Abstract
Purpose of the study:
The antiretroviral therapy (ART) has dramatically improved human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) treatment, prevention and also has been found to increase the lifespan of HIV/AIDS patients by providing durable control of the HIV replication in patients. Efavirenz is a non-nucleoside reverse transcriptase inhibitor of HIV-1. The purpose of this study is to formulate efavirenz-loaded bovine serum albumin nanoparticles to improve efavirenz delivery into various organs.
Materials and Methods:
Nanoparticles were prepared by desolvation technique and coated with polysorbate 80. Ethanol, glutaraldehyde, and mannitol were used as desolvating, cross linking agent, and cryoprotectant, respectively. Drug to polymer ratio was chosen at five levels from 1:2, 1:3, 1:4, 1:5, and 1:6 (by weight). The formulated nanoparticles were characterized for Fourier Transform Infrared (FT-IR) spectroscopy, differential scanning calorimetry (DSC) studies, entrapment efficiency, particle size, surface charge, surface morphology, in vitro drug release, release kinetics, stability studies, and biodistribution studies.
Results and Major Conclusion:
The particle size of the prepared formulations was found below 250nm with narrow size distribution, spherical in shape and showed good entrapment efficiency (45.62-72.49%). The in vitro drug release indicated biphasic release and its data were fitted to release kinetics models and release pattern was Fickian diffusion controlled release profile. The prepared nanoparticles increased efavirenz delivery into various organs by several fold in comparison with the free drug.
Keywords: Albumin nanoparticles, animal testing, desolvation technique, Efavirenz (NNRTIs), polysorbate 80
INTRODUCTION
Over the past decades human immunodeficiency virus (HIV) has emerged as one of the most serious public health problems in the world. HIV/acquired immunodeficiency syndrome (AIDS) is the fourth leading disease cause of death and more doctors are encountering infected individuals.[1] The HIV infects cells of the immune system, destroying these cells as well as the immune system's ability to fight off the invaders and its infection may increase the risk of many major health conditions experienced by aging adults and possibly accelerate the aging process.[2] The main need of antiretroviral therapy (ART) is to keep the amount of HIV in the body at low level, to reduce HIV associated morbidity, prolong the duration and quality of survival. Since 1996 ART for HIV infection has improved potently.[1] Effective ART has led to greater longevity in HIV-infected individuals resulting in an increasing number of older individuals living with HIV infection, stops any weakening of the immune system, or preserve immunologic function maximally, and also allows it to recover from any damage that HIV might have caused already.
Nanoparticles are one of the most promising drug delivery systems.[3] The primary goals are to target the drug at site of action, to enhance bioavailability without side effects,[4] greater safety and biocompatibility[5] due to its ability to deliver the drug to intercellular level of various organs. Various biodegradable polymers have been used to deliver the drug in controlled and sustained manner at the site of action and maintain the desired therapeutic concentration for long time. Albumin is the most abundant protein carrier for drug delivery and is biodegradable, bioacceptable, non toxic, non antigenic[6] and well tolerated due to their defined primary structure and high content of charged amino acids.[4] Moreover it is very cheap, easily available, and is a ideal material for fabrication of nanoparticles which will be more effective, easy to prepare, easy to monitor their size distribution.[7,8,9]
Different types of antiretroviral drugs are available and they act in different ways to prevent the replication of HIV in the human body. The non-nucleoside reverse transcriptase inhibitors (NNRTIs) inhibit the HIV reverse transcriptase by binding the hydrophobic pocket close to the active site (non-competitively) and preventing/blocking the conversion of ribonucleic acid (RNA) to deoxyribonucleic acid (DNA).[10] There are three first generation NNRTIs approved by US Food and Drug Administration (FDA) are efavirenz and nevirapine.[11] These drugs have been accepted as a component of fist line treatment in antiretroviral therapy with extensive experience, including some data suggesting good antiretroviral activity in severely immune suppressed patients and reduced adverse effects compared with most protease inhibitor based therapy. Efavirenz's (EFV) chemical formula is C14 H9 ClF3 NO2 and a benzoxazinone derivative.[4,12] It is a white crystalline powder and poorly soluble in water and aqueous fluids. Antiretrovirals s are known for their chronic and troublesome side effects such as pancreatitis, peripheral neuropathy, and skin rashes. EFV was chosen for the present study based on its effectiveness to provide viral suppression when compared with nevirapine, with fewer severe adverse effects.[13,14,15] The main objective of this study was to formulate albumin nanoparticles of Efavirenz with sustained release effect. The EFV incorporated nanoparticles are promising tools to improve the aqueous solubility of drugs, may minimize the side effects and also provide successful delivery/targeting of drugs to HIV infected immune cells.[16]
MATERIALS AND METHODS
Materials
EFV was a gift sample from Strides Arcolabs ltd., Bangalore, India. Bovine serum albumin (BSA) (fraction V, with purity of 98%) was purchased from Hi-media Pvt. ltd., Mumbai, India. Polysorbate 80 was commercially supplied by Sigma Aldrich. All the other chemicals used were analytical/high-performance liquid chromatography (HPLC) grade.
