Abstract
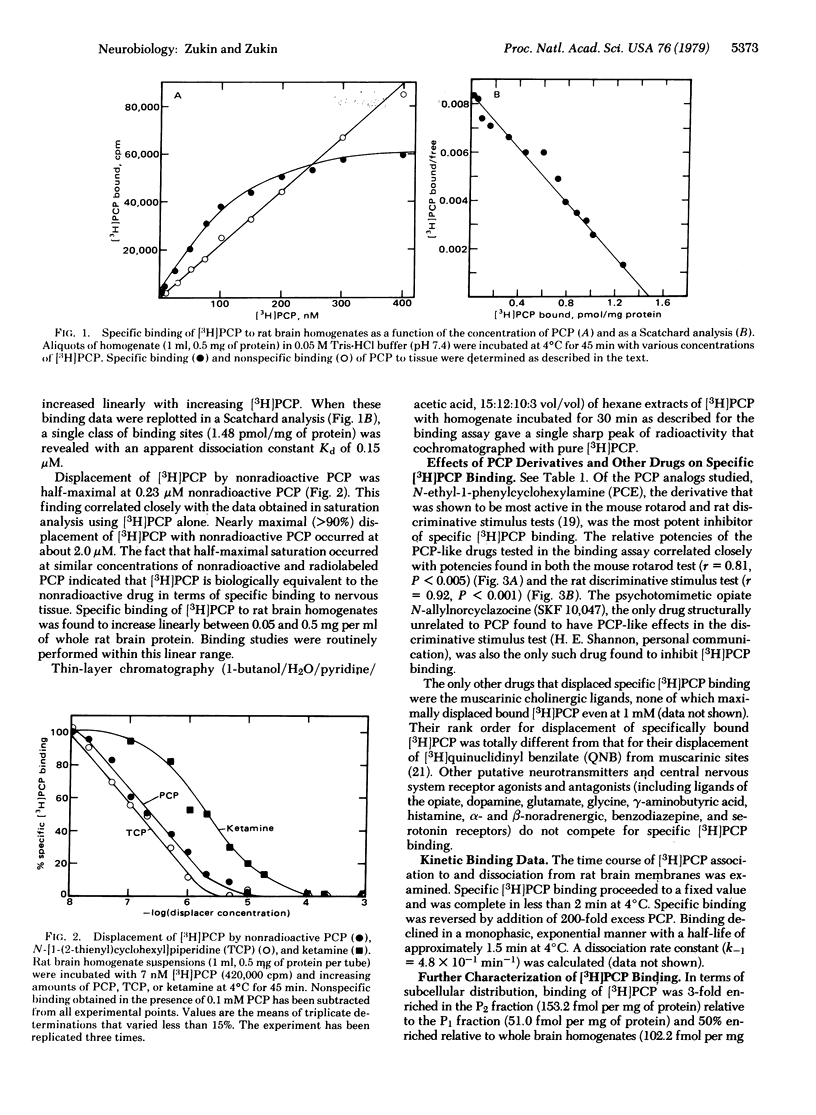
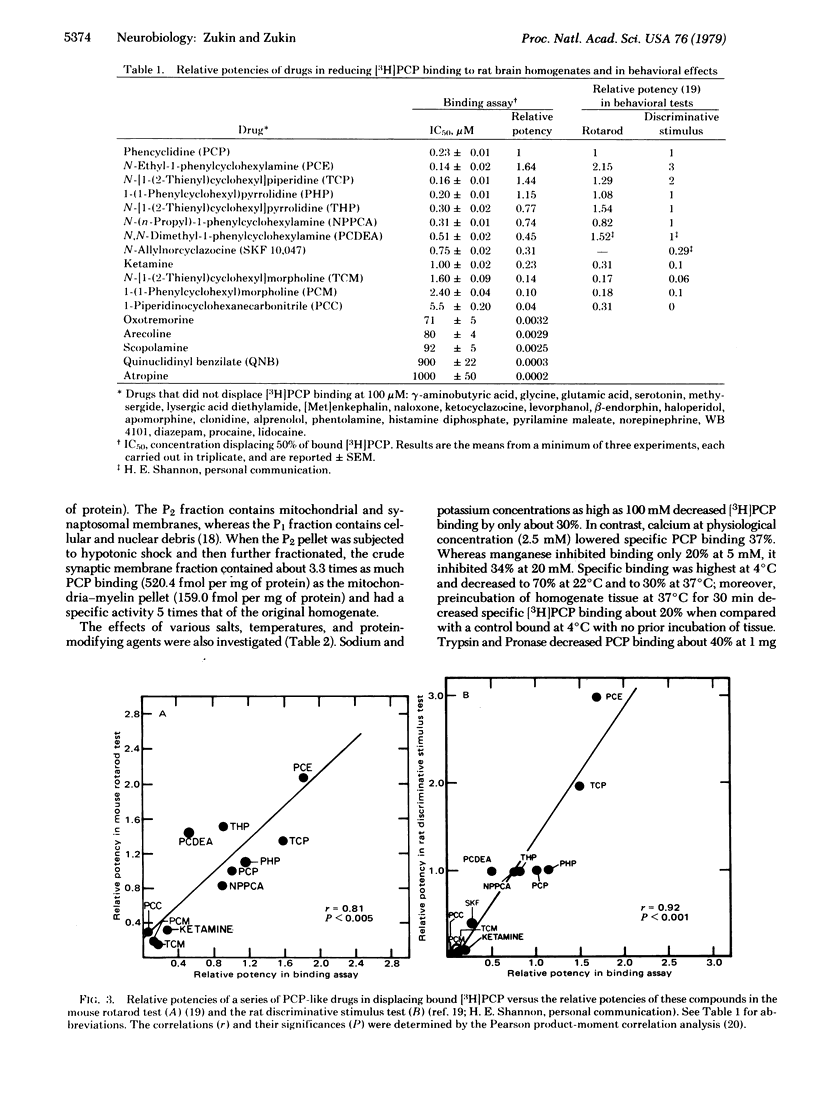
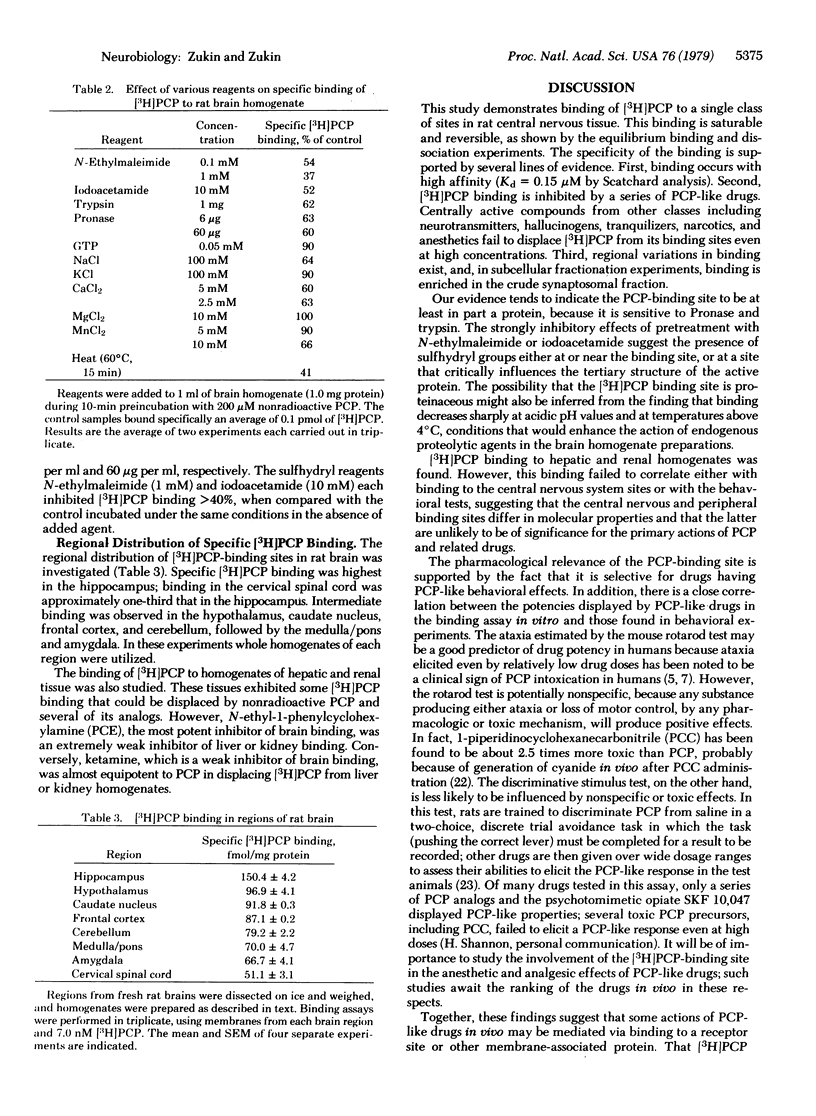
[3H]Phencyclidine (PCP) bound specifically and with high affinity (Kd = 0.15 microM at pH 7.4) to a single saturable class of binding sites in rat brain membrane preparations. Specific binding constituted approximately 70% of total binding at 0 degrees C and 33% of total binding at 37 degrees C (at 10 nM [3H]PCP). Bound [3H]PCP could be displaced by nonradioactive PCP, a series of its derivatives, and the psychotomimetic opiate N-allylnorcyclazocine (SKF 10,047) with relative potencies that closely paralleled those determined in animal behavioral tests. Muscarinic cholinergic ligands inhibited [3H]PCP binding, but only at 0.1 mM and in rank order at variance with that for binding to muscarinic sites or for pharmacological potencies. Other drugs, including opiates other than SKF 10,047, were unable to displace specifically bound [3H]PCP at 0.1 mM. [3H]PCP binding was most enriched in crude synaptosomal subcellular fractions, and was about three times higher in hippocampus (region of highest density) than in cervical spinal cord (region of lowest density). Trypsin and Pronase reduced specific [3H]PCP binding. Thus, PCP may exert its effects on the central nervous system via binding to specific brain receptor sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Spector S. Identification of inosine and hypoxanthine as endogenous ligands for the brain benzodiazepine-binding sites. Proc Natl Acad Sci U S A. 1979 Feb;76(2):977–981. doi: 10.1073/pnas.76.