Abstract
Background
The Pediatric Heart Network designed a clinical trial to compare aortic root growth and other short-term cardiovascular outcomes in children and young adults with Marfan syndrome randomized to receive atenolol or losartan. We report here the characteristics of the screened population and enrolled subjects.
Methods and results
Between 2007 and 2011, 21 clinical sites randomized 608 subjects, aged 6 months to 25 years who met the original Ghent criteria and had a body surface area–adjusted aortic root diameter z-score >3.0. The mean age at study entry was 11.2 years, 60% were male, and 25% were older teenagers and young adults. The median aortic root diameter z-score was 4.0. Aortic root diameter z-score did not vary with age. Mitral valve prolapse and mitral regurgitation were more common in females. Among those with a positive family history, 56% had a family member with aortic surgery, and 32% had a family member with a history of aortic dissection.
Conclusions
Baseline demographic, clinical, and anthropometric characteristics of the randomized cohort are representative of patients in this population with moderate to severe aortic root dilation. The high percentage of young subjects with relatives who have had aortic dissection or surgery illustrates the need for more definitive therapy; we expect that the results of the study and the wealth of systematic data collected will make an important contribution to the management of individuals with Marfan syndrome.
Marfan syndrome (MFS) is a systemic disorder of connective tissue caused by mutations in FBN1, the gene encoding fibrillin-1.1 Cardiovascular disease, mainly aortic root dilation and aortic dissection, is the leading cause of mortality in MFS. Although early diagnosis and refined medical and surgical management have improved median cumulative probability of survival from approximately 40 to 70 years, individuals with MFS continue to have high morbidity and early mortality.2
Transforming growth factor β (TGF-β) recently emerged as a potential mediator in the pathogenesis of MFS.3 The current hypothesis is that a deficiency of extracellular fibrillin-1 causes failure of matrix sequestration of the TGF-β large latent complex with consequent excessive TGF-β activation and signaling, resulting in the pleiotropic MFS manifestations including developmental emphysema, myxomatous valve disease, skeletal muscle myopathy, and aortic root aneurysm.3-6 Studies in an Fbn1-targeted mouse model of MFS with aortic disease similar to that seen in humans showed that treatment with losartan, an angiotensin II receptor blocker, normalized aortic root growth and aortic wall architecture and that these improvements correlated with reduced TGF-β activity.5
The National Heart, Lung, and Blood Institute (NHLBI)–funded Pediatric Heart Network designed a clinical trial to compare aortic root growth and other short-term cardiovascular outcomes in children and young adults with MFS randomized to receive losartan or atenolol, a β-blocker, which is the current standard of therapy at most centers.7 The primary aim of this trial is to compare the effect of atenolol therapy with that of losartan therapy on the rate of aortic root growth over 3 years. We report here the characteristics of the screened population and enrolled subjects. A detailed description of the echocardiographic methods and echocardiographic characteristics of the enrolled subjects is being reported separately.8
Methods
Screening and randomization protocol
The design of this trial has been reported.7 In brief, individuals 6 months to 25 years of age who met the original Ghent criteria9 and had a body surface area–adjusted aortic root diameter z-score >3.0 were eligible for inclusion (Table I). The study was designed to include subjects with this degree of aortic dilation at the time of enrollment because these individuals may be more likely than those with less aortic root dilation to show a treatment effect within the 3-year time frame of this study. Similarly, we excluded subjects with severe aortic dilation (≥5 cm) because they would likely withdraw before 3 years because of the need for surgery. To evaluate the effect of growth on change in z-score, we compared younger children who were still growing to older teenagers and young adults who were expected to have achieved final height at the time of study entry (≥16 years for males and ≥15 years for females).10 The study protocol was approved by the institutional review board or institutional ethics board at each participating center, and informed consent was obtained from the patient or a parent or legal guardian before trial enrollment.
Table I. Trial inclusion and exclusion criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
The study design included a multitiered screening, consent, and randomization process summarized in Figure 1. Revised diagnostic criteria for MFS were published after initiation of this trial.11 After completion of trial enrollment, we retrospectively compared the original and revised Ghent diagnostic criteria in the enrolled subjects and screened population. Nearly all the randomized subjects (603 of 608) and nonrandomized patients eligible for consent (42/43), all of whom had an aortic root diameter z-score >3.0, satisfied both the original and revised criteria.
Figure 1.
