h brimonidine tartrate is an α2 agonist, acts by decreasing the synthesis of aqueous humor and increasing the amount that drains from the eyes through uveoscleral outflow, has a half-life of 3 h. The above combination is marketed in the form of eye drops; however, due to problems such as rapid tear turnover, lachrymal drainage rate and drug dilution by tears, it has been demonstrated that 90% of the administered dose was cleared off within 2 min for an instilled volume of 50 μl. The ocular residence time of conventional solution is limited to few minutes, and the overall absorption is limited to 1-10%. Consequently most drugs get absorbed systematically through the nose or gut after drainage from the eye. This excessive systemic absorption not only reduces ocular bioavailability, but may also lead to unwanted side-effects and toxicity. The two main strategies for improving ocular absorption are increasing the corneal permeability and prolonging contact time on the ocular surface as well as combined medications, which provides additive effect of reducing IOP.[7]
With all the above aspects in mind the present work was aimed at investigating the potential of stimuli-sensitive hydrogel membranes containing a combination of timolol maleate and brimonidine tartrate as ocular drug delivery systems for the treatment of glaucoma so as to increase the contact time of the drug with the eye, reduce systemic side-effects, reduce the number of application, and better patient compliance.
In the present work, ophthalmic stimuli-sensitive hydrogels of timolol maleate and brominidine tartrate were prepared and evaluated for glaucoma treatment.
MATERIALS AND METHODS
Materials and animals
Timolol maleate and brimonidine tartarate were provided by FDC Ltd., Aurangabad, polyacrylic acid (carbopol C 934p) was provided by CIDF, Cochin, hydroxyl propyl methyl cellulose was made available by SD Fine-Chem Limited, Mumbai. Triethanolamine sodium chloride was provided by Loba Chemie Pvt. Ltd., Mumbai. Bezalkonium chloride was provided by Merck Ltd., Mumbai. Fluid thioglycolate medium and Soyabean Casein Digest were provided by Hi Media, Mumbai. All the reagents were of the analytical grade. White rabbits of both sexes, weighing between 2 and 3 kg were used for the study.
Preparation of hydrogel
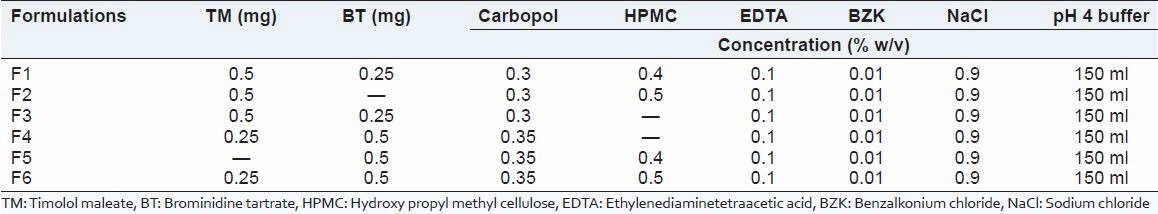
Stimuli-sensitive hydrogels were formulated by using timolol maleate and brimonidine tartrate (antiglaucoma agent), benzalkonium chloride (preservative), ethylenediaminetetraacetic acid (EDTA) (chelating agent), sodium chloride (tonicity contributors) and hydroxy propyl methyl cellulose (viscolizer). Weighed quantities of timolol maleate, brominidine tartrate, benzalkonium chloride, EDTA, NaCl were dissolved in the pH 4 phosphate buffers under aseptic conditions. Then poly acrylic acid was slowly added with continuous stirring with digital remi stirrer at speed of 1500-2000 rpm to minimize the formation of the lumps, then viscolizers was added with a slow stirring to avoid the foam formation. Stirring was continued until a clear dispersion was formed [Table 1].[8]
Table 1.
