Abstract
The extracellular potassium makes up only about 2% of the total body potassium store. The majority of the body potassium is distributed in the intracellular space, and of which about 80% is in skeletal muscle. Movement of potassium in and out of skeletal muscle thus plays a pivotal role in extracellular potassium homeostasis. The exchange of potassium between the extracellular space and skeletal muscle is mediated by specific membrane transporters. These include potassium uptake by Na+, K+-ATPase and release by inward rectifier K+ channels. These processes are regulated by circulating hormones, peptides, ions, and by physical activity of muscle as well as dietary potassium intake. Pharmaceutical agents, poisons and disease conditions also affect the exchange and alter extracellular potassium concentration. Here, we review extracellular potassium homeostasis focusing on factors and conditions that influence the balance of potassium movement in skeletal muscle. Recent findings that mutations of a skeletal muscle-specific inward rectifier K+ channel cause hypokalemic periodic paralysis provide interesting insights into the role of skeletal muscle in extracellular potassium homeostasis. These recent findings will be reviewed.
Keywords: Hypokalemic periodic paralysis; Thyrotoxic periodic paralysis; Inward rectifier K+ channel; Kir; Na+, K+-ATPase; Skeletal muscle; Hypokalemia-induced paradoxical depolarization
Introduction
The concentration of potassium in the extracellular fluid is a critical determinant of the resting membrane potential of cells and must be maintained within a narrow range, normally between 3.5 and 5 mM in plasma concentration. Potassium, however, is predominantly an intracellular cation and extracellular potassium homeostasis is intimately connected with cellular potassium homeostasis.
In humans, the total body potassium is about 50 mmole per kg of body weight.1 For a 70 kg adult human, the total body potassium is ~3500 mmole. About 98% of the total body potassium is stored in the intracellular space, with muscle containing 80% of the intracellular potassium with concentration around 160 mM.2 The remaining 20% is distributed in the bone, liver, and erythrocytes. Only 2% (70 mmole) of the total body potassium is circulated in the extracellular space, including interstitial space (75%, 53 mmole) and plasma (25%, 17 mmole).3
As the single largest pool of body potassium, skeletal muscle regulates extracellular potassium by taking up or releasing the ion. The uptake of potassium by muscle from the extracellular fluid is mediated predominantly by the ubiquitous Na+, K+-ATPase, and release from muscle into the extracellular fluid by potassium channels including several inwardly rectifier potassium channels (Kir) and voltage-gated potassium channels (Kv). This review will discuss function and regulation of these potassium transporters in physiological and diseased states and the significance in extracellular potassium homeostasis.
Na+, K+-ATPase and Extracellular Potassium Homeostasis
Function, Distribution, and Molecular Composition of Na+, K+-ATPase
Since the discovery by Skou in 1957, the role of Na+, K+-ATPase in skeletal muscle has been extensively studied and reviewed.1,4–8 Using the energy expenditure of hydrolysis of one ATP molecule to ADP, Na+, K+-ATPase extrudes three sodium ions out and brings two potassium ions in muscle cell via a ping-pong like mechanism (so-called Albers-Post cycle).9 Thus, Na+, K+-ATPase is primarily responsible for maintaining the high extracellular sodium and intracellular potassium concentration relative to the intracellular and extracellular space, respectively. These transmembrane potassium and sodium gradients are essential for normal resting membrane potential and active transport of substrate that relies on sodium gradient, respectively. In the resting state, Na+, K+-ATPase consumes 20–30% of the total body ATP.9 This huge amount of energy consumption reflects high abundance of Na+, K+-ATPase in tissues.
Using [3H] ouabain binding assay, it is estimated that skeletal muscle contains ~0.3 µmole Na+, K+-ATPase per kilogram skeletal muscle wet weight, which is only less than brain cortex (11 µmole/kg) and cardiomyocytes (0.7 µmole/kg) but higher than smooth muscle (< 0.1 µmole/kg) and other tissues.10 The capacity of potassium uptake through Na+, K+-ATPase is a function of the abundance and turnover rate of the pump. Assuming a maximal pump activity ~8000 turnover per minute, the maximal capacity of Na+, K+-ATPase-mediated potassium uptake into skeletal muscle is 4.8 mmole · kg−1 · min−1 (0.3 µmole · kg−1 × 8000 min−1 × 2).11,12 In an adult male with skeletal muscle about 42% of the body mass, the maximal potassium uptake rate in skeletal muscle amounts to ~134 mmole · min−1. Considering the total extracellular potassium is ~70 mmole, maximal uptake by Na+, K+-ATPase in skeletal muscle can lead to a complete turnover of the extracellular potassium about every half a minute.
Molecularly, Na+, K+-ATPase is made up of three components, α, β, and γ (also called FXYD) subunits. The minimal functional unit requires one α and one β subunit in heterodimer.9 The α subunit (~112 kDa) is a catalytic subunit and provides the binding sites for sodium, potassium, ATP and cardiac glycosides (inhibitors of the pump). The β subunit (~55 kDa) is required for maturation and stability of the α subunit. There are four α and four β isoforms transcribed by different genes.13 Different αβ isoforms are expressed in different fiber-type and in different subcellular distribution.
Regulation of Activity of Na+, K+-ATPase
Both the activity and abundance of Na+, K+-ATPase are regulated.10 Acute regulation tends to affect the pump activity, whereas chronic regulation affects the abundance of pump protein. The activity of Na+, K+-ATPase pump is stimulated by its own substrate, i.e. by increased intracellular sodium and extracellular potassium concentrations. The Km (concentration for half-maximal activation) of Na+, K+-ATPase for intracellular sodium is between 5–20 mM depending on fiber-type. This value is close to the resting intracellular sodium concentration (~15 mM) in most cells, making the pump sensitive to changes in the intracellular sodium concentration.1,14. In contrast, Km of Na+, K+-ATPase for extracellular potassium is estimated at 0.8–1.5 mM,15,16 suggesting that 70–85% of pumps are saturated in the normal range of plasma potassium concentration, and activity of the pump is not sensitive to physiological changes of plasma K+. This value for Km, however, is based on measurements performed in vitro, and its relevance to in vivo conditions remains unknown (see below).
Insulin is a well-known acute activator of Na+, K+-ATPase. The underlying mechanism is probably through increasing pump affinity for intracellular sodium (decreased Km) without changing its Vmax (maximal pump activity).17 This stimulation by insulin is independent of glucose uptake.18 Whether translocation of intracellular Na+, K+-ATPase to the plasma membrane contributes to insulin-induced acute potassium uptake remains debatable.19,20 Similar to insulin, catecholamines also stimulate Na+, K+-ATPase activity, probably also through increasing the affinity of pump for intracellular sodium.6,21 The stimulation by catecholamines is mediated via β2-adrenoceptor and through activation of adenylate cyclase to produce cAMP.22 Activation of protein kinase A by cAMP directly phosphorylates the pump and causes a conformational change of the pump and increases its affinity for intracellular sodium.21 This effect can be blocked by β-blockers (propranolol) and potentiated by cAMP enhancers (theophylline, forskolin).23 It has been noticed that high-dose catecholamine causes an increase in serum potassium concentration, which leads to the suggestion that activation of α–adrenoceptor (by high catecholamine concentration) inhibits the pump. This effect, however, is likely due to that α–adrenoceptor activation causes an increase in potassium efflux from liver independently of the pump rather than through inhibiting Na+, K+-ATPase.24 The effects of insulin and catecholamine on Na+, K+-ATPase are additive, indicating different mechanisms on Na+, K+-ATPase.25 Acid-base disturbances are also known to affect pump activity. This topic is recently reviewed,26 and is discussed elsewhere in this issue.