Flowchart of Pediatric Heart Network Marfan trial screened population. Abbreviations: LDS, Loeys-Dietz syndrome; SGS, Shprintzen-Goldberg syndrome. a, The most common exclusion criterion at this stage of the screening process (not mutually exclusive) was having an aortic root diameter z-score ≤3 (71%), followed by indication of a prior aortic surgery or dissection or aortic surgery planned within 6 months (10%) and intolerance to angiotensin receptor blocker, angiotensin-converting enzyme inhibitor, or β-blocker (10%). A relatively small proportion of ineligible patients indicated therapeutic usage of angiotensin-converting enzyme inhibitor, β-blocker, or calcium-channel blockers (6%), inability to complete study procedures (6%), current or planned pregnancy (3%), aortic root dimension >5 cm (2%), and diabetes or renal dysfunction (1%). b, Twenty patients were ineligible following consent to participate in the trial because of ineligible baseline echocardiogram, 24-hour ambulatory electrocardiogram (Holter), or laboratory studies.
Echocardiograms were performed under a standardized protocol and interpreted centrally by investigators blinded to treatment arm. Aortic root diameter was measured at the sinuses of Valsalva at its maximum dimension in systole, from inner edge to inner edge, in the parasternal long-axis view,12 and body surface area–adjusted aortic root diameter z-score was calculated.13
Statistical methods
Weight, height, and body mass index (BMI) z-scores were derived from 2000 CDC Growth Charts.14 Descriptive statistics are shown as means ± SDs and number of subjects (percentage); medians and interquartile ranges (IQRs) are presented for highly skewed measures. Continuous variables were compared with analysis of variance or its nonparametric analogue (Kruskal-Wallis) between groups. Categorical variables were compared between groups with a Fisher exact test; ordinal variables were compared with a Fisher exact test and the Mantel-Haenszel test for trend. Because of the large number of comparisons, 2-sided P values < .01 were considered to be statistically significant.
Results
Screening and randomization
Subjects were enrolled from January 2007 to February 2011 (Figure 1). The extremely high consent rate (97%) did not allow a robust comparison of randomized subjects to fully eligible patients who were not randomized solely because of lack of consent. However, a comparison of randomized subjects (n = 608) to all nonrandomized patients (n = 43) who met Ghent criteria showed no significant differences in baseline characteristics (data not shown).
Baseline characteristics of the randomized cohort
The mean age at randomization was 11.2 years, 60% of subjects were male, and 25% of subjects were older teenagers and young adults who were expected to have achieved their final height at the time of randomization (Table II).10 Most of the subjects were <18 years of age (85%).
Table II. Baseline demographic and clinical characteristics of randomized subjects.
| Baseline characteristic | Randomized subjects (n = 608) | Treatment A (n = 303) | Treatment B (n = 305) |
|---|---|---|---|
| Age at randomization, y | 11.2 ± 6.3 | 11.5 ± 6.5 | 11.0 ± 6.2 |
| Male ≥16 y/female ≥15 y | 151 (25%) | 76 (25%) | 75 (25%) |
| Male | 366 (60%) | 180 (59%) | 186 (61%) |
| Race | |||
| Asian | 16 (3%) | 6 (2%) | 10 (3%) |
| Black or African American | 46 (8%) | 21 (7%) | 25 (8%) |
| Other | 20 (3%) | 10 (3%) | 10 (3%) |
| White | 526 (87%) | 266 (88%) | 260 (85%) |
| Hispanic or Latino | 82 (14%) | 36 (12%) | 46 (15%) |
| No. of major Ghent criteria met | |||
| 2 | 282 (46%) | 137 (45%) | 145 (48%) |
| 3 | 253 (42%) | 125 (41%) | 128 (42%) |
| 4 | 69 (11%) | 39 (13%) | 30 (10%) |
| 5 (maximum) | 4 (1%) | 2 (1%) | 2 (1%) |
| Presence of causal FBN1 mutation | |||
| Yes | 189 (31%) | 97 (32%) | 92 (30%) |
| No | 79 (13%) | 41 (14%) | 38 (12%) |
| Unknown | 340 (56%) | 165 (54%) | 175 (57%) |
| Core echocardiogram laboratory reading | |||
| Maximum aortic root diameter, cm | 3.4 ± 0.7 | 3.4 ± 0.7 | 3.4 ± 0.7 |
| Maximum aortic root diameter z-score | 4.0 (3.4, 4.9) | 4.0 (3.5, 4.8) | 4.0 (3.4, 5.0) |
| Maximum aortic root diameter z-score ≥4.5 | 221 (36%) | 108 (36%) | 113 (37%) |
