Abstract
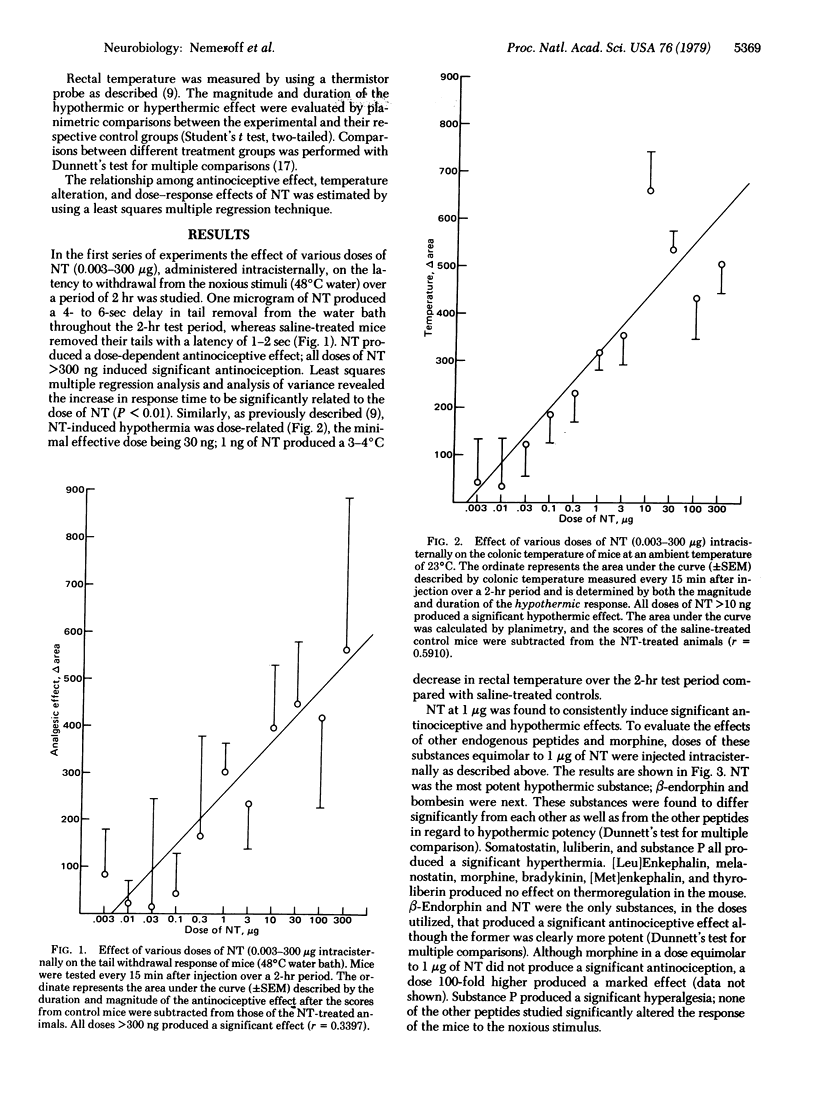
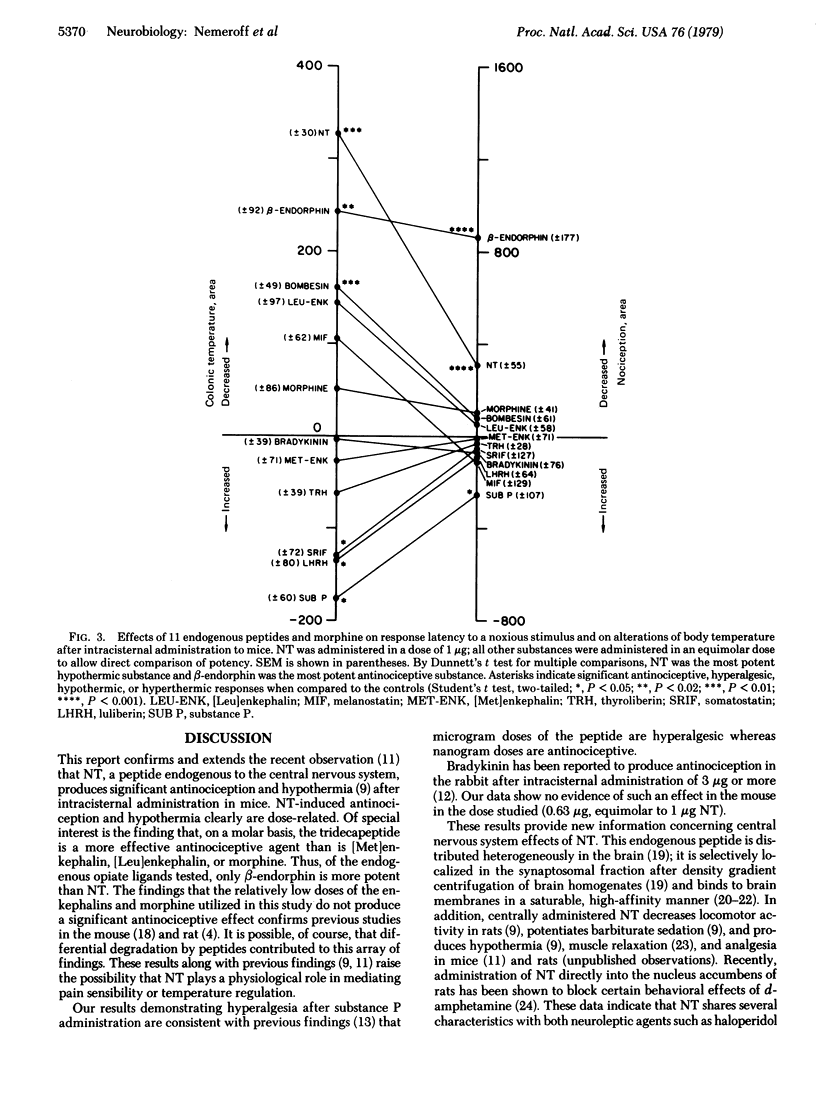
The antinociceptive and hypothermic effects of intracisternal administration of 11 endogenous neuropeptides and morphine were evaluated in mice. Of the substances tested, only neurotensin (NT) and beta-endorphin exerted significant antinociceptive and hypothermic effects; NT was the most potent in inducing hypothermia whereas beta-endorphin was the most potent antinociceptive agent via this route of administration. Both NT, and beta-endorphin were, on a molar basis, considerably more potent antinociceptive agents than morphine, [Met]enkephalin, or [Leu]enkephalin. NT-induced analgesia and hypothermia both were significantly dose-dependent. Substance P was found to produce significant hyperalgesia and hyperthermia. Bombesin produced a significant hypothermic effect, whereas somatostatin and luteinizing hormone-releasing hormone (luliberin) produced hyperthermia. None of the other peptides studies [bradykinin, thyrotropin-releasing factor (thyroliberin), melanocyte-stimulating hormone release-inhibiting factor (melanostatin), somatostatin, [Met]enkephalin, and [Leu]enkephalin] produced any significant alterations in colonic temperature or response to a noxious stimulus with the doses tested. These data demonstrate that NT and beta-endorphin, two endogenous brain peptides, are potent in inducing hypothermia and in producing an antinociceptive state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belluzzi J. D., Grant N., Garsky V., Sarantakis D., Wise C. D., Stein L. Analgesia induced in vivo by central administration of enkephalin in rat. Nature. 1976 Apr 15;260(5552):625–626. doi: 10.1038/260625a0. [DOI] [PubMed] [Google Scholar]
- Brown M., Rivier J., Vale W. Bombesin:potent effects on thermoregulation in the rat. Science. 1977 May 27;196(4293):998–1000. doi: 10.1126/science.860130. [DOI] [PubMed] [Google Scholar]
- Buscher H. H., Hill R. C., Römer D., Cardinaux F., Closse A., Hauser D., Pless J. Evidence for analgesic activity of enkephalin in the mouse. Nature. 1976 Jun 3;261(5559):423–425. doi: 10.1038/261423a0. [DOI] [PubMed] [Google Scholar]
