Abstract
After traumatic brain injury (TBI), proteolysis of Alpha II Spectrin by Calpain 1 produces 145 SBDPs (Spectrin Breakdown Products) while proteolysis by Caspase 3 produces 120 SBDPs. 145 and 120 SBDP immunoblotting reflects the relative importance of caspase-dependent apoptosis or calpain-dependent excitotoxic/necrotoxic cell death in brain regions over time. In the adult rat, controlled cortical impact (CCI) increased 120 SBDPs in the first hours, lasting a few days, and increased 145 SBDPs within the first few days lasting up to 14 days after injury. Little is known about SBDPs in the immature brain after TBI. Since development affects susceptibility to apoptosis after TBI, we hypothesized that CCI would increase 145 and 120 SBDPs in the immature rat brain relative to SHAM during the first 3 and 5 days, respectively. SBDPs were measured in hippocampi and cortices at post injury days (PID) 1, 2, 3, 5, 7 and 14 after CCI or SHAM surgery in the 17 day old Sprague Dawley rat. 145 SBDPs increased in both brain tissues ipsilateral to injury during the first 3 days, while changes in contralateral tissues were limited to PID2 cortex. 145 SBDPs elevations were more marked and enduring in hippocampus than in cortex. Against expectations, 120 SBDPs only increased in PID1 hippocampus and PID2 cortex. 145 SBDPs elevations occurred early after CCI, similar to previous studies in the adult rat, but resolved more quickly. The minimal changes in 120 SBDPs suggest that calpain-dependent, but not caspase-dependent, cell death predominates in the 17 day old rat after CCI.
Keywords: Controlled cortical impact, apoptosis, excitotoxicity, cell death, developmental
1. INTRODUCTION
Traumatic brain injury (TBI) affects nearly half a million children each year in the United States (Langlois et al., 2005) and is a leading cause of pediatric death and disability (Langlois et al., 2006). Survivors of pediatric TBI are at increased risk for prolonged, even life-long, neurologic impairments (Yeates et al., 2002; Yeates et al., 2005) (Anderson et al., 2012) (Anderson et al., 2005b) (Aitken et al., 2009). Despite the significant burden imposed by pediatric TBI on individuals and society, clinical therapies to directly improve neurologic outcome after pediatric TBI are lacking. In the experimental setting, blockade of specific cell death pathways improves neurologic outcome after TBI (Chen et al., 2012; Faden et al., 2005; O'Connor et al., 2007). Methods to identify specific cell death pathways activated after TBI may aid translation of candidate therapeutics into the clinical arena. One such method involves measuring Alpha II Spectrin breakdown products, or SBDPs.
Alpha II Spectrin is a membrane-associated protein with essential roles in neuronal development, synaptic plasticity and cytoskeletal remodeling (Yan et al., 2012). Proteolysis of the 280kDa Alpha II Spectrin by the calcium-dependent, cysteine protease Calpain I produces 150kDa and 145 kDa fragments that are indicative of necrotic /excitotoxic cell death (Yan et al., 2012). Proteolysis by Caspase 3, the predominant executioner caspase in neuronal apoptosis (D'Amelio et al., 2010), results in 150i and 120 kDa fragments that are indicative of caspasedependent apoptotic cell death (Yan et al., 2012) (Siman and Noszek, 1988). The two 150 kDa fragments, albeit the product of different cleavage sites in Alpha II Spectrin, are indistinguishable from each other via anti-Fodrin immunoblotting. However, anti-Fodrin immunoblotting allows direct comparison of caspase and calpain-dependent Alpha II Spectrin break down products, or SBDPs, in a single sample (Liu et al., 2006). In sum, measurement of 150, 145 and 120 SBDPs by anti-Fodrin immunoblotting provides information on the relative importance of caspase- or calpain-dependent cell death processes in a given brain region over time after injury.
In the adult rat brain, experimental TBI increases 150, 145 and 120 SBDPs. Experimental TBI elevates 150 and 145 SBDPs in brain regions characterized by contusion, neuronal death and/or axonal injury (McGinn et al., 2009; Pike et al., 1998; Pike et al., 2001; Ringger et al., 2004). Experimental TBI also increases 120 SBDPs, associated with increased apoptosis and caspase activation (Beer et al., 2000). 120 SBDP elevations occur in the first hours after TBI and last for a few days (Beer et al., 2000; Pike et al., 1998), while 145/150 SBDPs elevations occur in the first several days after CCI and can last as long as 7 and 14 days (McGinn et al., 2009; Pike et al., 1998; Valiyaveettil et al., 2014).
Less is known about SBDPs after experimental TBI in the immature brain. Aikman et al measured 150, 145 and 120 SBDPs in the cortex ipsilateral to injury during the first three days after controlled cortical impact (CCI) in the 9 day old rat (Aikman et al., 2006). To our knowledge, SBDP levels in other brain regions or beyond the first three days after experimental TBI in the immature brain have not been reported. The relative importance of apoptosis and necrosis after developmental TBI has been studied in the newborn and 7–10 day old rat (Bittigau et al., 2003) (Bayly et al., 2006) (Aikman et al., 2006; Bittigau et al., 1999; Bittigau et al., 2004; Rice and Barone, 2000), which are developmentally similar to a preterm and newborn human, respectively (Rice and Barone, 2000). Few studies have addressed the relative role of apoptotic versus necrotic cell death after brain injury in immature rats at later ages.