Formulation of stimuli-sensitive hydrogel
Evaluation of the hydrogels
Viscosity determination
Viscosity determinations of the prepared formulation were determined using Brookfields viscometer LVDV II (Elscolab Netherlands B.V Tolboomweg, The Netherlands). The viscosity of the stimuli-sensitive hydrogel was measured at different rpm. The correct viscosity of the stimuli-sensitive hydrogel was noted at particular spindles (10, 30, 50, 60, and 100) at which it shows maximum percent torque value.[9]
Drug polymer interaction studies
The successful formulation of a stable and effective dosage form depends on the careful selection of the excipients that are added to facilitate administration, promote the consistent release, improved bioavailability, and protects from degradation. Fourier transform infrared spectroscopy (IR) studies were followed to investigate and predict any physicochemical interactions between components in the formulation and therefore can be applied to the selection of suitable chemically compatible excipients.
In vitro release study
In vitro release study of timolol maleate and brominidine tartrate from the stimuli-sensitive hydrogels was determined by the diffusion process. A volume of 1 ml of the formulation was kept in the donar compartment over a cellophane membrane, which was rinsed and soaked for the 24 h in the diffusion medium.[10] The donar compartment was immersed in the receptor compartment containing 50 ml of the phosphate buffer of pH 7.4, the beaker containing diffusion medium (receptor compartment) was maintained at 37°C with the constant stirring at 22 rpm using the magnetic stirrer. One ml aliquots were withdrawn from the diffusion medium every hour for the 8 h and the same quantity of fresh, prewarmed diffusion medium was replaced for the amount withdrawn. The withdrawn samples were analyzed spectrophotometrically at 292 nm and 272.2 nm for timolol maleate and brominidine tartrate, respectively by using Shimazdu double beam ultra violet-visible spectrophotometer (UV-1700 Shimadzu corporation, Tokyo, Japan).[11] The experimental data were subjected to statistical analysis using one-way ANOVA. P < 0.05 was considered to be statistically significant.
Test for sterility
The sterility testing of the stimuli-sensitive hydrogels were performed for the aerobic, anaerobic bacteria and fungi by using alternative thioglycolate medium and soyabean casein digest medium. The medium was prepared by dissolving 500 mg of peptic digest of animal tissue (such as bacteriological peptone) or its equivalent in water to make 100 ml, and the pH was adjusted to 7.1 ± 0.2. The medium was filtered or centrifuged to clarify and dispensed into flasks in 10 ml quantities and was sterilized at 121°C for 20 min. The positive control (growth promotion) and negative control (sterility) test were also carried out. Bacillus subtilis, Bacteriodes vulgatus, and Candida albicans were used as test organisms in the aerobic bacteria, anaerobic bacteria and fungi test, respectively. Incubation was carried out in all cases and growth was observed.[12,13]
Ocular irritation studies
Ocular irritation study was performed on 6 white rabbits grouped, weighing 2-3 kg. Animals were housed in standard cages in a number of 2/cage. They were fed with suitable diet and water as much as required. A dark and light cycle of 12 h was maintained. The temperature and humidity were maintained at 28°C ± 2°C and 60°C ± 15°C, respectively. Of 6 formulations, the best ones were selected for the study.[14,15]
In vivo intraocular pressure lowering activity
Glaucoma was induced in rabbits by instilling prednisolone eye drops (1% w/v) up to 3-4 weeks. The study was performed on 12 white rabbits with weighing 2-3 kg divided into three groups. First group received the F2, second group received F6 and the third group received marketed combination eye drops in the right eye and the other eye was untreated. IOP was measured using a Schiötz tonometer (Rudolf Riester, GmbH & Co., K.G.Postfach 35, Jungingen, Germany) after instilling a drop of procaine hydrochloride local anesthetic (1% w/v). The left eye was used as control and treatment was carried out on the right eye. All the formulations were instilled into the lower conjunctival sac.[16,17] At regular intervals, the IOP was measured. Change in IOP was expressed as follows:
ΔIOP = IOP untreated eye − IOP treated eye.