Methods
Method of preparation
BSA nanoparticles of efavirenz were prepared by desolvation method.[17] Five batches of nanoparticles were prepared with varying concentrations of BSA. Briefly known quantity of bovine serum albumin powder was dissolved in 10 ml of distilled water and pH was adjusted to 8 with 0.1 M NaOH solution to get nanoparticles with smaller particle size. EFV was dissolved in ethanol. Ethanolic solution of EFV was added dropwise under magnetic stirring at 500 rpm at a rate of 1 ml/min into the BSA solution until turbidity occurred and the stirring was continued. After the desolvation process, 100μl of an aqueous solution of 8% glutaraldehyde was added to induce particle cross linking and stirring continued over a time period of 5 hours at room temperature. Finally, 2% w/v mannitol was added to the nanoparticle suspension as cryoprotectant. The EFV-loaded nanoparticles were separated from free drug by centrifugation (20,000 rpm for 30 min at 4°C) and subjected to freeze drying. For coating 1% w/v of polysorbate 80[18] was added to nanoparticle suspensions and incubated for 30 min with constant stirring.
Nanoparticle characterization
Determination of Process yield and Entrapment Efficiency (EE)
The process yield of EFV-loaded albumin nanoparticles with different drug polymer ratios was calculated using the following formula:[19]
% Process yield = (Weight of the nanoparticles/Total weight of drug + polymer) × 100
EE was determined by separating the free drug in the nanosuspensions and other fluid materials added by centrifugation at 20,000 rpm. The supernatant was collected and drug concentration in supernatant was determined by UV spectrophotometrically at 247nm after proper dilution. % EE was calculated by following equation.[19]
% EE = [(Total amount of drug added − amount of free drug in supernatant)/Total amount of drug] × 100
Determination of particle size distribution and zeta potentials
The particle sizes and zeta potential (surface charges) of EFV-loaded albumin nanoparticles were measured on a Zetasizer dynamic light scattering detector (Malvern Instruments, Malvern, UK) equipped with a 633 nm, 4mW Helium/Neon laser (red laser). All measurements were performed at 25° C at a detecting angle of 173°. Prior to measurements, the100 μL of nanosuspensions were diluted with 900 μL of milli-Q water and mixed by a vortex mixer for 10 minutes. 500 μL of samples was taken for better measurement in a clear disposable zeta cell at 25 ± 2°C.
Determination of particulate surface morphology
Scanning electron microscope (JSM-6100; JEOL, Tokyo, Japan) of prepared nanoparticles was also carried out to determine their morphological character.[20] Dried nanoparticles were placed on brass stub, which were gold coated in an ion sputter to render them electrically conductive. Photomicrographs of nanoparticles were taken at different magnifications from randomly selected fields.
Fourier transforms infrared spectroscopy (FT-IR) and differential scanning calorimetry study (DSC)
The FT-IR and DSC studies are the analytical techniques to detect chemical interaction, changes in crystallinity and confirm the thermal stability of drug. FT-IR (Shimadzu Corporation, Japan) spectrum of drug (EFV), polymer (BSA), physical mixture of drug and polymer, and EFV-loaded nanoparticles were recorded by using KBr pellet method to check drug polymer interaction. DSC (Mettler – Toledo star 822 systems, Switzerland) analysis was carried out for free EFV and freeze dried EFV-loaded BSA NPs.