Regulation of Abundance of Na+, K+-ATPase
For long-term regulation, protein synthesis of α and β subunits of Na+, K+-ATPase is altered at the transcriptional as well as post-transcriptional levels. Glucocorticoid and aldosterone both regulate the expression of Na+, K+-ATPase in skeletal muscle but the effects are relatively modest. Adrenalectomized rats have normal amount of [3H] ouabain binding site in their skeletal muscle, indicating that adrenal steroids are not essential for basal expression of Na+, K+-ATPase.27 Administration of dexamethasone to rats at 0.1 mg/kg per day (close to the regular dose in medical practice) for 14 days increases the abundance of the α2-, β1-subunit and [3H] ouabain binding site in skeletal muscle by ~50%.28,29 Patients with hyperaldosteronism have higher mRNA and protein of α2 and β1 subunits in their skeletal muscle. This increase is positively correlated with serum aldosterone levels and reversed by adrenalectomy.30 However, aldosterone treatment in animals decreases the abundance of Na+, K+-ATPase.31 This apparently paradoxical effect is probably due to that profound hypokalemia in aldosterone-treated animals suppresses the expression of Na+, K+-ATPase.27 Glucocorticoid response element (GRE) is present in the promoter of α1- and β1-subunit genes which in part explains the transcriptional regulation by glucocorticoid and mineralocorticoid.32,33 GRE has not been found in the promoter regions of other isoforms.
Thyroid hormone also increases mRNA and protein abundance of the α-subunits of Na+, K+-ATPase.34,35 Thyroid response element (TRE) is present in some but not all isoforms of Na+, K+-ATPase subunits, and the stimulation by thyroid hormones may involve transcriptional as well as post-transcriptional mechanisms.36,37 In general, the abundance of Na+, K+-ATPase is in good correlation with serum thyroid hormone levels.35,38
Physical training and dietary potassium intake also regulate the abundance of Na+, K+-ATPase. Muscle inactivity and low dietary potassium intake decrease the abundance of pump in skeletal muscle, and vice versa.6 The activity of voltage-gated sodium channel and intracellular sodium content may underlie the regulation of pump expression by muscle activity and dietary intake.39 Accumulation of potassium in the interstitium of skeletal muscle during repetitive action potential depolarizes membrane potentials and contributes to muscle fatigue. Upregulation of Na+, K+-ATPase will enhance muscle potassium uptake and reduce potassium accumulation in the interstitium during exercise, and explain why physical training increases exercise endurance. Up- and down-regulation of Na+, K+-ATPase are important in maintaining extracellular potassium homeostasis in response to high and low dietary potassium intake, respectively.1,6 The above-mentioned acute and chronic regulation could act alone or coordinately. Of note, many of them, like thyroid hormone, insulin, aldosterone and exercise, exert both acute and chronic regulation on Na+, K+-ATPase.
Disturbance of Extracellular Potassium Homeostasis Caused by Na+, K+-ATPase
Drugs that inhibit (such as cardiac glycosides digoxin or β-blockers) or stimulate (such as β2-agonists or cAMP enhancers theophylline and caffeine) Na+, K+-ATPase in skeletal muscle may cause hyperkalemia and hypokalemia, respectively.40 Clinically, inhibition of the pump by therapeutic doses of digoxin or β-blockers rarely cause hyperkalemia unless potassium excretion is impaired in patients with renal failure or with concomitant use of potassium-sparing diuretics. On the contrary, stimulation of the pump by β2-agonists, theophylline and caffeine may induce hypokalemia in the absence of decreased renal excretion. This is in part due to that these agents also cause inhibition of potassium release from skeletal muscle (see below). Changes in the abundance of Na+, K+-ATPase may also contribute to disturbance of extracellular potassium homeostasis. Patients with hypothyroidism, diabetes, heart failure, chronic potassium deficiency or elderly have reduced abundance of Na+, K+-ATPase in skeletal muscle,6 are more susceptible to drug-induced hyperkalemia and probably also exercise-induced hyperkalemia.41,42 On the other hand, patients of hyperthyroidism who have increased abundance of Na+, K+-ATPase is prone to develop hypokalemia and is known to be protected against development of hyperkalemia in conditions of digitalis overdose.43,44 Similar to β2-agonists, insulin stimulates potassium uptake via Na+, K+-ATPase and at the same time inhibits potassium release from potassium channel in skeletal muscle. The dual actions explain why insulin is very effective in causing potassium shift from the extracellular space to skeletal muscle and causing hypokalemia, especially in susceptible patients in hypokalemic periodic paralysis (see details below).45
K+ Channels and Extracellular Potassium Homeostasis
K+ Channels in Skeletal Muscle
There are many types of K+ channels in skeletal muscle, including voltage-gated K+ (KV) channels and inward-rectifying K+ (Kir) channels. Kv channels are closed at hyperpolarized membrane potentials and opened by membrane depolarization. Several types of voltage-gated K+ channels are expressed in skeletal muscle, but none are skeletal muscle-specific. Inward-rectifying potassium channels allow more inward than outward potassium fluxes when open. The mechanism of asymmetric conductance (i.e., rectification) is due to voltage-dependent block of channel pore by intracellular Mg2+ and polyamines.46 When the membrane potential (Em) is more positive than the equilibrium potential for K+ (Ek), intracellular Mg2+ or polyamines are driven into the channel pore blocking outward potassium flux.47 Kir channels are classified into seven subfamilies based on amino acid sequence homologies, rectification properties, and mechanisms of regulation by intracellular factors.46 Each subfamily has several membranes. Several Kir channel subtypes are present in skeletal muscle and play important roles in the function of muscle and release of potassium from muscle. These include three members of Kir2 subfamily (Kir2.1, Kir2.2, Kir2.6) and the ATP-sensitive Kir channels (Kir6.1 and 6.2, also known as KATP). Among these, Kir2.1, Kir2.2 and KATP are expressed in human skeletal muscle as well as many other tissues. In contrast, Kir2.6 is a skeletal muscle-specific Kir channel. The role of Kir channels in extracellular potassium homeostasis is best illustrated by the fact that mutations of Kir2.1 and Kir2.6 cause muscle paralysis and hypokalemia, a disease known as hypokalemic periodic paralysis.48,49
Hypokalemic Periodic Paralysis
Hypokalemic periodic paralysis (HypoPP) is a heterogeneous disease featured by episodic muscle paralysis associated with ictal hypokalemia during the period of the attacks as a result of shift of K+ from plasma into muscle cells.50 The mechanism of cellular K+ shift is related to the pathogenesis of muscle paralysis, and not caused by any known acid-base disorders or exogenously administrated substances, such as drugs that activate β-adrenergic receptors. The paralytic attack is frequently precipitated by factors that stimulate Na+, K+-ATPase and increase potassium uptake by muscle from plasma. As will be discussed in details below, the initial small decrease in serum K+ levels induced by precipitating factors cause paradoxical depolarization of sarcolemma, which sets off a vicious cycle of hypokalemia, further depolarization, and muscle paralysis.
