Abstract
Objective:
The objective of this study was to prepare and investigate better and stable amorphous ezetimibe nanosuspensions for oral bioavailability enhancement.
Materials and Methods:
Nanosuspensions of ezetimibe were prepared by solvent-antisolvent precipitation technique using the surfactant, Tween 80 as stabilizer. The nanosuspension preparation was optimized for particle size by investigating two factors that is, solvent:antisolvent ratio and surfactant concentration, at three levels. The formulations were characterized for particle size, surface morphology, crystallinity, zeta potential, saturation solubility, in vitro drug release and in vivo drug absorption.
Results:
The nanosuspensions of ezetimibe were successfully prepared using solvent-antisolvent precipitation. The two factors solvent:antisolvent ratio and surfactant concentration influenced the particle size of the nanosuspensions prepared. Nanosuspensions were smooth and spherical. The X-ray powdered diffraction and differential scanning calorimetry results indicated that the antisolvent-solvent method led to the amorphization of ezetimibe. Under storage, the amorphous ezetimibe nanosuspensions demonstrated significant physical stability. Ezetimibe nanosuspensions increased the saturation solubility to an extent of 4-times. Ezetimibe nanosuspensions completely dissolved in the dissolution medium within 1 h, while pure drug was dissolved up to 42% during same time. The Cmax with ezetimibe nanosuspension was approximately 3-fold higher when compared with that of ezetimibe conventional suspensions administered orally.
Conclusions:
Stable amorphous ezetimibe nanosuspensions were successfully prepared and these nanosuspensions demonstrated dramatic improvement in oral bioavailability of the active.
Keywords: Dissolution rate, ezetimibe, nanosuspension, oral bioavailability, solvent-antisolvent precipitation
INTRODUCTION
Poor solubility of drugs and new chemical entitity (NCE) is one of the major problems currently being addressed by pharmaceutical scientists at industry and academia.[1] At present, about 10% of the drugs in clinical use have bioavailability problems due to poor solubility. It is estimated that about 40% of the newly developed drugs will be poorly soluble with low oral bioavailability. The poor solubility even makes it very difficult to perform the pharmacological screening of compounds for potential drug effects. Hence, improving the saturation solubility and dissolution rate of poorly water-soluble drugs is very important and significantly challenging to pharmaceutical researchers seeking to achieve optimum absorption of new drug candidates.[2,3] In this research work, we addressed this issue by taking ezetimibe as a model compound. We particularly focused on amorphous nanosuspensions to enhance the bioavailability of ezetimibe. In term of solid state of the drug, two types of nanosuspensions exist. These include crystalline and amorphous nanosuspensions. In terms of oral bioavailability, amorphous nanosuspensions result in better results compared with crystalline counterpart because of enhanced solubility. Thus, amorphous nanosuspensions are recently gaining prominence.[4] They have less stability than crystalline nanosuspensions. If the stability issue is resolved, we have a better approach to enhance oral bioavailability in the form of amorphous nanosuspensions. Ezetimibe is a lipid-lowering compound that selectively inhibits the absorption of cholesterol and related phytosterols from the intestine. It is a Biopharmaceutical classification system (BCS) Class II drug.[5,6] The bioavailability of a BCS Class II drug is rate-limited by its dissolution. Therefore, an enhancement of the dissolution rate of the drug is thought to be a key factor for improving the bioavailability of BCS Class II drugs.[7,8] Ezetimibe shows 35% bioavailability due to poor solubility, rapid first-pass metabolism, and P-glycoprotein efflux.[9]
Over the years, several strategies have been developed to enhance the oral bioavailability of poorly soluble compounds.[10] These techniques offer benefits along with some drawbacks. Of these strategies, novel nanotechnology based formulations demonstrated significant promise with fewer drawbacks.[11] Nanoemulsions, liposomes, and nanosuspensions are some of these nanotechnology based techniques.[12] Nanoemulsions demonstrated enhancement in oral bioavailability of ezetimibe.[13,14,15,16] Nanoemulsions enhance oral bioavailability by different mechanisms, which include enhancement in solubility, enhancement in permeability, avoidance of efflux transporters, etc. On the other hand, similar nanotechnology based technique is nanosuspensions.[17] Nanosuspensions enhance the solubility and thereby oral bioavailability. They are currently gaining tremendous prominence in pharma research and already products are available in the market. According to the Noyes-Whitney equation, the saturation solubility and dissolution rate of poorly water soluble drugs can be enhanced by reducing the particle size to the nano-range, thus increasing the interfacial surface area.[11]
At present, the preparation of drug nanosuspensions can be basically classified into two categories: (a) The top-down approach and (b) the bottom-up approach. Top-down process is a common, scalable and effective mechanical process used to produce nanometer-sized particles of poorly aqueous soluble drug compounds and can be expensive.[18] The bottom-up process, antisolvent-solvent precipitation under sonication is a relatively rapid and low cost method. In this method, the drug is dissolved in an organic solvent and then added to an antisolvent in the presence of surfactants, polymers or a mixture of both as stabilizers.[19] Currently, nano-sized particles of some poorly water-soluble drugs have been produced by a top-down or bottom-up approaches. To the best of our knowledge no study regarding ezetimibe nanosuspensions prepared by antisolvent-solvent precipitation under vaccum was reported. Although it is not very difficult to prepare nanosuspensions of many poorly water-soluble drugs, the stability of drug nanosuspensions is very crucial to ensure the advantages of nano-sized particles. Therefore, it is imperative to produce ezetimibe nanosuspensions with long term stability under storage.