| Family history | |||
| Family history of Marfan | 360 (62%) | 180 (61%) | 180 (62%) |
| No. of biological relatives with Marfan | |||
| 1 | 126 (35%) | 58 (32%) | 68 (38%) |
| 2 | 157 (44%) | 78 (43%) | 79 (44%) |
| 3+ | 77 (21%) | 44 (24%) | 33 (18%) |
| Relative(s) with aortic dissection* | 107 (32%) | 54 (32%) | 53 (32%) |
| Relative(s) with aortic surgery* | 196 (56%) | 101 (57%) | 95 (56%) |
| Medical history | |||
| Cardiac surgery | 12 (2%) | 6 (2%) | 6 (2%) |
| Cardiovascular | 75 (12%) | 39 (13%) | 36 (12%) |
| Endocrine | 7 (1%) | 7 (2%) | 0 (0%) |
| Neurodevelopmental | 117 (19%) | 56 (19%) | 61 (20%) |
| Psychiatric | 39 (6%) | 23 (8%) | 16 (5%) |
| Medications history | |||
| β-Blocker | 343 (57%) | 173 (57%) | 170 (56%) |
| Angiotensin-converting enzyme inhibitor | 34 (6%) | 12 (4%) | 22 (7%) |
| Calcium-channel blocker | 8 (1%) | 4 (1%) | 4 (1%) |
| Angiotensin receptor blocker | 18 (3%) | 10 (3%) | 8 (3%) |
| Other antihypertensive | 2 (0.3%) | 0 (0%) | 2 (1%) |
| Any antihypertensive | 361 (59%) | 178 (59%) | 183 (60%) |
| Prerandomization washout required | 241 (40%) | 114 (38%) | 127 (42%) |
Data are shown as mean ± SD, median (interquartile range), or n (%). Baseline demographic and clinical characteristics are not statistically different between treatment arms (P > .2) with the exception of positive endocrine history (P = .007).
In subjects who reported a family history of MFS.
By design of the trial, all subjects had aortic root dilation and, therefore, met the major Ghent criterion for the cardiovascular system. The median aortic root diameter z-score was 4.0 (IQR 3.4-4.9) (Table II).
The most prevalent major criterion after the cardiovascular system was family or genetic history (76%, Figure 2), although FBN1 status was unknown in 56% of subjects (Table II). A family history of MFS (as defined by Ghent criteria) was reported in 62% of subjects, with 35% of those subjects reporting 1 relative, 44% reporting 2 relatives, and 21% reporting ≥3 relatives with MFS (Table II). Roughly a third of subjects with a family history of MFS also reported a family history of aortic dissection, and over half of subjects with a family history of MFS also reported a family history of aortic surgery.
Figure 2.
Prevalence of Ghent criteria in aggregate and by treatment arm. The prevalence of Ghent criteria, both major and involvement of organ systems, is shown. Prevalence of organ system involvement is calculated in subjects who do not meet major criteria in the respective organ system, with the exception of cardiovascular involvement. Because all subjects had aortic root dilation and, therefore, met the major criterion for the cardiovascular system, cardiovascular involvement indicates the prevalence of additional minor cardiovascular criteria of MVP and dilated main pulmonary artery in all randomized subjects.
Approximately half of the subjects met the major Ghent criteria for the skeletal or ocular systems (Figure 2). Fortysix percent of subjects met 2 major Ghent criteria, and 42% met 3 major criteria (Table II). Imaging for dural ectasia varied widely by site (0%-49%); among those with imaging (n = 95), the prevalence of dural ectasia was 34%.
Most subjects (57%) reported prior usage of β-blockers at any time before the trial, whereas relatively few reported prior use of other antihypertensive medications. Only 3% of subjects reported prior use of angiotensin receptor blockers.
Neurodevelopmental conditions, mainly learning disabilities, attention deficit disorder, and/or hyperactivity, were reported in 19%. Psychiatric disorders, mainly depression and anxiety, were reported in 6%.
As expected, the subjects, in general, were thin and tall, as indicated by their weight, height, and BMI z-scores (Table III). Specifically, BMI was 1 SD below normal, and height was, on average, 2 SDs above normal. The median arm span–to–height ratio was normal (1.03), and the median upper-to-lower segment ratio was 0.89. Most subjects (60%) had an abnormally reduced upper-to-lower segment ratio; young children (≤6 years) were much more likely to have a reduced ratio (online Appendix B Supplemental Table I).