HypoPP exists in familial and non-familial forms. Familial form is inherited in an autosomal-dominant pattern, and is predominantly caused by mutations in genes encoding for skeletal muscle-specific voltage-gated Na+ channel Nav1.4 (~20% cases) or the L-type Ca2+ channel Cav1.1 (~70%).51,52 About 10% of familial HypoPP is unmapped. Non-familial HypoPP can occur in the presence of hyperthyroidism, called thyrotoxic periodic paralysis (TPP) or in the absence of hyperthyroidism called spontaneous periodic paralysis (SPP).45 The clinical presentation of muscle weakness and acute hypokalemia in patients with non-familial HypoPP is indistinguishable from those with familial HypoPP. Serum K+ levels are normal in HypoPP patients at the baseline, and patients develop profound hypokalemia only during paralytic attacks. Recent studies have provided interesting insights into the mechanism of hypokalemia.49,53
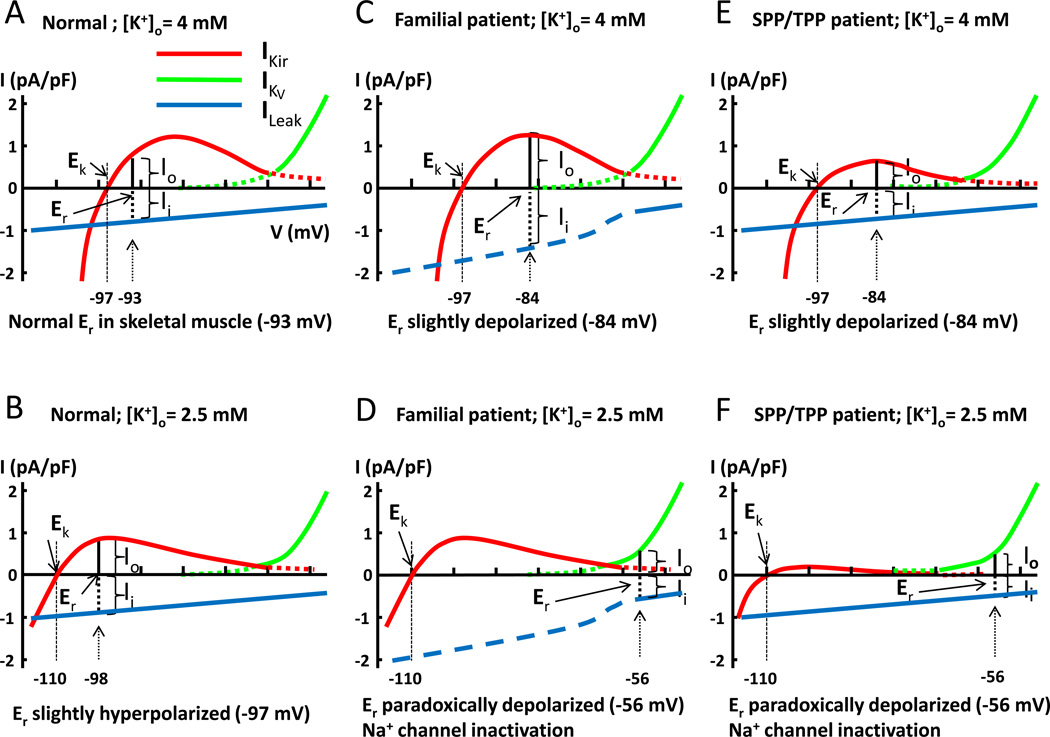
In skeletal muscle, the resting membrane potential (Er) of cells is determined by the balance between outward K+ currents (Io) and inward leak currents (Ii) (See Figure 1A, where “I” indicates current, and “o” and “i” indicate outward and inward, respectively). In the sarcolemma of skeletal muscle, the outward K+ currents are mediated by K+ efflux through two types of K+ channels: one is Kir and the other is the voltage-activated K+ channel (KV). Figure 1A illustrates this principle and the resting membrane voltage where Io = Ii, using the current-voltage (I-V) relationship curve for Kir (IKir, red curve), for KV (IKV, green curve), and for leak current (ILeak, blue line). Note that outward K+ current through IKir is hump-shaped because of reduced outward conductance at depolarized membrane potentials, which is due to strong inward rectification, and that outward K+ current through IKV becomes significant only at membrane potentials more depolarized than −65 mV, which is due to voltage-dependent activation of such channels. As shown in Figure 1A, in normal individuals with a normal serum K+ concentration 4 mM, the resting membrane potential is approximately −93 mV, where outward K+ current mediated by IKir and inward cation leak current (ILeak) are equal. Reducing serum K+ concentration to 2.5 mM has two effects (Figure 1B). One is to shift the equilibrium potential for K+ (EK) to hyperpolarization (−110 mV) according to Nernst equation (EK = −58 mV × log {[K]in/[K]out}). In addition, low extracellular K+ concentration alters channel gating and suppresses the overall conductance of Kir, which brings the I-V curve closer to the zero-current line.54 As a result, the resting membrane potential is shifted from −93 mV to −98 mV, where a new balance between outward K+ current and inward leak current is reached (Figure 1B).
Figure 1.
Models for paradoxical depolarization in patients with hypokalemic periodic paralysis. Current-voltage (I-V) relationship curves for inward rectifier K+ channel (Kir, red curve), voltage-gated K+ channel (KV, green curve), and leak current (blue line). The reversal potential of leak current is 0 mV. Please note that aberrant pore current from mutations of Ca2+ and Na+ channels in familial HypoPP patients only exists in hyperpolarized potentials. Thus, the total inward leak current in familial HypoPP patients is not linear, but is increased in hyperpolarized potentials (shown in interrupted blue line). The model is intended for conceptual understanding; numerical value may be slightly different from true in vivo value. Abbreviations: Ek: equilibrium potential for K+; Er: resting membrane potential; Io: outward cation current; Ii: inward cation current; IKir: current of inward rectifier K+ channel; IKV: current of voltage-gated K+ channel; ILeak: inward cation leak current; [K]o: extracellular potassium concentration. See text for further details.
The hallmark of HypoPP is depolarization of the sarcolemma induced by hypokalemia during attacks.55 This hypokalemia-induced depolarization is in contrast to the prediction based on Nernst equation that membrane potential hyperpolarizes during hypokalemia, and thus termed “paradoxical”. The paradoxical depolarization occurs during hypokalemia is central to the pathogenesis of hypokalemic periodic paralysis because it causes inactivation of voltage-gated Na+ channels in skeletal muscle and thus muscle inexcitability and paralysis.
The mechanism of hypokalemia-induced paradoxical depolarization in patients with familial HypoPP has been elucidated,56 and is shown in Figures 1C and 1D. Mutations of Cav1.1 or Nav1.4 channels in familial HypoPP create an aberrant conducting pore that allows passage of small cations (Na+ and H+) from outside into cells at (and only at) hyperpolarized resting membrane potentials (shown as interrupted blue line for ILeak in Figure 1C and 1D. Note that it occurs only at membrane potentials more negative than −65 mV, in which Cav1.1 or Nav1.4 channels are closed).57,58 This additional inward cation current through the aberrant conducting pore adds to the existing leak current thus increases the total inward leak current at hyperpolarized resting membrane potentials. In familial HypoPP patients at normal serum K+ concentration, the increase in leak current results in slight depolarization in the resting membrane potential (Figure 1C; Er shifts from −93 mV to −84 mV). The increase in the total leak current in familial HypoPP patients, however, poses a problem for the resting membrane during hypokalemia (when EK is left-shifted and Kir conductance is suppressed). Because of the increase in the total leak current at hyperpolarized membrane potentials, it is now impossible for outward K+ currents and inward leak currents to reach a new balance during hypokalemia simply by shifting Er to hyperpolarized membrane potentials (Figure 1D; note that outward K+ currents mediated by Kir [red curve] is always smaller than inward leak current at hyperpolarized membrane potentials [interrupted blue line]). As a result, the balance between inward and outward currents for Er can only be reached at depolarized membrane potential where outward K+ current is mediated by KV (green curve). This mechanism explains why paradoxical depolarization develops in patients with familial HypoPP when the initial hypokalemia is induced by precipitating factors. The same degree of initial hypokalemia does not cause paradoxical depolarization in normal healthy individuals because they do not have mutations in Ca2+ or Na+ channels that increase the leak current (see Figure 1A and 1B).