The objective of this study was to prepare stable ezetimibe nanosuspension using solvent-antisolvent precipitation, characterize the process and then investigate the enhancement in oral bioavailability of the active.
MATERIALS AND METHODS
Ezetimibe was obtained as a gift sample from Suven Nishtaa, Hyderabad, India. Ethanol, Tween 80 were obtained from SD fine Chemicals Limited, Mumbai, India. All other ingredients used were of analytical grade.
Preparation of ezetimibe nanosuspensions and optimization of preparation process
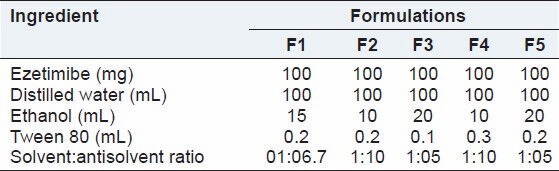
Several batches of ezetimibe nanosuspensions were prepared by solvent-antisolvent precipitation method. The levels of influencing factors investigated in such preparations are indicated in Table 1. The two factors: Surfactant concentration and solvent:antisolvent ratio were investigated at three levels to study their effect on particle size. Several previous studies indicated that these two factors significantly affect the particle size of fabricated nanosuspensions. Ethanol was used as solvent and Tween 80 was used as surfactant to stabilize the nanosuspension formulation. The drug was dissolved in solvent (ethanol). A 1% Tween 80 was dissolved in distilled water in a 250 ml Buchner flask as antisolvent. The butterfly syringe was filled with the prepared drug solution and injected dropwise into antisolvent (water containing Tween 80) with a constant stirring on a magnetic stirrer. Vaccum was applied by placing paper on Buchner flask, while stirring until the solvent evaporated to form the product. Finally, the product was filtered and dried at room temperature in a desiccator overnight. The product was centrifuged at 7000 rpm for 10 min. Particle size of drug nanosuspension after mixing on a bath sonicator was measured. The drug nanosuspension was freeze dried using Mini Lyodel Freeze Dryer (Delvac Pumps Pvt Ltd., Chennai, India).
Table 1.
Composition used to prepare ezetimibe nanosuspensions
In vitro characterization of nanosuspensions
Yield and encapsulation efficiency
To determine the yield, the weight of nanosuspensions obtained at the end of preparation was determined. The total weight of raw materials used to obtain this nanosuspension was determined to obtain the theoretical yield. Percentage yield was then determined using the formula:
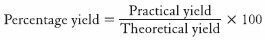
To determine the encapsulation efficiency, protocol developed in our laboratory was used. The amount of drug entrapped was estimated by dissolving the 100 mg of nanosuspensions in methonal and then sonicated under vigorous shaking for 1 h. The resultant solution was centrifuged. The drug content in supernatant solution was analyzed spectrophotometrically by using ultraviolet-visible (UV-VIS) spectrophotometer at 232 nm with further dilutions against appropriate blank. The amount of the drug entrapped in the nanosuspensions was calculated using the formula:
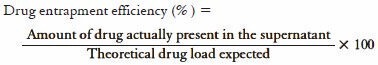
Particle size analysis
The mean particle size was determined using optical microscope. In this method, the size of 250 particles was determined and the average particle size was calculated. Optical microscope can detect particles of sizes >600 nm with accuracy. If particles produced are in this size range, this technique can be conveniently used to measure the particle size.
Scanning electron microscopy
In order to examine the particle surface morphology and shape, scanning electron microscopy (SEM) was used. A concentrated aqueous suspension was spread over a slab and dried under vacuum. The sample was shadowed in a cathodic evaporator with gold layer 20 nm thick. Photographs were taken using a JSM-5200 SEM (Joel Ltd., Tokyo, Japan) operated at 20 kV. The smallest size nanosupension was used for determining surface morphology.