Results are reported as mean (±standard error) ANOVA one-way statistical test was used to identify statistically significance at P < 0.05.[17]
RESULTS AND DISCUSSION
This study was undertaken to design, formulate and evaluate timolol maleate and brimonidine tartrate for stimuli-sensitive hydrogels. The formulations were evaluated for various parameters and the results obtained were within the range.
Viscosity determination
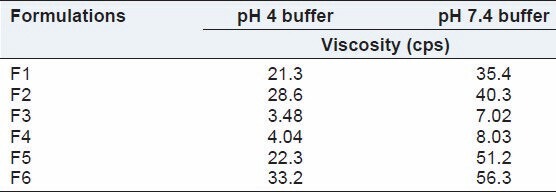
Viscosity results indicate that at acidic pH 4 phosphate buffer, hydrogel were less viscous and at pH 7.4 phosphate buffer (equivalent to pH of eye cavity) it changes into a highly viscous preparation. The literature suggested that the viscosity value in the range of 15-50 cps significantly improves the contact time of the formulation on the corneal surface and higher viscosity values offers no significant advantage [Table 2].
Table 2.
Viscosity of stimuli-sensitive hydrogel
DRUG POLYMER INTERACTION STUDIES
Drug polymer interaction studies were carried out by infrared spectral analysis. Timolol maleate showed a broad band appearing at 3302 cm−1 due to O-H stretching vibrations. The presence of Peak at 3047 cm−1 belongs to N-H stretching. The bands at 2968 cm−1, 2891 cm−1, and 2854 cm−1 are due to aliphatic C-H stretching vibrations. Acid carbonyl group of maleic acid and N-H bending vibrations gave the band at 1707 cm−1 and 1496 cm−1. The C=N stretching vibrations appears at 1621 cm−1. Bands at 1263 cm−1 and 1120 cm−1 are due to the =C-O-C and morpholino C-O-C stretching vibrations, respectively, while the bands at 1229 cm−1 and 954 cm−1 are due to O-H bending and hydroxyl C-O stretching vibrations, respectively. Brimonidine tartrate IR spectra obtained was elucidated for important groups. -NH stretching was obtained at 3438 cm−1 with a shoulder at 3437 cm−1, -CN stretching at 1600 cm−1, 1732 cm−1 indicates the presence of -C=O stretching.
The formulation showed broad peak at 3300 cm−1 indicated that O-H stretch of timolol maleate and the peak at 1704 cm−1 indicated the presence of C=O stretching of brimonidine tartrate. These are the distinguishing features of IR spectra of formulation indicated the absence of any interaction between the two drugs. Most of the other peaks such as N-H stretching and -CN stretching peaks of both the drugs are overlapped [Figure 1].
Figure 1.
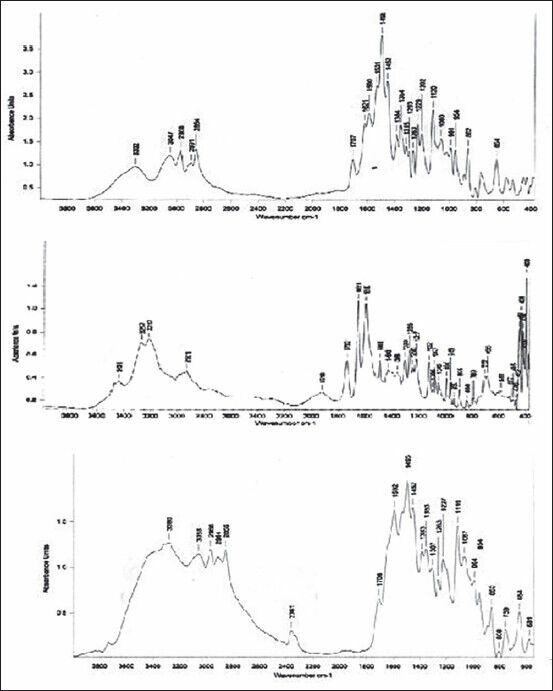
Drug interaction studies (Fourier transform infrared spectroscopy spectrum of timolol maleate, brimonidine tartrate and formulation)
In vitro release study
It is apparent from the table and figures that the drug release was governed by polymer content. An increase in the polymer content was associated with a decrease in drug release rates. The formulation F1, F2, and F3 releases the drug much faster than the formulation F4, F5, and F6. It is due to carbopol content, which was less in F1, F2, and F3 and slightly more in F4, F5, and F6. Drug release is also depends on the type of polymer used. The stimuli-sensitive hydrogel provides sustained release of drug up to 90% at the end of 8 h. The in vitro release studies did not show any significant difference in drug release due to the effect of the diffusion membrane. The prolonged release rate may be attributed largely to the drug transport by diffusion controlled mechanism from polymer. The in vitro drug release studies showed that, there was slow and prolonged release of drug from all the formulations following zero-order kinetics [Table 3 and Figure 2].