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS V. J., GOROSPE C. A., ROVENSTINE E. A. Intravenous nonbarbiturate, nonnarcotic analgesics: preliminary studies. 1. Cyclohexylamines. Anesth Analg. 1960 May-Jun;39:302–306. [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Garey R. E., Heath R. G. The effects of phencyclidine on the uptake of 3H-catecholamines by rat striatal and hypothalamic synaptosomes. Life Sci. 1976 May 15;18(10):1105–1110. doi: 10.1016/0024-3205(76)90145-4. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE M., EVANS V., BAIGEL S. Sernyl (CI-395) in clinical anaesthesia. Br J Anaesth. 1959 Oct;31:433–439. doi: 10.1093/bja/31.10.433. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUBY E. D., COHEN B. D., ROSENBAUM G., GOTTLIEB J. S., KELLEY R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959 Mar;81(3):363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Maayani S., Weinstein H., Ben-Zvi N., Cohen S., Sokolovsky M. Psychotomimetics as anticholinergic agents. I. 1-Cyclohexylpiperidine derivatives: anticholinesterase activity and antagonistic activity to acetylcholine. Biochem Pharmacol. 1974 Apr 15;23(8):1263–1281. doi: 10.1016/0006-2952(74)90330-x. [DOI] [PubMed] [Google Scholar]
- Maayani S., Weinstein H. Some structure activity relationships of phencyclidine derivatives as anticholinergic agents in vitro and in vivo. Prog Clin Biol Res. 1979;27:91–106. [PubMed] [Google Scholar]
- Rainey J. M., Jr, Crowder M. K. Letter: Prevalence of phencyclidine in street drug preparations. N Engl J Med. 1974 Feb 21;290(8):466–467. doi: 10.1056/nejm197402212900826. [DOI] [PubMed] [Google Scholar]