Zeta potential
The zeta potential is used to measure the electric charge at the surface of the particles, indicating the physical stability of colloidal systems. In this study, the zeta potential was assessed by determining the electrophoretic mobility of the particles. The zeta potential was measured using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Samples were diluted with the respective original dispersion medium, which provides information regarding the thickness of the diffuse layer. Diluted nanosuspension was added to the sample cell (quartz cuvette) and was put into the sample holder unit and zeta potential was measured.
Saturation solubility
Saturation solubility is defined as the maximum quantity of a compound (solute) that can be dissolved in a certain quantity of a specific solvent at a specified temperature. The saturation solubility of the prepared nanosuspension, oral suspension and the pure drug were determined by stirring on magnetic stirrer for 48 h in acetate buffer pH 4.5. The nanosuspension powder was dispersed in 10 mL of distilled water, kept for magnetic stirring for 48 h. Following this, 1.5 mL of nanosuspension was filled into centrifugation tube and centrifuged at 10,000 rpm for 30 min (Remi industries centrifuge, Mumbai). Supernatant was filtered and analyzed spectrophotometrically using UV-VIS spectrophotometer (UV-Shimadzu, Japan) at 232 nm. The UV method for drug assay in saturation solubility and dissolution studies was developed in our laboratory and further validated. Linearity was achieved over a range of 100 ng/mL-10 μg/mL. This assay was conveniently used to determine the drug samples. Saturation solubility of pure drug and an ezetimibe conventional suspension prepared using hydroxypropylmethylcellulose (HPMC) was also determined using the same method. The nanosuspension used was the smallest size nanosuspension obtained from the fabrication process.
X-ray powdered diffraction analysis
The drug crystalline state in the polymer sample was evaluated by X-ray powdered diffraction analysis. X-ray spectra were recorded with X'Pert-PRO multipurpose X-ray diffractometer (PANalytical, Tokyo, Japan) using Ni-filtered, Cu Ka radiation, a voltage of 45 kV, and a current of 40 mA with a scintillation counter. The instrument was operated in the continuous scanning speed of 4°/min over a 2θ range of 5-40°. The samples were grinded using a wedgwood mortar and pestle, placed into the cavity of an aluminum sample holder and packed smoothly using a glass slide. The smallest size nanosuspension was used in the study.
Differential scanning calorimetry
Thermal analysis and properties of the powder samples (Ezetimibe, Tween-80 and nanosuspension) were investigated with a differential scanning calorimetry (DSC) (DSC shimadzu model 60, Japan). Approximately, 10 mg of sample was analyzed in an open aluminum pan, and heated at scanning rate of 10°C/min between 0°C and 400°C under nitrogen atmosphere. Magnesia was used as the standard reference material.
In vitro drug release
In vitro dissolution studies of samples were carried out using USP apparatus II paddle method by dispersed powder technique. Accurately weighed samples were added to 500 mL of buffer media (acetate buffer pH 4.5) at 37°C ± 0.5°C and stirred at 50 rpm. An aliquot of 10 mL was withdrawn at different time intervals. The solid particles were prevented from pipetting by withdrawing the sample through a pipette fitted with a cotton plug. An equal volume of fresh dissolution medium was immediately replaced. The filtered samples were assayed spectrophotometrically at 232 nm. Acetate buffer pH 4.5 was used for in vitro dissolution studies. This is USFDA recommended dissolution medium for ezetimibe. The dissolution of nanosuspension was compared with the dissolution of equivalent amount of oral suspension and pure drug.
Stability studies
Physical stability
Ezetimibe nanosuspensions were kept in glass vials at room temperature to study the physical stability. The changes in appearance, particle size, polydispersity index, Ostwald ripening and settling behavior were recorded at predetermined time intervals until 50 days. Particle size measurements were performed using Zetasizer analyzer and surface morphology by SEM.
Chemical and photo stability
For the chemical stability studies, all the samples are kept in glass vials at 25°C (no light) for 1 month and the content was estimated using a HPLC method. To evaluate photo stability of ezetimibe all the samples were exposed to illuminance of fluorescent lamps for 7 days. At predetermined time intervals, approximately 10 μL of sample was withdrawn and analyzed using HPLC method as described previously.[20] Since we used a published method, we did not validate the method. However, we determined inter-day and intra-day variability in our laboratory conditions. Further, we also analyzed stressed samples over a period of 1 month. Routine protocols were used in generating stressed conditions to the samples. We determined that the method can be stability indicating and only then we used this assay method. HPLC analysis method of ezetimibe was performed on Shimadzu HPLC system equipped with an autosampler system and variable wavelength U.V detector. The detection wave length was set at 232 nm. Chromatographic separation was carried out with Eclipse XBD-C18 column (150 mm × 4.6 mm, 5 μm, shimadzu, Japan) with mobile phase of acetonitrile/5% acetic acid solution and acetate buffer pH 4.5 at 30°C. The flow rate was 1.0 mL/min. The calibration curve was linear within the range of 0.75-120 μg/mL and r = 0.9998. The detection limit (signal to noise ratio 2:1) and quantification limit (signal to noise ratio 8:1) were 0.015 μg/mL and 0.065 μg/mL, respectively.