Table III. Baseline anthropometric characteristics.
| Baseline characteristic | Randomized subjects (n = 608) | Treatment A (n = 303) | Treatment B (n = 305) |
|---|---|---|---|
| Weight, kg | 36.0 (22.4, 58.7) | 38.1 (23.0, 57.5) | 34.6 (21.8, 58.8) |
| Weight-for-age z-score (≤20 y)* | 0.3 ± 1.1 | 0.2 ± 1.2 | 0.3 ± 1.1 |
| Weight-for-height z-score (<120.5 cm)† | −1.1 ±1.6 | −1.1 ± 1.6 | −1.0 ± 1.6 |
| Height, cm | 155 (126, 178) | 160 (127, 178) | 152 (125, 178) |
| Height-for-age z-score (≤20 y)* | 2.0 ± 1.2 | 1.9 ± 1.1 | 2.0 ± 1.2 |
| BMI, kg/m2 | 16.7 ± 3.6 | 16.8 ± 3.7 | 16.6 ± 3.6 |
| BMI-for-age z-score (≤20 y)* | −1.2 ± 1.6 | −1.2 ± 1.7 | −1.2 ± 1.6 |
| Arm span, cm | 162 (128, 185) | 165 (130, 185) | 158 (128, 184) |
| Arm span–to–height ratio | 1.03 (1.00, 1.05) | 1.03 (1.01, 1.05) | 1.03 (1.00, 1.05) |
| US/LS ratio | 0.89 (0.81, 0.98) | 0.88 (0.81, 0.97) | 0.89 (0.81, 0.98) |
| Reduced US/LS ratio‡ | 360 (60%) | 179 (60%) | 181 (60%) |
Data are shown as mean ± SD, median (interquartile range), or n (%). All baseline anthropometric characteristics are not statistically different between treatment arms (P> .15).
Abbreviation: US/LS, Upper-to-lower segment.
Weight-for-age z-score, height-for-age z-score, and BMI-for-age z-score are not available for individuals >20 years of age.
Weight-for-height z-score is not available for individuals ≥120.5 cm.
Reduced upper-to-lower segment ratio defined as <1.5 for 0 to 1 year, <1.4 for 1 to 2 years, <1.3 for 2 to 3 years, <1.2 for 3 to 4 years, <1.1 for 4 to 5 years, <1.0 for 5 to 6 years, <0.95 for 6 to 7 years, <0.90 for 7 to 8 years, and <0.85 for >8 years.
Baseline demographic, clinical, and anthropometric characteristics (Table II and Table III) did not differ by assigned treatment arm (P > .2) with the exception of reported endocrine disorders (P = .007); the number of subjects reporting an endocrine disorder was small (n = 7).
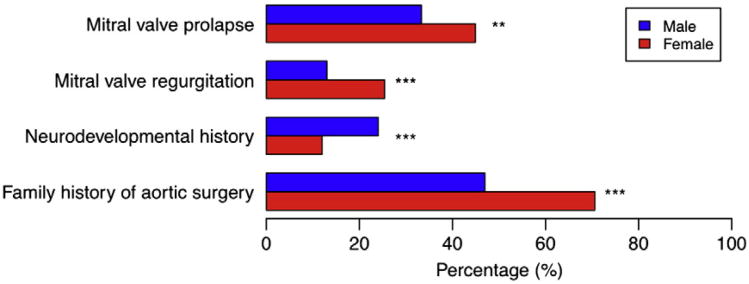
Gender differences
Among those with a family history of MFS, a family history of aortic surgery was more common in females than males (71% vs 47%, P < .001) (Figure 3). The prevalence of mitral valve prolapse (MVP) and measurable (mild or more) mitral regurgitation (MR) was higher among female subjects (MVP: females 45% vs males 33%, P = .006, MR: females 25% vs males 13%, P < .001). A history of neurodevelopmental disorders requiring therapy was more common in males than in females (24% vs 12%, P < .001). These associations with gender were not modified by age at randomization (gender by age interaction P > .1). Excluding anthropometric measurements, other characteristics, including aortic root diameter z-score, did not differ between females and males (online Appendix B Supplemental Table II).
Figure 3.
Gender differences. Gender differences in family history of aortic surgery, neurodevelopmental history, measurable (mild or more) mitral valve regurgitation, and MVP. Family history of aortic surgery was restricted to subjects who indicated a family history of MFS. **P < .01, ***P < .001.