In vitro drug release
In vitro drug release from drug-loaded nanoparticles was performed by simple dialysis method at 37 ± 1o C. Briefly, equivalent to 10 mg of the drug along with 5 ml of pH 7.4 phosphate buffer were taken in the dialysis bag (cut off 5 kDa, Himedia, Mumbai, India) which was tied at the both ends. The dialysis bag was dipped into the flask containing 100 ml of dissolution medium (pH 7.4 PBS) on a magnetic stirrer at 100 rpm. 5ml of sample was withdrawn at regular time intervals and same volume of dissolution medium was replaced in the flask to maintain sink condition. The withdrawn samples were filtered and the drug release was analyzed after proper dilution by measuring the absorbance at 247 nm UV-visible spectrophotometer. All samples were done in triplicate for each formulation.
Release kinetics
The in vitro release data were fitted to various kinetics models[21] such as zero order (Q = k0 t), first order (ln (100 − Q) = ln Q0 − k1 t), Higuchi model (Q = kH t½) and Korsmeyer-Peppas (Mt /M∞ = Ktn) in order to investigate the kinetics and drug release mechanism from albumin nanoparticles.
Stability studies
The stability study[22] of freeze dried EFV-loaded albumin nanoparticles (E1) was carried out to assess the stability and chemical interaction of drug in nanoparticles. For this purpose the samples were taken in borosilicate vials and sealed and the vials were stored at room temperature (25o to 30o C) and refrigerator (3o to 5o C) (relative humidity = 75%) over a period of 3 months. After specified period of time (0, 1, 2 and 3 months) the samples were checked for their drug content, physical appearance as well as chemical stability by Fourier transforms infrared (FT-IR) studies.
Evaluation of targeting efficiency of the formulations by in vivo studies on rats
The Biodistribution studies of EFV-loaded albumin nanoparticles were carried out on healthy adult albino rats weighing between 200-250 g. The rats were obtained from the animal house of Dayananda Sagar College of Pharmacy, Bangalore, India after obtaining approval from the institutional animal ethics committee and CPCSEA (DSCP/IAEC/PH.D/PH.CEUTICS/2009-2010). All animals were provided with proper care, food, water ad libitum and maintained in well ventilated large spacious cages throughout the study. The rats were divided randomly into three groups with six animals per group, Group 1 was injected with free EFV solution into the tail vein, Group 2 was received uncoated nanoparticles and Group 3 was given polysorbate 80 coated nanoparticles. All the formulations were given in a dose level equivalent to 20 mg/kg body weight.[23] One hour after injection, the rats were sacrificed by euthanized and organs such as liver, lung, kidney, heart and spleen were isolated. The organs were washed with buffer saline and absorbed dry with filter paper, then weighed immediately. Prior to the analysis organs homogenates were prepared and drug concentration in the organs were estimated by reverse-phase HPLC method.[24]
RESULTS AND DISCUSSIONS
Nanoparticles preparation
EFV-loaded albumin nanoparticles with different drug polymer ratios were prepared by desolvation techniques. Here, ethanol acts as an antisolvent for albumin to reduce its solubility in water and activate nanoparticle formation by desolvation. The nanoparticles formed were cross linked with glutaraldehyde to improve the stability. This method was optimized initially to get an optimized formula with different parameters like the albumin concentration, quantities of ethanol, pH, stirring speed, and volume of glutaraldehyde solution. Drug-loaded nanoparticles were characterized for compatibility study, process yield, encapsulation efficiency, particle size, polydispersity, surface morphology, zeta potential, in vitro drug release, release kinetics, stability studies, and biodistribution studies.
Process yield and encapsulation efficiency
The percentage process yield and percentage EE are reported in Table 1. The process yield was slightly increased with increasing polymer concentration and maximum process yield (79.92%) was obtained in E5 where BSA concentration was higher. The percentage drug encapsulation efficiency was decreased along with the increase amount polymer (albumin) concentration and ranged between 72.49-31.64%.
Table 1.