Animal studies
In vivo pharmacokinetic studies in rats: Animal experimental procedure
All the animal studies were conducted as per the guidelines of CPCSEA, India. The protocol was approved by Institutional Animal Ethics Committee of Geetanjali College of Pharmacy, Hyderabad (IAEC No. 1648/PO/a/12/CPCSEA). Wistar rats (weighed 180-220 g) were used as experimental animals. The smallest size ezetimibe nanosuspension was used in this study. Twelve rats randomly divided into two groups with 6 rats in each group. Prior to experimentation the rats were fasted for 12 h with free access to water. The next day, ezetimibe nanosuspension (F4), ezetimibe oral suspension prepared using sodium HPMC as suspending agent were given to the two groups of rats via the oral route. All the formulations contained 100 mg of drug. About 0.5 mL of blood samples were collected via the orbit vein at 0.25, 0.50, 0.75, 1, 2, 3, 4, 6, 8, 10, 12 and 24 h after administration. The collected blood samples were placed in heparinized tube and then separated immediately by centrifugation at 3000 rpm for 10 min and then stored at −20°C prior to analysis. The plasma samples were then extracted and ezetimibe was analyzed using HPLC method as described earlier in stability studies.
The preparation of plasma samples was as follows: Deproteinization method was employed. 400 mL methanol was added to each 200 μL rat plasma sample. The mixtures were vortexed (MS3, IKA, Germany) for 5 min and centrifugated (Ultracentifuge, Remi, India) at 3000 rpm for 5 min. Thereafter, 20 μL volume of supernatant was directly injected into HPLC system for analysis. The plasma concentration time profile for two batches was determined, the average and standard deviation at all the time points was determined and concentration versus time plot was plotted.
RESULTS AND DISCUSSION
Different batches of ezetimibe were prepared considering the solvent:antisolvent ratio and concentration of surfactant to obtain nanoparticles with a reduced particle size. The key to preparing nanosuspensions by antisolvent-solvent precipitation is to create conditions that favor very rapid particle formation and little or no particle size growth. Vaccum has been used to generate nano-sized drug particles by (a) intensifying the mass transfer avoiding inefficient mixing; (b) atomizing the drug solution with very fine droplets into the antisolvent resulting in the formation of smaller particles; (c) reducing the particle size of the newly formed particles and suppressing the agglomeration of fine particles. Ezetimibe nanosuspensions were successfully prepared using the solvent-antisolvent precipitation technique of this study. Optical microscopy was used in the determination of particle size of nanosuspensions, the particle size was verified using SEM and confirmed by zetasizer. The average particle size for all the batches, the yield and percentage encapsulation is given in Table 2. Optical microscopy at ×100 and oil immersion was successfully used to measure the particle size of our study since the particle size was >600 nm (cut-off size for optical microscopy). The hydrodynamic size as measured by zeta sizer was slightly higher than that measured by optical microscope. This is due to the electrical double layer that is also considered with zeta size measurements. From SEM, it was concluded that the average particle size for optimized formulation was found to be around 700 nm [Figure 1]. Surface morphology and shape were visualized. The particles were smooth and spherical. Surfactant levels as well as solvent:antisolvent ratio were found to have a significant influence on the size. Increase in the concentration of surfactant reduced the particle size. The particle size was also reduced as the solvent:antisolvent ratio was decreased.
Table 2.
Characterization of the prepared nanosuspensions
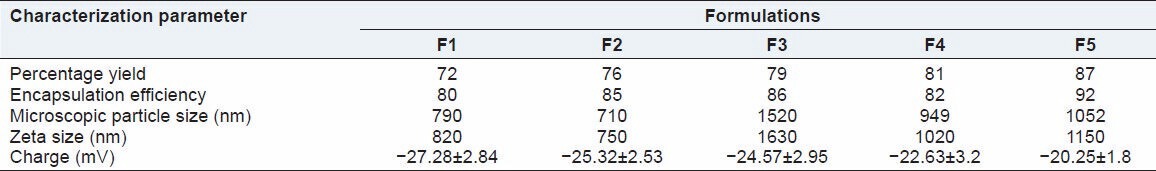
Figure 1.