- Clineschmidt B. V., McGuffin J. C. Neurotensin administered intracisternally inhibits responsiveness of mice to noxious stimuli. Eur J Pharmacol. 1977 Dec 15;46(4):395–396. doi: 10.1016/0014-2999(77)90236-9. [DOI] [PubMed] [Google Scholar]
- Frederickson R. C., Burgis V., Harrell C. E., Edwards J. D. Dual actions of substance P on nociception: possible role of endogenous opioids. Science. 1978 Mar 24;199(4335):1359–1362. doi: 10.1126/science.204012. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Endorphins: physiology and clinical implications. Ann N Y Acad Sci. 1978;311:49–58. doi: 10.1111/j.1749-6632.1978.tb16762.x. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Ling N., Lazarus L., Burgus R., Minick S., Bloom F., Nicoll R., Siggins G., Segal D. The endorphins, novel peptides of brain and hypophysial origin, with opiate-like activity: biochemical and biologic studies. Ann N Y Acad Sci. 1977 Oct 28;297:131–157. doi: 10.1111/j.1749-6632.1977.tb41850.x. [DOI] [PubMed] [Google Scholar]
- Kitabgi P., Carraway R., Van Rietschoten J., Granier C., Morgat J. L., Menez A., Leeman S., Freychet P. Neurotensin: specific binding to synaptic membranes from rat brain. Proc Natl Acad Sci U S A. 1977 May;74(5):1846–1850. doi: 10.1073/pnas.74.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R. M., Brown M., Vale W. Regional distribution of neurotensin and somatostatin in rat brain. Brain Res. 1977 May 13;126(3):584–588. doi: 10.1016/0006-8993(77)90613-8. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Brown M. R., Perrin M. H. Distribution, localization and characteristics of neurotensin binding sites in the rat brain. Neuropharmacology. 1977 Sep;16(9):625–629. doi: 10.1016/0028-3908(77)90033-8. [DOI] [PubMed] [Google Scholar]
- Li C. H., Yamashiro D., Tseng L. F., Loh H. H. Synthesis and analgesic activity of human beta-endorphin. J Med Chem. 1977 Mar;20(3):325–328. doi: 10.1021/jm00213a001. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Bissette G., Prange A. J., Jr, Loosen P. T., Barlow T. S., Lipton M. A. Neurotensin: central nervous system effects of a hypothalamic peptide. Brain Res. 1977 Jun 17;128(3):485–496. doi: 10.1016/0006-8993(77)90173-1. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Prange A. J., Jr Peptides and psychoneuroendocrinology. A perspective. Arch Gen Psychiatry. 1978 Aug;35(8):999–1010. doi: 10.1001/archpsyc.1978.01770320093009. [DOI] [PubMed] [Google Scholar]