This model can be applied to explain the mechanism of paradoxical depolarization in non-familial HypoPP. Recent studies show that loss-of-function mutations in Kir2.6 cause non-familial HypoPP, TPP and SPP.49,53 As illustrated in Figure 1E and 1F, reduced IKir current through mutant Kir would have the same impact as enhanced ILeak with respect to the effect of creating imbalance between outward K+ current and inward leak current. That is, during hypokalemia in patients with TPP and SPP, outward K+ currents through mutant Kir is always smaller than the inward leak current, and balance between outward and inward currents can only be reached if resting membrane potential is shifted to depolarized membrane potential where outward K+ current is mediated by KV (Figure 1F). This model explains why patients with TPP and SPP also have hypokalemia-induced paradoxical depolarization as in familial HypoPP.
Mechanism of Hypokalemia in Hypokalemic Periodic Paralysis
Muscle paralysis and hypokalemia in HypoPP patients are frequently precipitated by strenuous exercise, high carbohydrate food, etc and in the case of TPP is associated with an increase in thyroid function. These precipitating factors stimulate Na+, K+-ATPase and increase potassium uptake by muscle from plasma. Increased uptake of potassium by Na+, K+-ATPase alone does not cause significant hypokalemia because of compensation by increased K+ efflux through K+ channels. Insulin and catecholamines (released during exercise and by high carbohydrate food) can cause hypokalemia because they inhibit Kir channels in skeletal muscle besides stimulation of Na+, K+-ATPase.45 To develop severe hypokalemia with serum K+ concentration ~2 mM or lower as typically seen in patients with HypoPP, an additional mechanism is likely involved. Mutations of Kir in patients with non-familial HypoPP may contribute to hypokalemia, but cannot be solely responsible because their serum K+ levels are normal between attacks. The mechanism of paradoxical depolarization in TPP and SPP patients has provided unique insights into the pathogenesis of severe hypokalemia in HypoPP.53
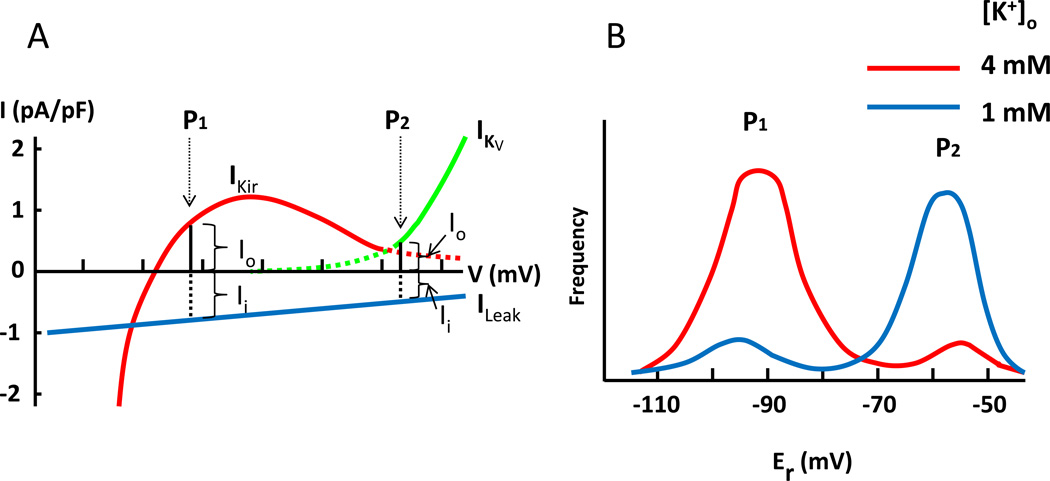
As shown in Figure 2A, potassium efflux at the paradoxically depolarized membrane potential (labeled “P2” in Figure 2A) is smaller than that at the normal, more hyperpolarized resting membrane potentials (“P1”). This is due to the fact that leak current at paradoxical depolarization is smaller compared to that at hyperpolarized resting membrane potential (and therefore the balancing outward K+ current is smaller). Muscle fibers are heterogeneous, and in vivo individual fiber develops paradoxical depolarization at different extracellular K+ concentrations. Indeed, the percentage of muscle fibers that develop paradoxical depolarization increases proportional with decreasing serum K+ concentration.59 This is illustrated in Figure 2B, which shows bi-modal distribution of resting membrane potentials of a population of muscle fibers. At 4 mM [K+], the majority of fibers have a normal resting membrane potential (“P1”). At 1 mM [K+], most fibers are paradoxically depolarized (“P2”). Thus, in HypoPP patients, the initial mild hypokalemia caused by the precipitating factors will recruit more fibers to paradoxical depolarization. This would lead to further hypokalemia because potassium efflux (which is the outward K+ current that balances the inward leak current shown in Figure 2A) at the paradoxically depolarized membrane potential is smaller than that at the normal more hyperpolarized resting membrane potentials. The process repeats itself in a positive feedback cycle leading to severe hypokalemia (Figure 3).
Figure 2.
Decreased potassium efflux at paradoxical depolarization (A) and bi-modal distribution of resting membrane potentials (B). (A) Leak current is a function of membrane potential. Inward leak current at the normal hyperpolarized membrane potential (“P1”) is larger than that at the paradoxically depolarized membrane potential (“P2”). Thus, K+ efflux at P1 (mediated by Kir channel) is larger than that at P2 (mediated by KV channel). (B) Because of heterogeneity, muscle fibers develop paradoxical depolarization at different [K+]o. This is reflected by bi-modal distribution of resting membrane potentials (Er) of a population of muscle fibers. Red and blue curve represent the distribution of Er at [K+]o 4 mM and 1 mM, respectively. Note that shown here is the distribution of normal muscle fibers, in which paradoxical depolarization occurs at extreme hypokalemia ([K+]o ~1 mM). In HypoPP, muscles fibers develop paradoxical depolarization at a relatively higher [K+]o, ~2.5 mM. That is, increased inward leak current as produced by mutations of Ca2+ and Na+ channel in familial HypoPP or decreased outward K+ current as caused by mutations of Kir2.6 in TPP and SPP predispose muscle fibers to paradoxical depolarization. See text for further details.