Table 3.
Drug release pattern of various formulations
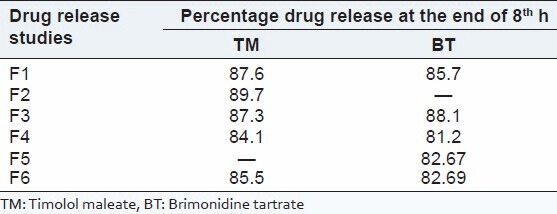
Figure 2.
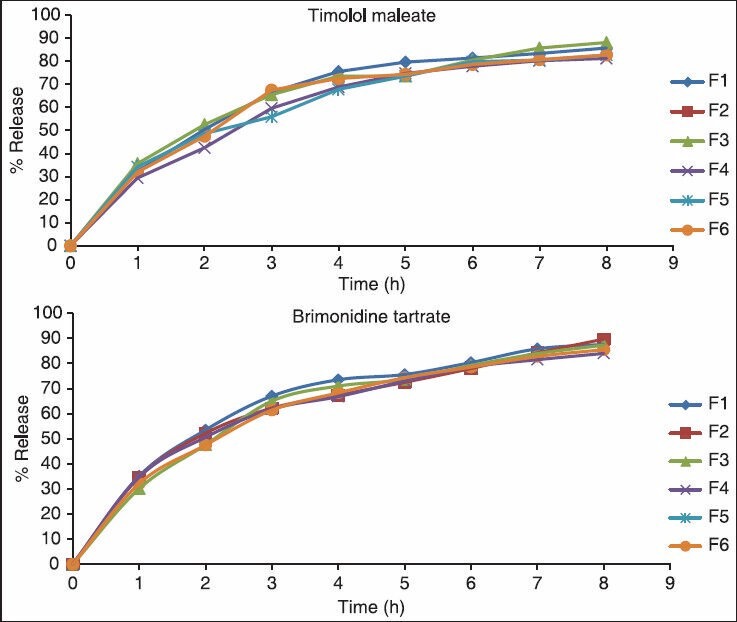
In vitro study of timolol maleate and brimonidine tartrate
Test for sterility
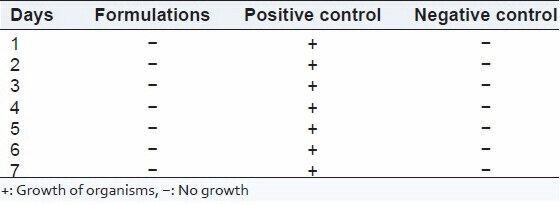
The test was performed as per the procedure given in the methodology and Pharmacopeia of India, 1996. Both positive and negative controls were prepared. The results of the sterility when compared with positive and negative control showed that the medium used was sterile and provided necessary nutrients for the microorganism. Further, it could also be interpreted that the presence of drugs did not show any antimicrobial or antifungal activity since the growth of organism found to be equal in growth promotion test (positive control) and test for bacteriostasis/fungistasis test. The results for the test for sterility are given in Tables 4 and 5. After examination, there was no macroscopic evidence of microbial growth. Hence, it passes the test for sterility.