Data of % Process yield and encapsulation efficiency of efavirenz-loaded albumin nanoparticles

Fourier transforms infrared spectroscopy (FT-IR) and differential scanning calorimetry study (DSC)
The FT-IR spectra of the EFV, BSA, physical mixture, and formulated nanoparticles are shown in Figure 1. The FT-IR spectrum of EFV showed the characteristic peaks of alkyne at 2249.07 cm−1, CH2 rock at 1215. 15 cm−1, CH2 scissor at 1458.18 cm−1, C-F stretch at 1384.94 cm−1, N-H stretch at 3319.60 cm−1, C-O at 1082.1 cm−1, and C = O stretch at 1749.49 cm−1. The major peaks of EFV were not changed in the drug-loaded nanoparticles which indicates no interaction the drug and polymer during the nanoparticles preparation. Figure 2 shows the DSC thermograms of EFV, BSA, and lyophilized drug-loaded nanoparticles. Efavirenz showed a sharp endothermic peak at 142.06°C where BSA displayed a broad melting endothermic peak at 220.06°C and the drug-loaded lyophilized nanoparticles exhibit small endothermic peak at 140.19°C suggesting that the drug was from the desolvation technique. FT-IR and DSC studies demonstrated the compatibility, stability of the drug in the formulation, and also confirmed the drug loading into the nanoparticles.
Figure 1.
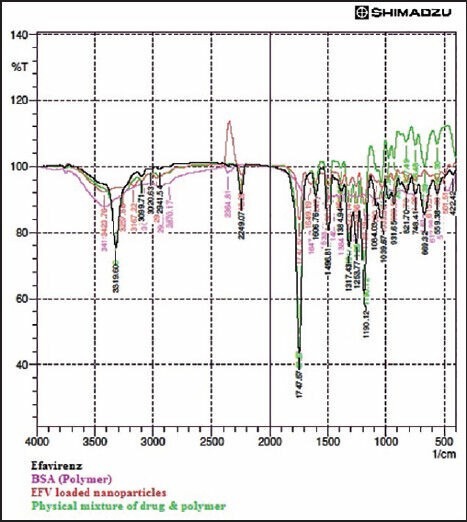
Merged FT-IR spectra of Efavirenz, BSA, Physical mixture and formulated nanoparticles
Figure 2.

DSC thermogram of Efavirenz and formulated nanoparticles
Particle size distribution and zeta potential
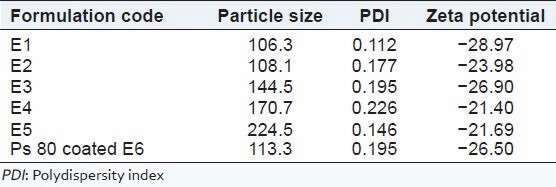
Particle surface characteristics and charge play important role in the particle's physical state, stability in different media, agglomeration tendencies and interaction with biological systems. The morphology of albumin nanoparticles was studied by SEM. Figure 3 shows the SEM image of freeze dried EFV-loaded albumin nanoparticles. The SEM studies revealed that the nanoparticles were spherical in shape with smooth surface. The average particle size of EFV-loaded nanoparticles E1 to E5 were found to be below 230 nm with low polydispersity index (PDI). The low PDI indicates the narrow size distribution of the nanoparticles [Table 2]. The pH of the BSA solution before desolvation was adjusted to pH 8 with 0.1M NaOH to get smaller size particles. Zeta potential provides indirect measure of the net charge and its value of EFV-loaded nanoparticles was ranging from −0.112 to −0.226. This value of zeta potential is sufficient to produce strong repulsive forces between nanoparticles to avoid aggregation. Figure 3a and 3b show the particles size distribution and zeta potential of E1 formulation
Figure 3.

Scanning electron microscopic image of efavirenz loaded albumin nanoparticles. (a) particles size distribution of efavirenz loaded albumin nanoparticles (E1), (b) zeta potential of efavirenz loaded albumin nanoparticles (E1)
Table 2.