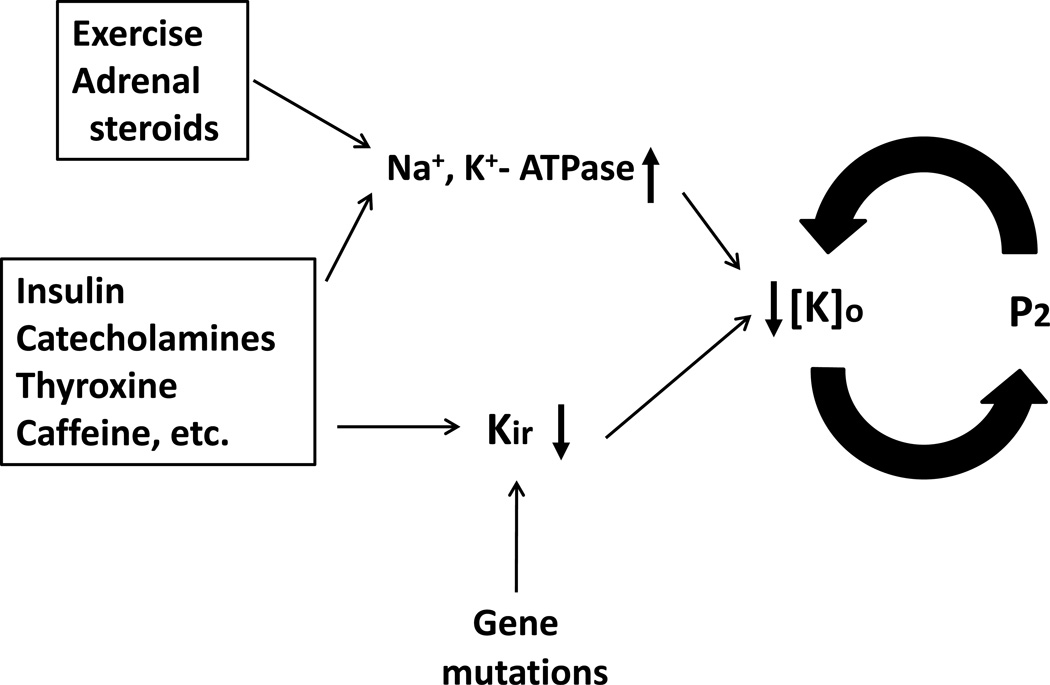
Figure 3.
A positive feedback cycle for development of severe hypokalemia via paradoxical depolarization of skeletal muscle membrane potential. Exercise and adrenal steroids stimulate Na+, K+-ATPase. Insulin, catecholamines, thyroid hormones, caffeine, etc can both stimulate Na+, K+-ATPase and inhibit Kir. These effects can lead to hypokalemia. Loss-of-function mutations of Kir predispose to the development of severe hypokalemia through a positive feedback cycle between paradoxical depolarization and hypokalemia. Combined stimulation of Na+, K+-ATPase and inhibition of Kir by endogenous and/or exogenous factors may cause significant hypokalemia to set in motion the positive feedback cycle for paradoxical depolarization without Kir gene mutation. See text for further details.
Hypokalemia in Barium Poisoning
It has been known for decades that hypokalemia and muscle paralysis develops in patients with barium intoxication.60 Barium inhibits many types of K+ channels, but is most potent for Kir2 subfamily of channels which are present in skeletal muscle and the heart.46,61 Struyk et al show that in isolated muscle fibers inhibition of IKir by barium predisposes sarcolemma to the development of paradoxical depolarization,62 supporting the notion that the mechanism of hypokalemia in barium poisoning is similar to that in TPP and SPP with mutations of Kir2.6.53 The effect of barium on Kir channels in the heart explains why cardiac arrhythmia develops in patients with barium poisoning. Indeed, mutations of Kir2.1 (which is present in cardiac as well as skeletal muscle) cause Andersen’s disease featured by cardiac arrhythmia, skeletal muscle paralysis, and hypokalemia.48
It is interesting to consider the role of Na+, K+-ATPase in the development of severe hypokalemia and muscle paralysis in barium poisoning. The clinical syndrome of barium poisoning was initially described by Dr. Allen in early 1940’s as an endemic illness in rural China where villagers develop symptoms after ingestion of a large amount of food contaminated with barium chloride.60 In these scenarios after large meals, insulin release and stimulation of Na+, K+-ATPase are invariable. On the other hand, a large inhibition of myocyte K+ efflux by barium may be sufficient to trigger a vicious cycle of hypokalemia and paradoxical depolarization. Of note, mutations of Kir2.6 in TPP and SPP patients are so far heterozygous. A recent computer modeling study suggests that 50% of total Kir current is required for maintaining normal plasma potassium concentration.63
Barium sulfate used for radiographic contrast is poorly absorbable by the gastrointestinal tract, but ingestion of soluble salts such as barium carbonate (in rodent-killers), barium sulfide and nitrate used in various purposes can cause poisoning.64,65 Overdose of other cation blocker of Kir such as cesium (used in the treatment of brain tumor) or drugs that inhibit Kir channels, such as anti-malarial chloroquine or barbiturate (thiopental) can cause hypokalemia by inhibiting potassium efflux.66–68
Implications for Hypokalemia Without Kir2.6 Mutations
Mutations of Kir2.6 are detected in only a small portion (< 33%) of patients with TPP and SPP in different populations.49,53 Polymorphism and intron mutations (such as those generate new abnormal splice sites) may explain some of patients without mutations within the coding region of Kir2.6 gene. There are multiple endogenous and exogenous inhibitors of Kir2.6. It is conceivable that inhibition of Kir2.6 by these regulators, singly or in combination, may be sufficient to trigger paradoxical depolarization of sarcolemma and cause HypoPP.
Ryan et al reports that a thyroid response element (TRE) is present in the promoter of Kir2.6 gene and thyroid hormone increases the expression of Kir2.6.49 Multiple Kir channels including Kir2.1, Kir2,2, and Kir2.6 are present in skeletal muscle and they likely form functional heteromultimers in the sarcolemma.53,69 Thyroid hormone is not known to regulate Kir2.1 or Kir2.2. Increased expression of a less active Kir2.6 subunit (either caused by gene mutation or inhibited by regulators) would be expected to decrease the total Kir currents through dominant-negative inhibition of fully functional Kir subunits.53 This effect on Kir2.6, together with stimulation of Na+, K+-ATPase, likely explains why thyroid hormone increases the susceptibility for hypokalemia and paralytic attacks in patients with TPP.
Overall, these concepts indicate that functions of Kir and Na+, K+-ATPase in skeletal muscle play important roles in the regulation of extracellular K+ homeostasis in individuals without gene mutations as well as those with mutations (see Figure 3).