Table 4.
Results of test for sterility in fluid thioglycolate medium
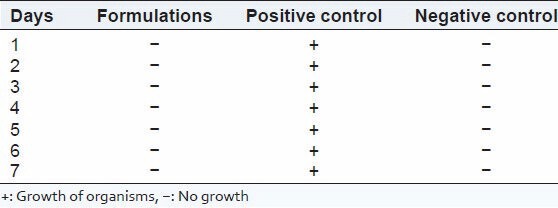
Table 5.
Results of test for sterility in soyabean casein digest medium
Ocular irritation studies
The formulation was applied into the cul-de-sac region once a day for a period of 7 days and the rabbits were monitored periodically for irritation, inflammation, etc., by the naked eye or by means of a pen torch. The test may be considered positive if there are one or more positive reactions at any observation period. One eye was used as test and other as control. Rabbits were grouped into three (2 + 2 + 2). F2 and F6 were selected for the ocular irritation studies because F2 contains timolol maleate and F6 contains both timolol maleate and brimonidine tartrate. For the first group containing two rabbits formulation F2 were applied to one eye and the other eye was kept as a control (to which nothing applied). For the second group containing two rabbits formulation F6 was applied to one eye and the other eye was kept as control. For the third group containing two rabbits marketed hydrogel was instilled to one eye and the other eye was kept as control. During the time of the examination period, each rabbit was scored for ocular reaction. The cornea, iris, and conjunctivae were evaluated for several parameters such as opacity and its degree of density, opaqueness (in case of cornea), swelling (in case of iris), redness, chemosis, and discharge (in case of conjunctivae) and allotted with maximum scores of 80, 10, and 20, respectively. The total maximum score was 110. The results of the ocular irritation studies indicate that all formulations are nonirritant to the eye. Excellent ocular tolerance was noted. No ocular damage or abnormal signs to the cornea, iris, and conjunctiva was visible [Table 6 and Figure 3].
Table 6.
Scoring of ocular irritation studies

Figure 3.
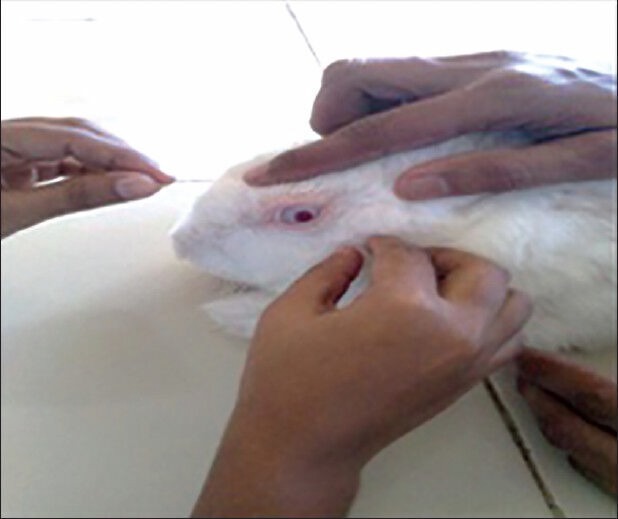
Ocular irritation studies
In vivo intraocular pressure lowering activity
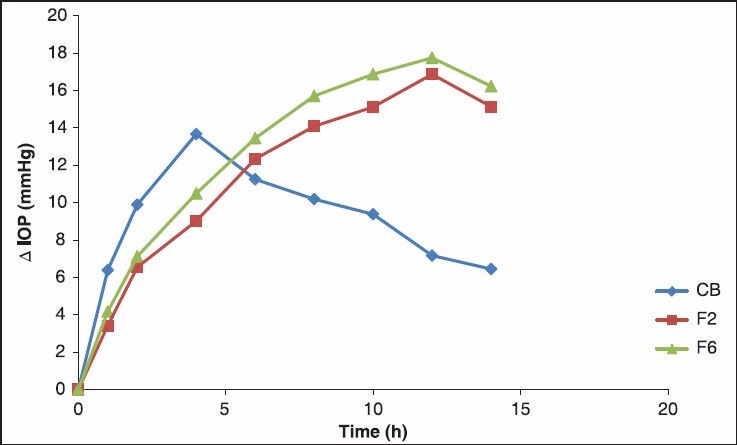
The marketed eye drops suddenly lowered the IOP to a minimum and afterwards, there was a sudden increase in the IOP, whereas the stimuli-sensitive hydrogels lowered the IOP slowly to the original and thereafter, a gradual increase in the IOP was observed. Combigen (marketed formulation) decreases IOP by 6.59 mmHg whereas F2 decreases IOP by 3.421 mmHg and F6 decreases 4.181 mmHg at the end of 1 h. Marketed formulation showed a decrease in IOP up to 14 mmHg at the end of 5 h, but then there was an increase in the IOP, which may be due to the elimination of the drug from the site of action. Hence, it was unable to sustain the activity for a long period of time, which calls for frequent administration of the formulation. F2 