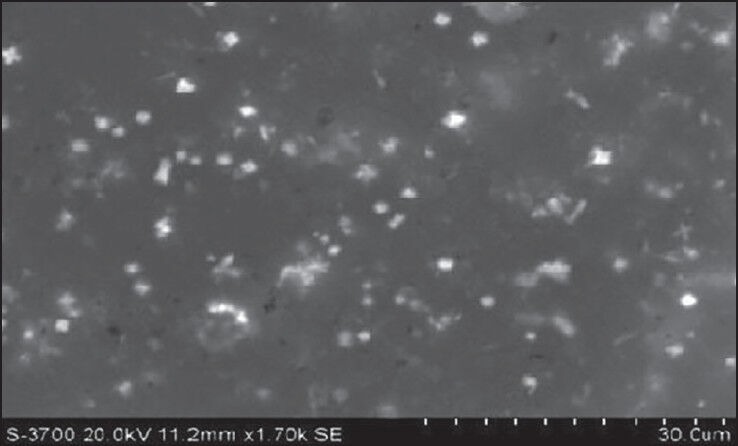
Scanning electron microscopy pictures of ezetimibe nanosuspensions
X-ray powdered diffraction was used to investigate the physical nature of the encapsulated drug upon fabrication. From Figure 2, it was observed that the crystallinity of the drug was changed in the nanosuspensions. The peaks obtained for pure drug were very clear and sharp, and the intensity of the peaks was very high when compared with peaks of ezetimibe nanosuspensions. Reduction in the peak intensity indicates amorphization. DSC further confirmed the solid state of the drug. Ezetimibe showed a melting peak at 184°C as shown in Figure 3. In the thermogram of the optimized nanosuspension, the drug peak disappeared and this is due to change in crystallinity to amorphous state. A very small peak of Tween 80 was present at 165°C. This was not present in nanosuspension peak and this may be due to the very low concentration of Tween 80 present in the formulation. The zeta potential is used to predict the storage stability of colloid dispersed systems during the shelf storage and it reflects the electrostatic barriers, which would prevent nanosuspension from aggregation and agglomeration. Particle aggregation will also likely to occur if particles had low zeta potential. In general, zeta potential of particles should be at least −30 mV for electrostatically stabilized systems or −20 mV for sterically stabilized systems to obtain a physically stable nanosuspension. At the end of preparation of nanosuspensions and at all points of storage for 50 days, the zeta potential was in the stable range. In general, zeta potential value of ±20 mV is sufficient for stability of nanosuspension prepared using Tween 80. The zeta potential of optimized nanosuspension was found to be −25.32 ± 2.53 mV, indicating that the prepared nanosuspension do not suffer from instability problems. Zeta potential of nanosuspension exhibited no essential changes before and after the storage and stability studies. It indicates that formulation is physically stable and this may be due to sufficient thickness of diffusive layer to prevent particle aggregation. Ostwald ripening and settling behavior were observed at predetermined time intervals (1, 7, 14 and 50 days). The observations indicate no crystal growth and no ostwald ripening is noted during period of physical stability studies. No change in appearance and settling behaviour was observed. The results indicate that nanosuspension was physically stable. Chemical stability studies also demonstrated similar results. Control solutions showed degradation of ezetimibe over a period of 1 month when stored at 25°C. Upon exposure to light, a reduction in ezetimibe drug content and obvious discoloration was also observed in the solution over 1 month period. In contrast, ezetimibe nanosuspension stored at same conditions showed no significant change in drug content and in color over the monitoring period. Hence, it was concluded that the nanosuspension is also chemically stable. Similar results were observed in the photo stability studies, which showed reduction in drug content for ezetimibe solution but no significant reduction was found for ezetimibe nanosuspension. Thus, chemically and physically stable amorphous ezetimibe nanosuspensions were prepared using solvent-antisolvent precipitation employed in this study.
Figure 2.
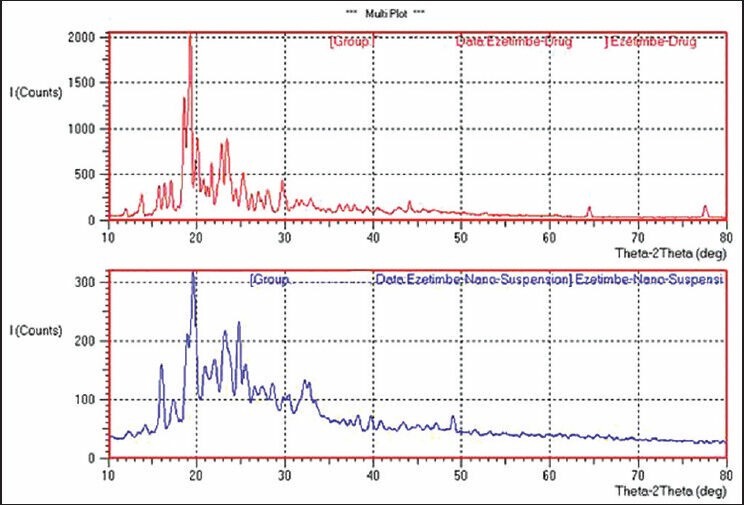
X-ray powdered diffraction of pure drug and ezetimibe nanosuspension
Figure 3.