Physicochemical characterization of efavirenz-loaded albumin nanoparticles
In vitro drug release study
The cumulative percentage of in vitro EFV release profile from all formulations over 36 h in phosphate buffered solution (pH 7.4) are shown in Figure 4. The EFV-loaded albumin nanoparticles exhibited a biphasic dug release pattern with burst effect initially (17% to 27.7% in the first 1 hour) due to drug dispersed close to the surface of the nanoparticles followed by a sustained release (56.9% to 75.1%). It was also found that the rate of drug release was affected by increase in polymer (albumin) content in the nanoparticles. Polysorbate 80 coating was slightly retarded the drug release than the uncoated nanoparticles.
Figure 4.

In vitro drug release profile of EFV loaded BSA nanoparticles in pH7.4 PBS (Data shown as mean ± SD, n = 3)
Release kinetics
The release kinetics was determined by fitting the in vitro data to various kinetic models. The values of the release exponent (n) and the correlation coefficient (r2) of the all formulations are tabulated in Table 3. The 'r2” values were found to be higher for the Korsmeyer-Peppas model and the value of “n” were below 0.45. The result suggested that release of drug from the albumin nanoparticles was governed by diffusion and the release mechanism was Fickian. In vitro release kinetics study showed that albumin nanoparticles are capable of releasing efavirenz in a slow sustained manner over a period of 36 hour.
Table 3.
Release kinetics of efavirenz-loaded BSA nanoparticles

Stability studies
All albumin nanoparticles formulations were sealed in ampoules and stored at room temperature (25-30°C) and refrigerator (3-5°C). The effect of storage condition on physical appearance, particle size, PDI, zeta potential, and drug content are shown in Table 4. There were no significant changes in drug content and FT-IR spectra. The physical as well as chemical characteristics of the formulation were not affected at storage conditions, i.e., 3-5°C and 25-30°C over a period of 3 months. The above results indicated that the developed albumin nanoparticles are physically and chemically stable and retain their pharmaceutical properties at various temperatures over a period of 3 months.
Table 4.
Stability studies of efavirenz-loaded albumin nanoparticles (E1)

Evaluation of targeting efficiency of the formulations by in vivo studies on rats
The concentration of efavirenz (ng/g) achieved in various organs after administering free efavirenz solution, the coated and uncoated EFV-loaded albumin nanoparticles by intravenous administration in rats are shown in Figure 5. It is clear that the concentration of EFV was higher in tissue level in most of the organs from polysorbate 80 coated albumin nanoparticles, while the concentration measured in various organs with free drug solution. This may be due to rapid uptake of polysorbate-80-coated albumin nanoparticles by macrophage-rich organs in comparison to uncoated nanoparticles and free drug solutions. EFV concentration in brain was sevenfold higher and in lymph nodes sixfold higher from polysorbate than that of free drug solution.
Figure 5.

Biodistribution of efavirenz in rats organs after i.v injection of BSA nanoparticles. EFV: Efavirenz, EFV Np: EFV loaded nanoparticles, Ps 80-ddi Np: Polysorbate coated EFV loaded nanoparticles. (mean ± SD, n = 3)
CONCLUSION
Efavirenz-loaded BSA nanocarriers was successfully prepared by desolvation technique and then coated with 1% v/v polysorbate 80. EFV-loaded albumin nanocarriers could release the antiretroviral drug to the macrophages in a sustained manner for a prolong period of time. This study may offer as ideal approach for the treatment of HIV and could potentially improve patient compliance and the life style of HIV infected patients. Nanoparticles are able to protect and promote the uptake of non-orally administered antiretroviral drugs into the cellular level of various organs where the HIV reside and also prolong the drug residence in the body.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Naidoo P. Barriers to HIV care and treatment by doctors: A review of the literature. SA Fam Pract. 2006;48:55. [Google Scholar]
- 2.Piot P, Bartos M, Ghys PD, Walker N, Schwartländer B. The global impact of HIV/AIDS. Nature. 2001;410:968–73. doi: 10.1038/35073639. [DOI] [PubMed] [Google Scholar]