Age effects
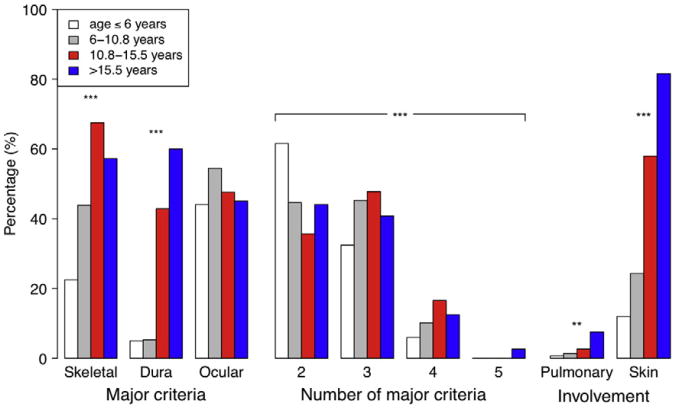
Aortic root diameter z-score was not dependent on age (online Appendix B Supplemental Table I). Prior administration of β-blockers was more common in older subjects (P < .001). As expected, older subjects were also more likely to report a positive history of cardiovascular and psychiatric disorders (P ≤ .006). Although older subjects met a higher number of major Ghent criteria and were more likely to meet major criteria in the skeletal system and dura specifically, the prevalence of was not dependent on age (Figure 4). Older subjects were also more likely to show involvement of the pulmonary system and skin.
Figure 4.
Differences by age at randomization. Prevalence of major Ghent criteria in the skeletal, dura, and ocular organ systems; number of major Ghent criteria met; and involvement of pulmonary and skin organ systems by age at randomization quartiles. **P < .01, ***P < .001.
Aortic root diameter z-score
We examined the associations of quartiles of aortic root diameter z-score at randomization with other baseline characteristics (online Appendix B Supplemental Table III). Subjects with higher aortic root diameter z-scores were more likely to have other cardiovascular involvement (MVP and/or dilated main pulmonary artery, P = .006). In contrast, subjects with aortic root diameter z-scores in the first quartile (aortic root diameter z-score ≤3.42) were more likely to have a family history of MFS than subjects with larger aortic root diameter z-scores (75% vs 57%, P < .001). Aortic root diameter z-score was negatively correlated with weight-for-age z-score (P < .001) and BMI (both raw and BMI-for-age z-score, P < .001). No other significant associations were identified.
Family history
Data on family history were available for all but 24 subjects. Among subjects with available data, 62% (n = 360) had a positive family history of MFS, a characteristic that showed no association with age at randomization (positive family history 11.0 ± 6.8 years of age, no family history 11.6 ± 5.7 years of age, P = .3). Of note, among those with a positive family history, 56% had a family member with aortic surgery, and 32% had a family member with a history of aortic dissection (Table II). Those with a positive family history also had slightly lower median aortic root diameter z-scores (3.9 vs 4.2, P < .001) and were less likely to meet major criteria in the skeletal system (41% vs 59%, P < .001). No other significant associations were identified.
FBN1 mutation status
The frequency of FBN1 testing varied widely by site (range 19%-96%), and FBN1 mutation status was unknown (FBN1 testing not done) in 56% of the randomized subjects, precluding robust analysis. FBN1 mutation status was associated with age at randomization (presence of FBN1 mutation 9.3 ± 5.6 years, absence of FBN1 mutation 12.8 ± 6.5 years, and FBN1 mutation status unknown 12.0 ± 6.4 years, P < .001). Among those with FBN1 testing, (n = 268), 71% had a defined FBN1 mutation, and 29% did not.
Ectopia lentis was more likely in subjects with unknown FBN1 status (55% vs 39% in subjects with a documented mutation and 42% in subjects without a mutation, age-adjusted P = .001). Subjects with a documented mutation were less likely than those without a mutation to have a family history of MFS (46% vs 64%, age-adjusted P = .002). No other significant associations were identified.
Discussion
We have successfully enrolled a very large cohort of pediatric and young adult patients with MFS from 21 international sites into this trial comparing cardiovascular outcomes in subjects randomized to receive atenolol or losartan. This cohort is different from most previously published large series of MFS patients15-17 because of the range of age (6 months-25 years) and aortic root diameter z-score requirement for enrollment. The high percentage of subjects <18 years of age in this large cohort is novel.