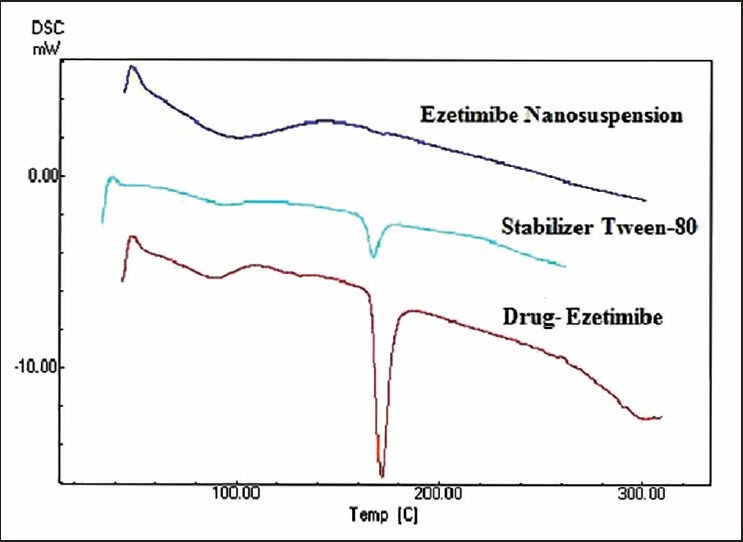
Differential scanning calorimetry studies of drug, excipient and nanosuspension
The saturation solubility of the particles is essential as it affects the bioavailability of the drug, the rate of drug release into dissolution medium and consequently, the therapeutic efficiency of the pharmaceutical product. The saturation solubility was measured for the pure drug, oral suspension and nanosuspension in water. Reduction in the particle size to nanometer range led to the increase in saturation solubility of nanosuspensions. The values of saturation solubility of nanosuspension, oral suspension and pure drug are as follows: 13.54 ± 0.78 μg/mL 4.55 ± 0.61 μg/mL, 0.81 ± 0.63 μg/mL, respectively. The results of dissolution studies were expressed in terms of % drug dissolved as the function of time. The data obtained from Figure 4 revealed that the onset of dissolution of drug from oral suspension was 20.56% in 1 h and from pure drug it was 42% in 1 h. Nanosizing of drugs can lead to a dramatic increase in the dissolution rate. From the data obtained, it was observed that nanosuspensions released the drug to an extent of 98% in 1 h. It clearly indicates that nanosuspension formulation has been a successful technique to improve the dissolution rate of ezetimibe and thereby can enhance bioavailability. The in vivo study in Wistar rats was performed to demonstrate in vivo bioavailability. After orally administering formulations, suspensions and nanosuspensions, the plasma concentration were obtained and compared [Figure 5]. The plasma concentration profile for nanosuspension represented significant improvement in drug absorption compared with the oral suspension. The Cmax and area under the curve (AUC) 0-24 h values of nanosuspensions were approximately 3-fold, greater than that of the oral suspension, indicating a remarkable improvement in the oral absorption of ezetimibe nanosuspension when administered in the form of amorphous nanosuspensions. The Cmax was found to be 495 ng/mL at 2 h for nanosuspension. The enhancement in oral bioavailability can be attributed to the adhesiveness of the drug nanosuspension, increased surface area due to reduction in particle size, increased saturation solubility, leading to an increased concentration gradient between the gastrointestinal tract lumen and blood, and increased dissolution velocity.
Figure 4.
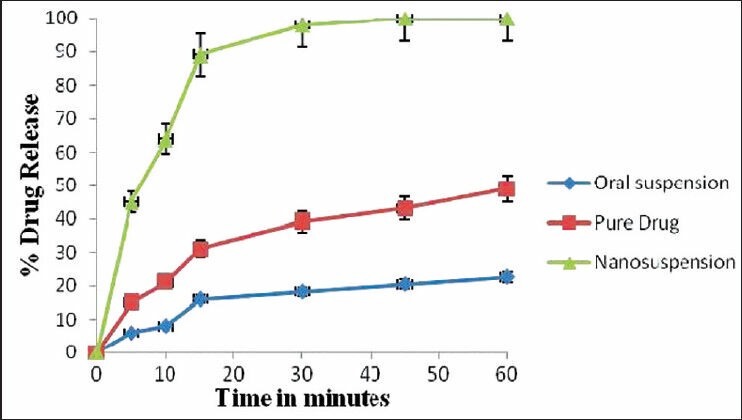
Graphical presentation of dissolution profile of pure drug and nanosuspension
Figure 5.