decrease 12 mmHg and F6 decrease 13 mmHg at the end of 6 h and the values were not statistically significant. F2 and F6 maintain the sustained effect up to 12 h. The decrease in IOP was greater in stimuli-sensitive hydrogel and when compared with combination decrease in IOP was better and sustained effect was maintained for more time. This may be due to the difference between the carbopol concentration and presence of two drug candidate. Hence, the IOP lowering activity of the combination of timolol maleate and brimonidine tartrate in stimuli-sensitive hydrogel was better when compared with marketed formulation. All values are negative, indicating that IOP returns to normal. The baseline IOP did not show any significant change during the course of a study indicating the absence of systemic side-effects. All values for all formulations are statistically significant (P < 0.05) except F2 and F6. The formulations F2 and F6 showed a significant difference when compared to the marketed formulations [Table 7 and Figures 4 and 5].
Table 7.
ΔIOP at various time intervals
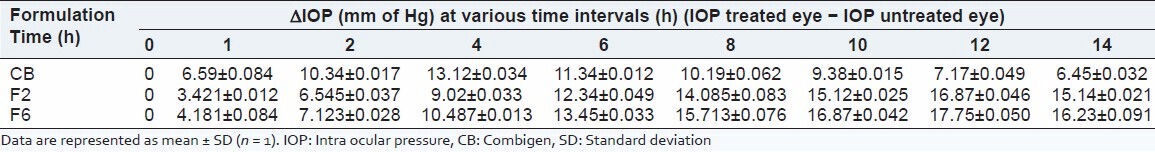
Figure 4.
Intraocular pressure measurement
Figure 5.
Effect of formulation on intraocular pressure
CONCLUSION
Maintaining an adequate concentration of the medications in the eye has remained a serious practical problem to the ophthalmologist since they exhibit many disadvantages, which include poor bioavailability because of rapid precorneal elimination, conjunctival adsorption, solution drainage due to induced lacrimation, tear evaporation, tear turnover, metabolism, limited corneal area and poor corneal permeability, binding of lachrymal proteins, etc. To enhance the amount of active substance reaching the target tissue or exerting a local effect in the cul-de-sac the residence time of the film should be lengthened. Moreover, combination medication provides additive effect for lowering IOP. Hence, a once a day combination formulation of hydrogel membrane was formulated. Timolol maleate (β-blocker) and brimonidine tartrate (α-agonist) were chosen as drug candidates for lowering the IOP.
Stimuli-sensitive hydrogels was successfully formulated and evaluated for rheological studies, drug release studies, drug interaction studies, sterility studies, ocular irritation studies, and in vivo studies. Stimuli-sensitive hydrogels show an increase in viscosity due to change in pH and provides a sustained drug release up to 90% until 8 h period. The hydrogel membranes were found to be sterile, nonirritant to the eye. IOP lowering activity of the combination of timolol maleate and brimonidine tartrate in stimuli-sensitive hydrogel was better as compared with alone medication, which shows the additive effect through combination medication.