With a few exceptions, the prevalence rates of the major and minor Ghent criteria were similar in our cohort compared with previously published series. By study design, all subjects had aortic root dilation and, therefore, met major Ghent criteria for the cardiovascular system. Older studies reported a higher prevalence of ectopia lentis of 60% to 70%, probably as result of selection bias; the prevalence of ectopia lentis in our series (48%) was similar to recently published large series (47%-54%).15-17 Imaging for dural ectasia was available in only 95 subjects (16%), and the prevalence was lower in our cohort compared with previous reports (34% vs 63%-92%).1 The prevalence of striae was similar (44% vs 47%),16 but pulmonary involvement was lower compared with published reports (3% vs 4%-15%).1
Older subjects in this trial were more likely to exhibit more major and minor manifestations than younger subjects, but the prevalence of ectopia lentis was not dependent on age, consistent with the concept that ectopia lentis is most commonly detected at a young age in MFS.18 Similarly, aortic root diameter z-score did not vary with age in our cohort of children and young adults selected for moderate to severe aortic dilation. Although a selection bias cannot be completely excluded, the absence of variation in aortic root diameter z-score with age in our cross-sectional sample is consistent with previous longitudinal studies that have shown that aortic root diameter z-score is stable (z-score change per year close to 0), at least in young individuals with MFS.19 It is important to emphasize that, in growing individuals, the aortic root continues to enlarge despite a stable z-score. Subjects with higher aortic root diameter z-scores were more likely to have additional cardiovascular involvement—MVP and/or dilation of the main pulmonary artery.
That 60% of our cohort is male is intriguing, given that MFS is an autosomal dominant disorder and a balanced gender ratio is expected. The male predominance was present throughout the screening process including the initial medical record review, suggesting that the gender ratio reflected the general Marfan population, but the reason for this is unclear. Several studies in children and adults have also shown a male predominance (54%-60%).15-17,20
Mitral valve prolapse and measurable (mild or more) MR were more common in females in our cohort. In contrast, Detaint et al20 found no gender differences in MVP or MR among 965 probands with pathogenic FBN1 mutations, but their cohort had a median age at diagnosis of 22 years, leaving open the possibility of an increased risk for MVP/MR in younger females. Of note, Detaint et al also found that men presented earlier and with more severe aortic dilation and related complications compared with the women. Similar trends of earlier and more severe aortic disease are observed in male Fbn1-deficient mice (personal communication, Harry C. Dietz, MD, 2012). However, aortic root diameter z-score was not dependent on gender in our randomized cohort. Thus, more severe disease in males cannot completely account for the greater proportion of males in the overall MFS population.
Hofman et al21 evaluated the neurodevelopmental status and cognitive ability of 30 consecutive school-aged children with MFS (70% male) and found ≥1 neuropsychologic deficits—learning disability, attention deficit disorder with or without hyperactivity, neuromaturational immaturity, and verbal performance discrepancy—in half of them (33% of the girls and 62% of the boys). Neurodevelopmental issues were more common in males in our cohort, but our estimates of prevalence were based on medical history and not on the results of formal testing. Whether the male predilection is specific for MFS or reflective of general population trends is unclear; further investigation with formal neuropsychologic testing and long-term follow-up is warranted.
Limitations
The study design only included individuals with at least moderate aortic root dilation and excluded patients at the extremes of the spectrum of aortic disease in MFS. Patients with aortic root diameter z-scores ≤3 and those with previous or impending aortic surgery were excluded. Although the randomized subjects were well characterized, the screening process did not include detailed data collection on all screened patients as this was beyond the scope of the study. Therefore, a robust comparison between the randomized subjects and the screened population was not feasible.
In summary, we have characterized the largest cohort of pediatric and young adult patients with MFS. We found that aortic root z-score did not vary with age and was not dependent on gender. Among those with a family history of MFS, a family history of aortic surgery was more common in females.
The very high consent rate among eligible subjects is notable. The clinical profile of the cohort is representative of patients in this population with moderate to severe aortic root dilation. As expected with randomization, baseline demographic, clinical, and anthropometric characteristics of the study cohort are not different between treatment groups. As such, our multicenter trial is in an excellent position to evaluate the efficacy and safety of atenolol and losartan in young patients with MFS and to determine if the benefit of losartan in the mouse model of MFS translates to humans. The high percentage of young subjects with relatives who have had aortic dissection or surgery illustrates the need for more definitive therapy. We expect that the results of the study and the wealth of systematic data collected will make an important contribution to the management of individuals with MFS.