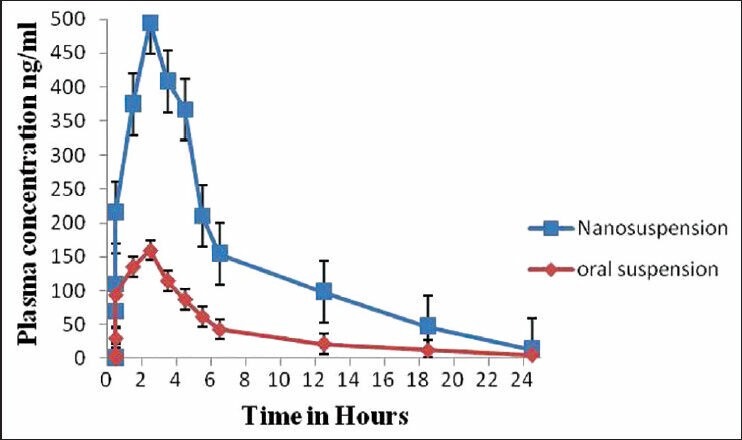
Graphical presentation of bioavailability studies of oral suspension and nanosuspension
Ezetimibe is the first member of a novel class of lipid-lowering agents that selectively inhibits the absorption of biliary and dietary cholesterol as well as other phytosterol from the intestine without affecting the absorption of fat-soluble vitamins, triglycerides or bile acids. It demonstrated less and variable oral bioavailability. To solve these problems, oral ezetimibe nanosuspensions have been prepared. Compared with conventional oral nanosuspensions, there was a 3-fold increase in oral bioavailability with nanosuspensions. This can be attributed to the reduction in the particle size. Further, there was a conversion of drug to amorphous form upon preparation of nanosuspensions. Thus, we produced amorphous nanosuspensions for ezetimibe. Amorphous nanosuspensions were produced using solvent-antisolvent precipitation technique, which is a bottom-up approach. There are two methods for preparation of nanosuspensions, which include top-down approach and bottom-up approach. These techniques are discussed in introduction as well as in several papers previously published.[18,19] Literature also revealed that top-down approaches generally retain the crystallinity of the drug while crystallinity is altered in bottom-up approach.[21] Our studies corroborated these earlier results. Further, the stability studies were conducted with the nanosuspensions prepared in the study. This is especially necessary for amorphous nanosuspensions, which are deemed to be less stable. The stability studies revealed that stable amorphous nanosuspensions were prepared for ezetimibe. Thus, we propose amorphous nanosuspensions for ezetimibe for improvement in bioavailability. Due to smaller particle size and much larger surface to volume ratio, oral nanosuspensions are especially used to enhance the bioavailability and reduce the fluctuations in the oral bioavalability. By using standard manufacturing techniques, drug nanosuspensions can be simply incorporated into various dosage forms. Although several techniques have been investigated the enhancement in oral bioavailability and reduction in its variability, nanosuspensions for ezetimibe were not previously reported. Further, nanosuspensions can be conveniently commercialized as there are already several nanosuspension products in the market.
Several nanosuspension formulations were previously prepared and evaluated. For instance, Kakran et al. have reported fabrication of drug nanoparticles by evaporative precipitation of nanosuspension (EPN) by varying ratio of solvent to antisolvent. EPN was used to fabricate nanoparticles of a poorly water-soluble antimalarial drug, artemisinin (ART), with an aim to increase the dissolution rate. The best dissolution percent was found to be 75.9%, at the drug concentration of 15 mg/mL and solvent to antisolvent ratio (by volume) of 1:20. The dissolution of EPN prepared ART nanoparticles was markedly increased when compared to the original ART powder.[19] Xu et al. have reported enhanced dissolution and oral bioavailability of aripiprazole (APZ) using nanosuspension technology. Nanosuspensions were prepared by nanoprecipitation/homogenization based on acid-base neutralization. Nanosuspensions significantly increased the solubility as well as the dissolution of APZ due to the decreased particle size. APZ nanosuspensions had greater absorption rate and extent when compared with APZ commercial tablet and suspensions. This technique has the potential to prepare nanosuspensions of insoluble drugs with pH-dependent solubility.-[22] Xia et al. prepared stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. The in vivo test demonstrated that the Cmax and AUC (0→12) values of nanosuspension in rats were approximately 6.1-fold and 5.0-fold greater than that of commercial tablets, respectively. The in vitro dissolution rate of nitrendipine was significantly increased by reducing the particle size.[23] These are some of the success stories with nanosuspensions for enhancement in oral bioavailability. Our study is one additional speck in the technology of nanosuspensions for enhancement in oral bioavailability. The technique of amorphous nanosuspensions can be conveniently used in drug discovery stages to weed out/reduce preclinical/clinical failures of NCEs due to the low bioavailability. The technique can also be used to improve existing formulations or develop improved generics.