Hence, stimuli-sensitive hydrogel membranes offer a promising avenue to fulfill the need for an ophthalmic drug delivery system that can localize and maintain drug activity at the site of action for a longer period of time thus allowing a sustained action; minimizing frequency of drug administration with patient compliance.
ACKNOWLEDGMENTS
The authors would like to thank the Shree Devi College of Pharmacy, Mangalore (Karnataka) India for providing necessary facilities and financial support to carry out this project. The authors are also thankful to FDC Ltd., Aurangabad, Maharashtra (India) for providing free gift sample of drugs.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kumar A, Malviya R, Sharma PK. Recent trends in ocular drug delivery: A Short Review. Eur J Appl Sci. 2011;3:86–92. [Google Scholar]
- 2.Coleman AL, Kodjebacheva G. Risk factors for glaucoma needing more attention. Open Ophthalmol J. 2009;3:38–42. doi: 10.2174/1874364100903010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta H. Updates on drug bioavailability and delivery to posterior segment of eye. J Pharm Bioallied Sci. 2013;5:173–4. doi: 10.4103/0975-7406.116792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang JC. Recent developments in ophthalmic drug delivery: Conventional ocular formulations. Adv Drug Deliv Rev. 1995;16:39–43. [Google Scholar]
- 5.Kumarasamy NA, Lam FS, Wang AL, Theoharides TC. Glaucoma: Current and developing concepts for inflammation, pathogenesis and treatment. Eur J Inflamm. 2006;4:129–37. [Google Scholar]
- 6.Hoare TR, Daniel SK. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49:1993–2007. [Google Scholar]
- 7.Syed MF, Loucks EK. Update and optimal use of a brinzolamide-timolol fixed combination in open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2011;5:1291–6. doi: 10.2147/OPTH.S13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V, Bushetti SS, Appala R, Shareef A, Imam SS, Singh M. Stimuli-sensitive hydrogels: A novel ophthalmic drug delivery system. Indian J Ophthalmol. 2010;58:477–81. doi: 10.4103/0301-4738.71677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh V, Bushetti SS, Appala R, Shareef A, Ahmed R, Singh M. In vitro and in vivo evaluation of stimuli sensitive hydrogel for ophthalmic drug delivery. Indian J Pharm Educ Res. 2010;44:380–5. [Google Scholar]
- 10.Ali A, Sharma SN. Fabrication of through flow apparatus for the in vitro determination of drugs from ophthalmic preparation. Indian drugs. 1991;29:157–60. [Google Scholar]
- 11.Hsiue GH, Hsu SH, Yang CC, Lee SH, Yang IK. Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials. 2002;23:457–62. doi: 10.1016/s0142-9612(01)00127-2. [DOI] [PubMed] [Google Scholar]
- 12.Soppimath KS, Aminabhavi TM, Dave AM, Kumbar SG, Rudzinski WE. Stimulus-responsive "smart" hydrogels as novel drug delivery systems. Drug Dev Ind Pharm. 2002;28:957–74. doi: 10.1081/ddc-120006428. [DOI] [PubMed] [Google Scholar]
- 13.Pharmacopoeia of India. Vol. II. Delhi: Controller of Publications, Ministry of Health and Family Welfare; 1996. [Google Scholar]
- 14.Mundada AS, Shrikhande BK. Controlled release gel of ciprofloxacin HCl for ophthalmic administration. Indian Drugs. 2006;43:9–12. [Google Scholar]
- 15.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12–7. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal D, Garg A, Kaur IP. Development of a topical niosomal preparation of acetazolamide: Preparation and evaluation. J Pharm Pharmacol. 2004;56:1509–17. doi: 10.1211/0022357044896. [DOI] [PubMed] [Google Scholar]
- 17.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–34. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]