- Simantov R., Snyder S. H. Morphine-like peptides in mammalian brain: isolation, structure elucidation, and interactions with the opiate receptor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2515–2519. doi: 10.1073/pnas.73.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P., Marangos P. J., Goodwin F. K., Edwards M., Paul S. Identification of inosine and hypoxanthine as endogenous inhibitors of [3H] diazepam binding in the central nervous system. Life Sci. 1978 Oct 9;23(14):1473–1480. doi: 10.1016/0024-3205(78)90128-5. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Meltzer H. Y., Arora R. C., Davis J. M. Effects of phencyclidine on [3H]catecholamine and [3H]serotonin uptake in synaptosomal preparations from rat brain. Biochem Pharmacol. 1977 Aug 1;26(15):1435–1439. doi: 10.1016/0006-2952(77)90370-7. [DOI] [PubMed] [Google Scholar]
- Taube H. D., Montel H., Hau G., Starke K. Phencyclidine and ketamine: comparison with the effect of cocaine on the noradrenergic neurones of the rat brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):47–54. doi: 10.1007/BF00510820. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Cavey D., Kamenka J. M., Geneste P., Lazdunski M. Interaction of phencyclidines with the muscarinic and opiate receptors in the central nervous system. Brain Res. 1978 Aug 18;152(1):176–182. doi: 10.1016/0006-8993(78)90145-2. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin S. R., Young A. B., Snyder S. H. Gamma-aminobutyric acid binding to receptor sites in the rat central nervous system. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4802–4807. doi: 10.1073/pnas.71.12.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]