- Osbahr A. J., 3rd, Nemeroff C. B., Manberg P. J., Prange A. J., Jr Centrally administered neurotensin: activity in the Julou-Courvoisier muscle relaxation test in mice. Eur J Pharmacol. 1979 Mar 1;54(3):299–302. doi: 10.1016/0014-2999(79)90090-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro S. A., Corrado A. P., Graeff F. G. Antinociceptive action of intraventricular bradykinin. Neuropharmacology. 1971 Nov;10(6):725–731. doi: 10.1016/0028-3908(71)90087-6. [DOI] [PubMed] [Google Scholar]
- Sewell R. D., Spencer P. S. Antinociceptive activitiy of narcotic agonist and partial agonist analgesics and other agents in the tail-immersion test in mice and rats. Neuropharmacology. 1976 Nov;15(11):683–688. doi: 10.1016/0028-3908(76)90037-x. [DOI] [PubMed] [Google Scholar]
- Stewart J. M., Getto C. J., Neldner K., Reeve E. B., Krivoy W. A., Zimmermann E. Substance P and analgesia. Nature. 1976 Aug 26;262(5571):784–785. doi: 10.1038/262784a0. [DOI] [PubMed] [Google Scholar]
- Terenius L. Endogenous peptides and analgesia. Annu Rev Pharmacol Toxicol. 1978;18:189–204. doi: 10.1146/annurev.pa.18.040178.001201. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Snyder S. H. Regional and subcellular distributions of brain neurotensin. Life Sci. 1976 Dec 15;19(12):1827–1832. doi: 10.1016/0024-3205(76)90114-4. [DOI] [PubMed] [Google Scholar]
- Verebey K., Volavka J., Clouet D. Endorphins in psychiatry: an overview and a hypothesis. Arch Gen Psychiatry. 1978 Jul;35(7):877–888. doi: 10.1001/archpsyc.1978.01770310083006. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H., Berger P. A., Barchas J. D. Some observations on the opiate peptides and schizophrenia. Arch Gen Psychiatry. 1979 Jan;36(1):35–41. doi: 10.1001/archpsyc.1979.01780010041004. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H., Berger P. A., Barchas J. D. Some observations on the opiate peptides and schizophrenia. Arch Gen Psychiatry. 1979 Jan;36(1):35–41. doi: 10.1001/archpsyc.1979.01780010041004. [DOI] [PubMed] [Google Scholar]