- 3.Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–55. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 4.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168–82. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 5.De Jong WH, Borm PJ. Drug delivery and nanoparticles: Applications and hazards. Int J Nanomedicine. 2008;3:133–49. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnedo A, Espuelas S, Irache JM. Albumin nanoparticles as carriers for a phosphodiester oligonucleotide. Int J Pharm. 2002;244:59–72. doi: 10.1016/s0378-5173(02)00300-9. [DOI] [PubMed] [Google Scholar]
- 7.Weber C, Coester C, Kreuter J, Langer K. Desolvation process and surface characterization of protein nanoparticles. Int J Pharm. 2000;194:91–102. doi: 10.1016/s0378-5173(99)00370-1. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Banerjee R, Bellare J. Aspirin loaded albumin nanoparticles by coacervation: Implications in drug delivery. Trends Biomater. 2005;18:203–12. [Google Scholar]
- 9.Merodio M, Arnedo A, Renedo MJ, Irache JM. Ganciclovir loaded albumin nanoparticles: Characterization and in vitro release properties. Eur J Pharm Sci. 2001;12:251–9. doi: 10.1016/s0928-0987(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 10.Idemyor V. Using non nucleoside reverse transcriptase inhibitors in treating HIV disease: Where are the therapeutic niches? J Natl Med Assoc. 2000;92:99–101. [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins JC, Noble S. Efavirenz. Drugs. 1998;56:1055–64. doi: 10.2165/00003495-199856060-00014. [DOI] [PubMed] [Google Scholar]
- 12.Rakhmanina NY, van den Anker JN. Efavirenz in the therapy of HIV infection. Expert Opin Drug Metab Toxicol. 2010;6:95–103. doi: 10.1517/17425250903483207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk MB, Notheis G, Schuster T, Elanjkal Z, von Hentig N, Sturmer M, et al. Effect of first line therapy including and two nucleoside reverse inhibitors in HIV-infected children. Eur J Med Res. 2005;10:503–8. [PubMed] [Google Scholar]
- 14.Arribas JR, Pulido F, Miró JM, Costa MA, González J, Rubio R, et al. EfaVIP Cohort Study Group. High effectiveness of efavirenz-based highly active antiretroviral therapy in HIV-1- infected patients with fewer than 100 CD4 cells/microl and opportunistic diseases: The EFAVIP study (Efavirenz in Very Immuno suppressed Patients) AIDS. 2002;16:1554–6. doi: 10.1097/00002030-200207260-00014. [DOI] [PubMed] [Google Scholar]
- 15.Csajka C, Marzolini C, Fattinger K, Décosterd LA, Fellay J, Telenti A, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 16.Chiappetta DA, Alvarez-Lorenzo C, Rey-Rico A, Taboada P, Concheiro A, Sosnik A. N- alkylation of poloxamines modulates micellar assembly and encapsulation and release of the antiretroviral efavirenz. Eur J Pharm Biopharm. 2010;76:24–37. doi: 10.1016/j.ejpb.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhao D, Zhao X, Zu Y, Li J, Zhang Y, Jiang R, et al. Preparation, characterization, and in vitro targeted delivery of folate- decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int J Nanomedicine. 2010;5:669–77. doi: 10.2147/ijn.s12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, et al. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–53. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- 19.Zu Y, Zhang Y, Zhao X, Zhang Q, Liu Y, Jiang R. Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM) Int J Nanomedicine. 2009;4:321–33. doi: 10.2147/ijn.s8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal A, Kapoor DN, Kapil R, Chhabra N, Dhawan S. Design and development of paclitaxel loaded bovine serum albumin nanoparticles for brain targeting. Acta Pharm. 2011;61:141–56. doi: 10.2478/v10007-011-0012-8. [DOI] [PubMed] [Google Scholar]
- 21.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 22.Wilson B, Samanta MK, Santhi K, Kumar KP, Ramasamy M, Suresh B. Chitosan nanoparticles as a new delivery system for anti-Alzheimer drug tacrine. Nanomedicine. 2010;6:144–52. doi: 10.1016/j.nano.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly (DL -lactide-co-glycolide) nanoparticles in mice. J Antimicrob Chemother. 2010;65:2183–7. doi: 10.1093/jac/dkq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery ER, Edmanson AL, Cook SC, Hovsepian PK. Development and validation of reverse phase HPLC method for analysis and its related substances in the drug substance and in a capsule formulation. J Pharm Biomed Anal. 2001;25:267–84. doi: 10.1016/s0731-7085(00)00495-7. [DOI] [PubMed] [Google Scholar]