Exercise and Potassium Homeostasis
The action potential in skeletal muscle begins with a depolarization phase from opening of voltage-gated Na+ channel and Na+ influx and followed by a repolarization phase due to opening of voltage-gated K+ channel and K+ efflux.70 Repeated action potential and skeletal muscle contraction thus result in release of considerable amount of potassium into the extracellular space. If potassium released by muscle completely enters the systemic circulation, the venous potassium concentration after 5 minute of cycling exercise can reach a level 7-fold higher than the pre-exercise level.6 The highest reported plasma potassium concentration during strenuous exercise is around 8 mM,71 indicating that most of exercise-induced potassium release is retained in the extracellular space of muscle, especially in the interstitium of transverse (T)-tubules. T-tubules are unique, tortuous structure of muscle that makes up a large extracellular space amounts up to ~10–15% of the total muscle volume.1 It is estimated that potassium concentration in the interstitium of T-tubule may be up to 26 to 52 mM during exercise.72 Several groups have shown that diffusion of extracellular potassium of skeletal muscle into systemic circulation is limited; the diffusion coefficient is less than 20% of its value in free solution.73
The high potassium concentration in T-tubule depolarizes membrane potential, which leads to inactivation of voltage-gated sodium channel and contributes to muscle fatigue. To counter the high potassium concentration, Na+, K+-ATPase in muscle is activated during exercise and in periods following exercise. In addition to the high extracellular potassium concentration, several exercise-induced changes in muscle, such as intracellular sodium and lactic acid accumulation, high beta-adrenergic activity, increased muscle temperature, released calcitonin gene-related peptide from nerve endings, contribute to the stimulation of Na+, K+-ATPase activity.74 Exercise may also induce translocation of Na+, K+-ATPase from cytoplasm to sarcolemma.75 As mentioned earlier, physical activity of muscle causes upregulation of the abundance of Na+, K+-ATPase, and explains why athletic training improves exercise endurance. Overall, increased uptake of potassium by Na+, K+-ATPase in the T-tubule of skeletal muscle in conditioned individuals helps avoiding precipitous rise of serum potassium concentration and cardiac arrhythmia during exercise.76,77 The sustained upregulation of Na+, K+-ATPase activity in post-exercise periods also explains why HypoPP may be triggered by strenuous exercise.
Skeletal Muscle as a Buffer for Acute Dietary Potassium Load
Besides the transcellular potassium shift, a major challenge for maintaining plasma potassium concentration is dietary potassium intake. Without buffering or fast excretion by kidney, acute addition of 35 mmole potassium into the extracellular fluid (70 mmole total) from a potassium-rich meal will increase potassium concentration by 50%. In reality, the postprandial plasma potassium concentration only increases by 0.5 mM at most.78 Several hypotheses have been put forward to explain this finding of relatively unchanged plasma potassium concentration following ingestion of a large load. Among these is a feed forward renal kaliuretic response induced by gut potassium sensor,3 which will be discussed in a separate chapter of this series. Immediate disposal by splanchnic and hepatic uptake followed by redistribution to skeletal muscle mediated by insulin and other hormones has also been proposed.79,80
With respect to the redistribution to skeletal muscle, it is important to point out that at the maximal capacity skeletal muscle can turn over the entire extracellular potassium in half a minute (see discussion in section of Na+, K+-ATPase earlier). Thus, skeletal muscle, with its storage capacity of 2800 mmole vs. 70 mmole extracellular potassium, can potentially take up potassium added to plasma without a significant increase in plasma concentration. Thereafter, potassium stored in muscle may be released slowly and excreted by kidney over many hours without causing significant changes in the plasma concentration. One challenge for this idea is that Km of Na+, K+-ATPase for extracellular K+ is estimated at ~1 mM,15,16 suggesting that activity of pump is not sensitive to changes in plasma potassium concentration at the normal physiological range. This value of Km, however, is determined in vitro. The activity of Na+, K+-ATPase is stimulated by many factors in vivo. It will be interesting to investigate in the future whether the activity of Na+, K+-ATPase is more sensitive to extracellular potassium in vivo and potassium uptake by muscle can adequately defend plasma potassium from dietary load.
Concluding Remarks
Other membrane potassium transporters, such as Na+-K+-2Cl- cotransporter NKCC, K+-Cl- cotransporter KCC, ATP-sensitive K+ channel, and calcium-activated K+ channel are also present in the skeletal muscle, and probably contribute to extracellular potassium homeostasis. This review focuses on the role of Na+, K+-ATPase and Kir2.6 inward rectifier K+ channel, two better understood players. Recent understanding of the pathogenesis of hypokalemic periodic paralysis provides unique insights into the role of these two transporters in extracellular potassium homeostasis.
Acknowledgements
Work in Chou-Long Huang’s lab is supported by the National Institutes of Health (DK59530, DK85726, DK79328.). CLH holds the Jacob Lemann Professorship in Calcium Transport of University of Texas Southwestern Medical Center. CJC is supported by a scholarship grant from the Ministry of Defense, Taiwan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- 2.Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985;248:R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- 3.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 5.Skou JC. Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiol Rev. 1965;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- 6.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- 7.McDonough AA, Youn JH. Role of muscle in regulating extracellular K+ Semin Nephrol. 2005;25:335–342. doi: 10.1016/j.semnephrol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Clausen T. Role of Na+,K+-pumps and transmembrane Na+,K+-distribution in muscle function. The FEPS lecture - Bratislava 2007. Acta Physiol (Oxf) 2008;192:339–349. doi: 10.1111/j.1748-1716.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen PL, Hakansson KO, Karlish SJ. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol. 2003;65:817–849. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- 10.Clausen T. Clinical and therapeutic significance of the Na+,K+ pump. Clin Sci (Lond) 1998;95:3–17. [PubMed] [Google Scholar]
- 11.Clausen T, Everts ME, Kjeldsen K. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J Physiol. 1988;388:163–181. doi: 10.1113/jphysiol.1987.sp016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clausen T. Hormonal and pharmacological modification of plasma potassium homeostasis. Fundam Clin Pharmacol. 2010;24:595–605. doi: 10.1111/j.1472-8206.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 13.Pestov NB, Zhao H, Basrur V, Modyanov NN. Isolation and characterization of BetaM protein encoded by ATP1B4--a unique member of the Na,K-ATPase β-subunit gene family. Biochem Biophys Res Commun. 2011;412:543–548. doi: 10.1016/j.bbrc.2011.07.112. [DOI] [PubMed] [Google Scholar]