Supplementary Material
Supplemental Table I. Baseline characteristics by quartiles of age at randomization
Abbreviation: US/LS, Upper-to-lower segment.
* In subjects who did meet not major criteria in the respective organ system.
† In all randomized subjects.
‡ P values obtained from a Fisher exact test and Mantel-Haenszel test for trend, respectively.
Supplemental Table II. Baseline characteristics by gender
* In subjects who reported a family history of MFS.
Supplemental Table III. Baseline characteristics by quartiles of aortic root diameter z-score
* In all randomized subjects.
Acknowledgments
This study was supported by U01 grants from the NHLBI (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057) and the FDA Office of Orphan Products Development. Additional support was provided by the National Marfan Foundation, Merck & Co, Inc, and Teva Canada Limited. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents. The contents do not necessarily represent the official views of NHLBI or National Institutes of Health.
Appendix A.
Appendix of Investigators and Contributors
National Heart, Lung, and Blood Institute
Gail Pearson, Mario Stylianou, Victoria Pemberton
Network Chair
Lynn Mahony, University of Texas Southwestern Medical Center
Data Coordinating Center
New England Research Institutes, Lynn Sleeper (PI), Sharon Tennstedt (PI), Steven Colan, Gloria Klein, Lin Guey, Lisa Wruck*, Thomas Travison*, Shan Chen, David F. Teitel
Core Clinical Site Investigators
Children's Hospital Boston, MA: Jane Newburger (PI), Ronald V. Lacro (Study Co-chair), Martha King, Carolyn Dunbar-Masterson, Jill Handisides, Andrea Posa*, Quincy Nang; Children's Hospital of New York, NY: Daphne Hsu (PI)*, Wyman Lai (PI), William Hellenbrand*, Beth Printz*, Mary Roman, Richard Devereux, Rosalind Korsin, Greysi Sherwood*; Children's Hospital of Philadelphia, PA: Victoria Vetter (PI), Stephen Paridon, Marie Gleason, Reed Pyeritz; Nicole Mirarchi, Sandra DiLullo*, Agbenu Ejembi, Ruth Morgan*, Tonia Morrison, Cincinnati Children's Medical Center, OH: D. Woodrow Benson (PI), Larry Markham*, William Border*, James Cnota, Haleh Heydarian, Michelle Hamstra, Kathryn Hogan, Lois Bogenschutz; North Carolina Consortium, Durham, Greenville, and Winston-Salem, NC: Page A. W. Anderson (PI) - deceased, Jennifer S. Li (PI), Stephanie Burns Wechsler, Amanda Cook*, Charles Sang, Wesley Covitz, Mingfen Xu, Lori Jo Sutton, Kari Crawford*, Summer Roberts*, Deborah Palmer; Medical University of South Carolina, Charleston, SC: J. Philip Saul (PI), Andrew Atz, Geoffrey Forbus, Teresa Atz, Patricia Infinger, Aparna Choudhury; Primary Children's Medical Center and the University of Utah, Salt Lake City, UT: LuAnn Minich (PI), Richard Williams, Angela Yetman, Marian Shearrow, Michelle Robinson, June Porter*; Hospital for Sick Children, Toronto, Canada, Brian McCrindle (PI), Timothy Bradley, Jennifer Russell, Jack Colman, Elizabeth Radojewski, Svetlana Khaikin, Nancy Slater; Johns Hopkins University School of Medicine, MD: Harry C. Dietz (Study Co-chair), Mary Rykiel, Elisabeth Sparks, Gretchen Oswald, Jennifer Leadroot*
Auxiliary Site Investigators
Washington University School of Medicine, St Louis, MO: Charles Canter (PI), Angela Sharkey*, Alan Braverman, Cheryl Rainey; Texas Children's Hospital Houston, TX: Jeffrey Towbin*, John L. Jeffries*, Timothy Slesnick*, Aimee Liou (PI), Hugo Martinez*, Andres Menesses*, Tunu Tenende; Stanford University Medical Center, Stanford, CA: David Liang (PI), Daniel Murphy, Elisabeth Merkel; Ghent University Hospital, Ghent, Belgium: Bart Loeys*, Julie De Backer (PI), Anne De Paepe, Sylvia De Nobele, Jan Maarten Cobben (Amsterdam), Thierry Sluysmans (Brussels); Mount Sinai School of Medicine, New York, NY: Bruce D. Gelb (PI), Shubhika Srivastava, Constance G. Weismann, Emily Lawrence, Stephanie Chin, Tejani Mendiz-Ramdeen, H. Helen Ko, Jen Le Yau; Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA: Steven Webber (PI), Stacey Drant (co-PI), Jane Luce, Kevin Stiegler; Vanderbilt University, Nashville, TN: Larry Markham (PI), Cheryl Kinnard*, Cheri Stewart, Sue Sommers, Carol Madison; Children's Memorial Hospital, Chicago, IL: Luciana Young (PI), Megan Domenico, Kathryn Waitzman, Carla Lozano; Children's Hospital and Clinics of Minnesota, St Paul, MN: Mary Ella Pierpont (PI), Charles Baker, Erin Zielinski, Heidi Vander Velden; Seattle Children's, Seattle, WA: Mark Lewin (PI), Aaron Olson, Amy Payne; Cedars-Sinai Medical Center, Los Angeles, CA: David Rimoin (PI) -deceased, Mitchel Pariani, Robert Siegel, Asim Rafique*; Rady Children's Hospital, UCSD, San Diego, CA: Paul Grossfeld, Arlene Smith, Terri McLees-Palinkas