CONCLUSION
Nanosuspensions are suitable delivery systems to improve the bioavailability of drugs with low water solubility. This has been confirmed with the model drug of this study, ezetimibe.
ACKNOWLEDGMENT
The authors would like to acknowledge Management of Mother Teresa College of Pharmacy for providing necessary support to the conduction of this work. Also, the authors would like to acknowledge Department of Technology, Osmania University, Hyderabad, for providing analytical support to this project.
Footnotes
Source of Support: Management, Mother Teresa College of Pharmacy, Hyderabad for providing financial support to this project, Department of Technology, Osmania University, Hyderabad, for providing analytical support to this project.
Conflict of Interest: None declared.
REFERENCES
- 1.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int J Pharm. 2011;420:1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Lakshmi PK, Srinivas Ch, Kalpana B. Preparation and comparative evaluation of liquisolid compacts and solid dispersions of valsartan. Stamford J Pharm Sci. 2012;4:47–57. [Google Scholar]
- 3.Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4:403–16. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 4.Stegemann S, Leveiller F, Franchi D, de Jong H, Lindén H. When poor solubility becomes an issue: From early stage to proof of concept. Eur J Pharm Sci. 2007;31:249–61. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 5.Mauro VF, Tuckerman CE. Ezetimibe for management of hypercholesterolemia. Ann Pharmacother. 2003;37:839–48. doi: 10.1345/aph.1C209. [DOI] [PubMed] [Google Scholar]
- 6.Bruckert E, Giral P, Tellier P. Perspectives in cholesterol-lowering therapy: The role of ezetimibe, a new selective inhibitor of intestinal cholesterol absorption. Circulation. 2003;107:3124–8. doi: 10.1161/01.CIR.0000072345.98581.24. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulou V, Valsami G, Dokoumetzidis A, Macheras P. Biopharmaceutics classification systems for new molecular entities (BCS-NMEs) and marketed drugs (BCS-MD): Theoretical basis and practical examples. Int J Pharm. 2008;361:70–7. doi: 10.1016/j.ijpharm.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Dressman JB, Reppas C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11(Suppl 2):S73–80. doi: 10.1016/s0928-0987(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 9.Oswald S, Haenisch S, Fricke C, Sudhop T, Remmler C, Giessmann T, et al. Intestinal expression of P-glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate-glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clin Pharmacol Ther. 2006;79:206–17. doi: 10.1016/j.clpt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Krishniah YS. Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J Bioequiv Availab. 2010;2:28–36. [Google Scholar]
- 11.Kesisoglou F, Panmai S, Wu Y. Nanosizing – Oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–44. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Aukunuru J. Drug Absorption Enhancement in Oral Drug Delivery Technology. 2nd ed. Hyderabad, India: Pharma Book Syndicate; 2012. pp. 213–237. [Google Scholar]
- 13.Bandyopadhyay S, Katare OP, Singh B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf B Biointerfaces. 2012;100:50–61. doi: 10.1016/j.colsurfb.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Bali V, Ali M, Ali J. Novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. J Drug Target. 2010;18:506–19. doi: 10.3109/10611860903548362. [DOI] [PubMed] [Google Scholar]
- 15.Bali V, Ali M, Ali J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf B Biointerfaces. 2010;76:410–20. doi: 10.1016/j.colsurfb.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Bali V, Ali M, Ali J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: In vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int J Pharm. 2011;403:46–56. doi: 10.1016/j.ijpharm.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Müller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 18.Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top down production of nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. PMID: 18721869. [DOI] [PubMed] [Google Scholar]
- 19.Kakran M, Sahoo NG, Li L, Judeh Z, Wang Y, Chong K, et al. Fabrication of drug nanoparticles by evaporative precipitation of nanosuspension. Int J Pharm. 2010;383:285–92. doi: 10.1016/j.ijpharm.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Shrikrishna BB, Erande RS, Shaikh SG. Analytical method development and validation for estimation of ezetimibe from tablet dosage forms by using RP-HPLC. Int J Res Pharm Biomed Sci. 2011;2:833–8. [Google Scholar]
- 21.Ali HS, York P, Ali AM, Blagden N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J Control Release. 2011;149:175–81. doi: 10.1016/j.jconrel.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Liu X, Lian R, Zheng S, Yin Z, Lu Y, et al. Enhanced dissolution and oral bioavailability of aripiprazole nanosuspensions prepared by nanoprecipitation/homogenization based on acid-base neutralization. Int J Pharm. 2012;438:287–95. doi: 10.1016/j.ijpharm.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Xia D, Quan P, Piao H, Piao H, Sun H. Preparation of stable nitrindepine nanosuspensions using precipitation-ultrasonication method for enhancement of dissolution of oral bioavailability. Adv Drug Deliv Rev. 2011;47:19–25. [Google Scholar]