- 14.Juel C. Na+-K+-ATPase in rat skeletal muscle: muscle fiber-specific differences in exercise-induced changes in ion affinity and maximal activity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R125–R132. doi: 10.1152/ajpregu.90760.2008. [DOI] [PubMed] [Google Scholar]
- 15.Sugden AL, Bean BL, Straw JA. Effects of high potassium or low sodium diet on vascular Na+,K+-ATPase activity and blood pressure in young spontaneously hypertensive rats. Hypertension. 1987;9:571–575. doi: 10.1161/01.hyp.9.6.571. [DOI] [PubMed] [Google Scholar]
- 16.Juel C. Na+-K+-ATPase in rat skeletal muscle: muscle fiber-specific differences in exercise-induced changes in ion affinity and maximal activity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R125–R132. doi: 10.1152/ajpregu.90760.2008. [DOI] [PubMed] [Google Scholar]
- 17.Kitasato H, Sato S, Marunaka Y, Murayama K, Nishio K. Apparent affinity changes induced by insulin of Na-K transport system in frog skeletal muscle. Jpn J Physiol. 1980;30:603–616. doi: 10.2170/jjphysiol.30.603. [DOI] [PubMed] [Google Scholar]
- 18.Choi CS, Lee FN, McDonough AA, Youn JH. Independent regulation of in vivo insulin action on glucose versus K(+) uptake by dietary fat and K(+) content. Diabetes. 2002;51:915–920. doi: 10.2337/diabetes.51.4.915. [DOI] [PubMed] [Google Scholar]
- 19.Al-Khalili L, Yu M, Chibalin AV. Na+, K+-ATPase trafficking in skeletal muscle: Insulin stimulates translocation of both alpha 1- and alpha 2-subunit isoforms. FEBS Lett. 2003;536:198–202. doi: 10.1016/s0014-5793(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 20.Clausen T. Regulatory role of translocation of Na+-K+ pumps in skeletal muscle: hypothesis or reality? Am J Physiol Endocrinol Metab. 2008;295:E727–E728. doi: 10.1152/ajpendo.90494.2008. [DOI] [PubMed] [Google Scholar]
- 21.Clausen T, Flatman JA. β2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K-transport in rat soleus muscle. Br J Pharmacol. 1980;68:749–755. doi: 10.1111/j.1476-5381.1980.tb10868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kockskämper J, Erlenkamp S, Glitsch HG. Activation of the cAMP-protein kinase A pathway facilitates Na+ translocation by the Na+-K+ pump in guinea-pig ventricular myocytes. J Physiol (Lond) 2000;3:561–574. doi: 10.1111/j.1469-7793.2000.t01-2-00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clausen T, Flatman JA. The effect of catecholamines on Na-K-transport and membrane potential in rat soleus muscle. J Physiol (Lond) 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro-Tavares J. Effects of isoprenaline and phenylephrine on plasma potassium: role of the liver. Arch Int Pharmacodyn Ther. 1975;218:110–119. [PubMed] [Google Scholar]
- 25.Li KX, Sperelakis N. Isoproterenol- and insulin-induced hyperpolarization in rat skeletal muscle. J Cell Physiol. 1993;157:631–636. doi: 10.1002/jcp.1041570324. [DOI] [PubMed] [Google Scholar]
- 26.Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981–1989. doi: 10.1681/ASN.2011040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dørup I, Clausen T. Effects of adrenal steroids on the concentration of Na(+)-K+ pumps in rat skeletal muscle. J Endocrinol. 1997;152:49–57. doi: 10.1677/joe.0.1520049. [DOI] [PubMed] [Google Scholar]
- 28.Ravn HB, Dørup I. The concentration of sodium, potassium pumps in chronic obstructive lung disease (COLD) patients: the impact of magnesium depletion and steroid treatment. J Intern Med. 1997;241:23–29. doi: 10.1046/j.1365-2796.1997.69891000.x. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CB, Dørup I, Ahn J, Leong PK, McDonough AA. Glucocorticoids increase sodium pump alpha(2)- and beta(1)-subunit abundance and mRNA in rat skeletal muscle. Am J Physiol Cell Physiol. 2001;280:C509–C516. doi: 10.1152/ajpcell.2001.280.3.C509. [DOI] [PubMed] [Google Scholar]
- 30.Phakdeekitcharoen B, Kittikanokrat W, Kijkunasathian C, Chatsudthipong V. Aldosterone increases Na+-K+-ATPase activity in skeletal muscle of patients with Conn's syndrome. Clin Endocrinol (Oxf) 2011;74:152–159. doi: 10.1111/j.1365-2265.2010.03912.x. [DOI] [PubMed] [Google Scholar]
- 31.Kjeldsen K, Nørgaard A, Clausen T. Effect of K-depletion on 3H-ouabain binding and Na-K-contents in mammalian skeletal muscle. Acta Physiol Scand. 1984;122:103–117. doi: 10.1111/j.1748-1716.1984.tb07488.x. [DOI] [PubMed] [Google Scholar]
- 32.Derfoul A, Robertson NM, Lingrel JB, Hall DJ, Litwack G. Regulation of the human Na/K-ATPase beta1 gene promoter by mineralocorticoid and glucocorticoid receptors. J Biol Chem. 1998;273:20702–20711. doi: 10.1074/jbc.273.33.20702. [DOI] [PubMed] [Google Scholar]
- 33.Kolla V, Robertson NM, Litwack G. Identification of a mineralocorticoid/glucocorticoid response element in the human Na/K ATPase alpha1 gene promoter. Biochem Biophys Res Commun. 1999;266:5–14. doi: 10.1006/bbrc.1999.1765. [DOI] [PubMed] [Google Scholar]
- 34.Azuma KK, Hensley CB, Tang MJ, McDonough AA. Thyroid hormone specifically regulates skeletal muscle Na+-K+-ATPase α2- and β2-isoforms. Am J Physiol Cell Physiol. 1993;265:C680–C687. doi: 10.1152/ajpcell.1993.265.3.C680. [DOI] [PubMed] [Google Scholar]
- 35.Phakdeekitcharoen B, Phudhichareonrat S, Pookarnjanamorakot C, Kijkunasathian C, Tubtong N, Kittikanokrat W, Radinahamed P. Thyroid hormone increases mRNA and protein expression of Na+-K+-ATPase alpha2 and beta1 subunits in human skeletal muscles. J Clin Endocrinol Metab. 2007;92:353–358. doi: 10.1210/jc.2006-0552. [DOI] [PubMed] [Google Scholar]
- 36.Feng J, Orlowski J, Lingrel JB. Identification of a functional thyroid hormone response element in the upstream flanking region of the human Na,K-ATPase beta 1 gene. Nucleic Acids Res. 1993;21:2619–2626. doi: 10.1093/nar/21.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajpai M, Mandal SK, Chaudhury S. Identification of thyroid regulatory elements in the Na-K-ATPase alpha3 gene promoter. Mol Biol Rep. 2001;28:1–7. doi: 10.1023/a:1011986418897. [DOI] [PubMed] [Google Scholar]
- 38.Kjeldsen K, Gotzsche CO, Norgaard A, Thomassen A, Clausen T. Effect of thyroid function on number of Na-K pumps in human skeletal muscle. Lancet. 1984;2:8–10. doi: 10.1016/s0140-6736(84)91996-2. [DOI] [PubMed] [Google Scholar]
- 39.Fambrough DM, Wolitzky BA, Tamkun MM, Takeyasu K. Regulation of the sodium pump in excitable cells. Kidney Int. 1987;32:S97–S112. [PubMed] [Google Scholar]
- 40.Perazella MA. Drug-induced hyperkalemia: old culprits and new offenders. Am J Med. 2000;109:307–314. doi: 10.1016/s0002-9343(00)00496-4. [DOI] [PubMed] [Google Scholar]
- 41.Ponce SP, Jennings AE, Madias NE, Harrington JT. Drug-induced hyperkalemia. Medicine (Baltimore) 1985;64:357–370. doi: 10.1097/00005792-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Schaafsma IA, van Emst MG, Kooistra HS, Verkleij CB, Peeters ME, Boer P, Rijnberk A, Everts ME. Exercise-induced hyperkalemia in hypothyroid dogs. Domest Anim Endocrinol. 2002;22:113–125. doi: 10.1016/s0739-7240(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 43.Lin SH, Huang CL. Pathogenesis of thyrotoxic periodic paralysis. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2012010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surawicz B. Factors affecting tolerance to digitalis. J Am Coll Cardiol. 1985;5:69–81. doi: 10.1016/s0735-1097(85)80465-4. [DOI] [PubMed] [Google Scholar]
- 45.Lin SH. Thyrotoxic periodic paralysis. Mayo Clin Proc. 2005;80:99–105. doi: 10.1016/S0025-6196(11)62965-0. [DOI] [PubMed] [Google Scholar]