Echocardiography Core Laboratory
Children's Hospital Boston: Steven D. Colan (Director), Seda Selamet Tierney, Jami Levine, Shari Trevey, Marga Rivera
Protocol Review Committee
Michael Artman, Chair; Judith Massicot-Fisher*, Executive Secretary; Erle Austin, H. Scott Baldwin, Daniel Bernstein, Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne, Kenneth G. Zahka
Data and Safety Monitoring Board
Johner, Chair; Rae-Ellen Kavey*, David Gordon, Executive Secretaries; David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb*
*No longer at the institution listed.
References
- 1.Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965–76. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–60. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- 3.Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 4.Ng CM, Cheng A, Myers LA, et al. TGF-beta–dependent pathogen-esis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–92. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn RD, Van EC, Habashi JP, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta–induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–10. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacro RV, Dietz HC, Wruck LM, et al. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J. 2007;154:624–31. doi: 10.1016/j.ahj.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selamet Tierney ES, Levine JC, Chen S, et al. Echocardiographic methods, quality review and measurement accuracy in a randomized multicenter clinical trial of Marfan syndrome. J Am Soc Echocardiogr. 2013 doi: 10.1016/j.echo.2013.02.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–26. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Erkula G, Jones KB, Sponseller PD, et al. Growth and maturation in Marfan syndrome. Am J Med Genet. 2002;109:100–15. doi: 10.1002/ajmg.10312. [DOI] [PubMed] [Google Scholar]
- 11.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 12.Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–95. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 15.Loeys B, Nuytinck L, Delvaux I, et al. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med. 2001;161:2447–54. doi: 10.1001/archinte.161.20.2447. [DOI] [PubMed] [Google Scholar]
- 16.Faivre L, Collod-Beroud G, Loeys BL, et al. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet. 2007;81:454–66. doi: 10.1086/520125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faivre L, Masurel-Paulet A, Collod-Beroud G, et al. Clinical and molecular study of 320 children with Marfan syndrome and related type I fibrillinopathies in a series of 1009 probands with pathogenic FBN1 mutations. Pediatrics. 2009;123:391–8. doi: 10.1542/peds.2008-0703. [DOI] [PubMed] [Google Scholar]
- 18.Maumenee IH. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc. 1981;79:684–733. [PMC free article] [PubMed] [Google Scholar]
- 19.Selamet Tierney ES, Feingold B, Printz BF, et al. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr. 2007;150:77–82. doi: 10.1016/j.jpeds.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Detaint D, Faivre L, Collod-Beroud G, et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur Heart J. 2010;31:2223–9. doi: 10.1093/eurheartj/ehq258. [DOI] [PubMed] [Google Scholar]
- 21.Hofman KJ, Bernhardt BA, Pyeritz RE. Marfan syndrome: neuropsychological aspects. Am J Med Genet. 1988;31:331–8. doi: 10.1002/ajmg.1320310210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. Baseline characteristics by quartiles of age at randomization
Abbreviation: US/LS, Upper-to-lower segment.
* In subjects who did meet not major criteria in the respective organ system.
† In all randomized subjects.
‡ P values obtained from a Fisher exact test and Mantel-Haenszel test for trend, respectively.
Supplemental Table II. Baseline characteristics by gender
* In subjects who reported a family history of MFS.
Supplemental Table III. Baseline characteristics by quartiles of aortic root diameter z-score
* In all randomized subjects.