- 46.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 47.Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 48.Plaster NM, Tawil R, Tristani-Firouzi M, Canún S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptácek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 49.Ryan DP, da Silva MR, Soong TW, Fontaine B, Donaldson MR, Kung AW, Jongjaroenprasert W, Liang MC, Khoo DH, Cheah JS, Ho SC, Bernstein HS, Maciel RM, Brown RH, Jr, Ptácek LJ. Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell. 2010;140:88–98. doi: 10.1016/j.cell.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin SH, Lin YF, Chen DT, Chu P, Hsu CW, Halperin ML. Laboratory tests to determine the cause of hypokalemia and paralysis. Arch Intern Med. 2004;164:1561–1566. doi: 10.1001/archinte.164.14.1561. [DOI] [PubMed] [Google Scholar]
- 51.Ptácek LJ, Tawil R, Griggs RC, Engel AG, Layzer RB, Kwieciński H, McManis PG, Santiago L, Moore M, Fouad G, et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994;77:863–868. doi: 10.1016/0092-8674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 52.Bulman DE, Scoggan KA, van Oene MD, Nicolle MW, Hahn AF, Tollar LL, Ebers GC. A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology. 1999;53:1932–1936. doi: 10.1212/wnl.53.9.1932. [DOI] [PubMed] [Google Scholar]
- 53.Cheng CJ, Lin SH, Lo YF, Yang SS, Hsu YJ, Cannon SC, Huang CL. Identification and functional characterization of Kir2.6 mutations associated with non-familial hypokalemic periodic paralysis. J Biol Chem. 2011;286:27425–27435. doi: 10.1074/jbc.M111.249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu TA, Chang HK, Shieh RC. Extracellular K+ elevates outward currents through Kir2.1 channels by increasing single-channel conductance. Biochim Biophys Acta. 2011;1808:1772–1778. doi: 10.1016/j.bbamem.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F. Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Arch. 2010;460:239–248. doi: 10.1007/s00424-010-0814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cannon SC. Voltage-sensor mutations in channelopathies of skeletal muscle. J Physiol. 2010;588:1887–1895. doi: 10.1113/jphysiol.2010.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- 58.Matthews E, Labrum R, Sweeney MG, Sud R, Haworth A, Chinnery PF, Meola G, Schorge S, Kullmann DM, Davis MB, Hanna MG. Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology. 2009;72:1544–1547. doi: 10.1212/01.wnl.0000342387.65477.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurkat-Rott K, Weber MA, Fauler M, Guo XH, Holzherr BD, Paczulla A, Nordsborg N, Joechle W, Lehmann-Horn F. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc Natl Acad Sci U S A. 2009;106:4036–4041. doi: 10.1073/pnas.0811277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen AS. Pa Ping, or Kiating paralysis. Chinese Medical Journal. 1943;61:296–301. [Google Scholar]
- 61.Alagem N, Dvir M, Reuveny E. Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001;534:381–393. doi: 10.1111/j.1469-7793.2001.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Struyk AF, Cannon SC. Paradoxical depolarization of BA2+- treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve. 2008;37:326–337. doi: 10.1002/mus.20928. [DOI] [PubMed] [Google Scholar]
- 63.Seebohm G, Strutz-Seebohm N, Ursu ON, Preisig-Müller R, Zuzarte M, Hill EV, Kienitz MC, Bendahhou S, Fauler M, Tapken D, Decher N, Collins A, Jurkat-Rott K, Steinmeyer K, Lehmann-Horn F, Daut J, Tavaré JM, Pott L, Bloch W, Lang F. Altered stress stimulation of inward rectifier potassium channels in Andersen-Tawil syndrome. FASEB J. 2012;26:513–522. doi: 10.1096/fj.11-189126. [DOI] [PubMed] [Google Scholar]
- 64.Johnson CH, VanTassell VJ. Acute barium poisoning with respiratory failure and rhabdomyolysis. Ann Emerg Med. 1991;20:1138–1142. doi: 10.1016/s0196-0644(05)81393-9. [DOI] [PubMed] [Google Scholar]
- 65.Sigue G, Gamble L, Pelitere M, Venugopal S, Arcement L, Rab ST, Thakur V. From profound hypokalemia to life-threatening hyperkalemia: a case of barium sulfide poisoning. Arch Intern Med. 2000;160:548–551. doi: 10.1001/archinte.160.4.548. [DOI] [PubMed] [Google Scholar]
- 66.Dalal AK, Harding JD, Verdino RJ. Acquired long QT syndrome and monomorphic ventricular tachycardia after alternative treatment with cesium chloride for brain cancer. Mayo Clin Proc. 2004;79:1065–1069. doi: 10.4065/79.8.1065. [DOI] [PubMed] [Google Scholar]
- 67.Clemessy JL, Favier C, Borron SW, Hantson PE, Vicaut E, Baud FJ. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;346:877–880. doi: 10.1016/s0140-6736(95)92711-5. [DOI] [PubMed] [Google Scholar]
- 68.Neil MJ, Dale MC. Hypokalaemia with severe rebound hyperkalaemia after therapeutic barbiturate coma. Anesth Analg. 2009;108:1867–1868. doi: 10.1213/ane.0b013e3181a16418. [DOI] [PubMed] [Google Scholar]
- 69.Preisig-Müller R, Schlichthörl G, Goerge T, Heinen S, Brüggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc Natl Acad Sci U S A. 2002;99:7774–7779. doi: 10.1073/pnas.102609499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hopkins PM. Skeletal muscle physiology. Contin Educ Anaesth Crit Care Pain. 2006;6:1–6. [Google Scholar]
- 71.Lindinger MI. Potassium regulation during exercise and recovery in humans: implications for skeletal and cardiac muscle. J Mol Cell Cardiol. 1995;27:1011–1022. doi: 10.1016/0022-2828(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 72.Clausen T. Clearance of extracellular K+ during muscle contraction—roles of membrane transport and diffusion. J Gen Physiol. 2008;131:473–481. doi: 10.1085/jgp.200809971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shorten PR, Soboleva TK. Anomalous ion diffusion within skeletal muscle transverse tubule networks. Theor Biol Med Model. 2007;4:18. doi: 10.1186/1742-4682-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clausen T, Nielsen OB, Clausen JD, Pedersen TH, Hayward LJ. Na+,K+-pump stimulation improves contractility in isolated muscles of mice with hyperkalemic periodic paralysis. J Gen Physiol. 2011;138:117–130. doi: 10.1085/jgp.201010586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galuska D, Kotova O, Barrès R, Chibalina D, Benziane B, Chibalin AV. Altered expression and insulin-induced trafficking of Na+-K+-ATPase in rat skeletal muscle: effects of high-fat diet and exercise. Am J Physiol Endocrinol Metab. 2009;297:E38–E49. doi: 10.1152/ajpendo.90990.2008. [DOI] [PubMed] [Google Scholar]
- 76.Knochel JP, Blachley JD, Johnson JH, Carter NW. Muscle cell electrical hyperpolarization and reduced exercise hyperkalemia in physically conditioned dogs. J Clin Invest. 1985;75:740–745. doi: 10.1172/JCI111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kjeldsen K, Nørgaard A, Hau C. Exercise-induced hyperkalaemia can be reduced in human subjects by moderate training without change in skeletal muscle Na,K-ATPase concentration. Eur J Clin Invest. 1990;20:642–647. doi: 10.1111/j.1365-2362.1990.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 78.Rabinowitz L, Green DM, Sarason RL, Yamauchi H. Homeostatic potassium excretion in fed and fasted sheep. Am J Physiol Regul Integr Comp Physiol. 1988;254:R357–R380. doi: 10.1152/ajpregu.1988.254.2.R357. [DOI] [PubMed] [Google Scholar]
- 79.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238:E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 80.Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol. 1981;240:F257–F268. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]