The immature brain has a heightened susceptibility to pathologic apoptosis after injury (Polster et al., 2003; Zhu et al., 2005). In previous experimental studies of pediatric TBI, apoptotic cell death peaked in the first 24–48 hours (Bayly et al., 2006; Bittigau et al., 1999) and lasted as long as five days (Bittigau et al., 1999). Excitotoxic, or calpain-mediated, cell death occurs acutely after trauma in both the mature and immature rodent brain (Bayly et al., 2006; Bittigau et al., 2004; Hinzman et al., 2012; Zhu et al., 2005). We therefore hypothesized that 145 and 120 SBDPs would increase during the first three and five days after experimental TBI, respectively, in the hippocampus and cortex of the immature rat ipsilateral to injury.
To test this hypothesis, we used CCI in 17 day old rats to model developmental TBI. CCI produces contusion and neuronal death in the cortex and hippocampus immediately beneath the impact as well as diffuse axonal injury (Dixon et al., 1991; Gobbel et al., 2007; Raghupathi et al., 2000; Raghupathi and Huh, 2007). SBDP levels were measured in dissected hippocampi and cortices ipsilateral and contralateral to impact on post injury days (PID) 1, 2, 3, 5, 7 and 14 using anti-Fodrin immunoblotting on brain samples of equal volume and protein content. Equal protein loading was additionally confirmed by tubulin protein levels as further detailed in the Methods section.
2. RESULTS
Consistent with previous work using this model, survival and weight gain did not differ between CCI and sham-operated rats (SHAM) groups (Schober et al., 2010). SBDPs were quantified relative to tubulin levels, since tubulin did not differ between CCI and SHAM hippocampus and cortex at any time point. Statistical analyses were performed between CCI and SHAM groups as outlined in the Methods. SBDP levels in CCI rats are presented as a percent of corresponding SBDP values obtained in age-matched rats after sham surgery on the same post-surgery day.
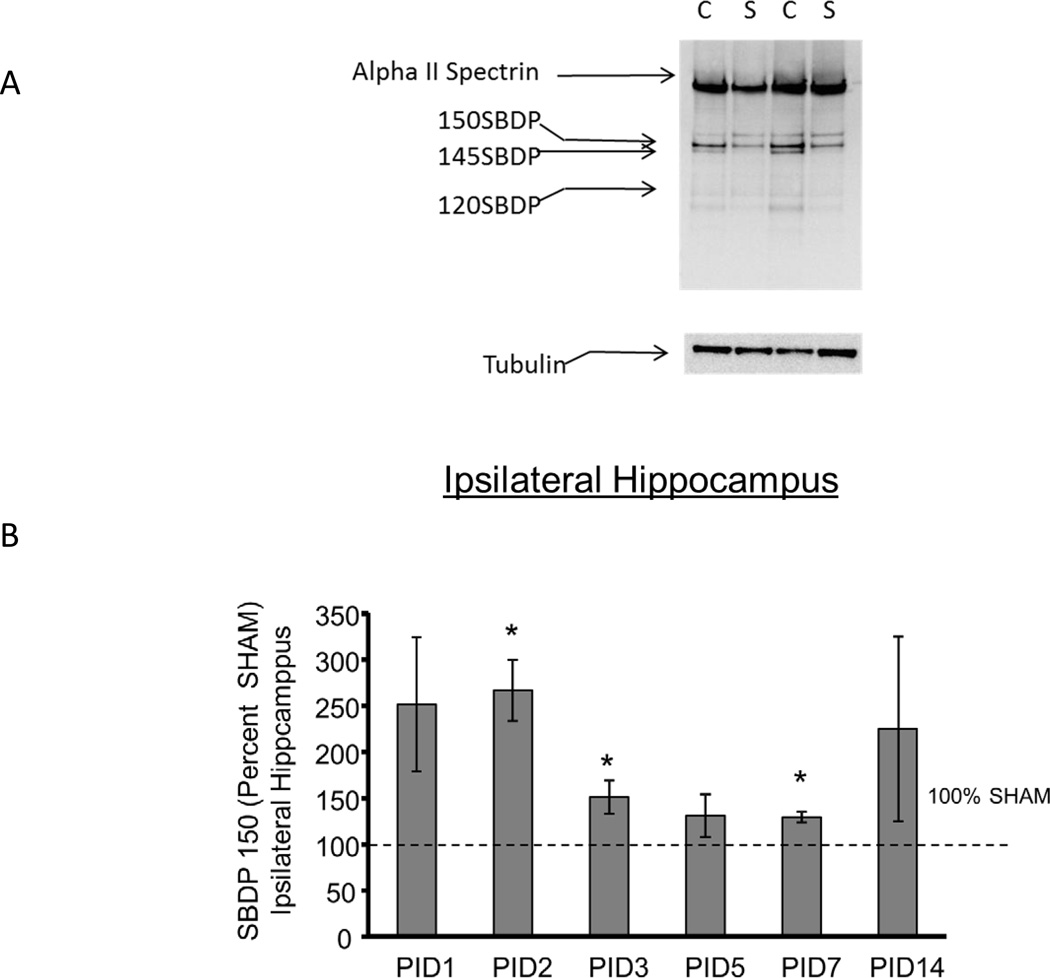
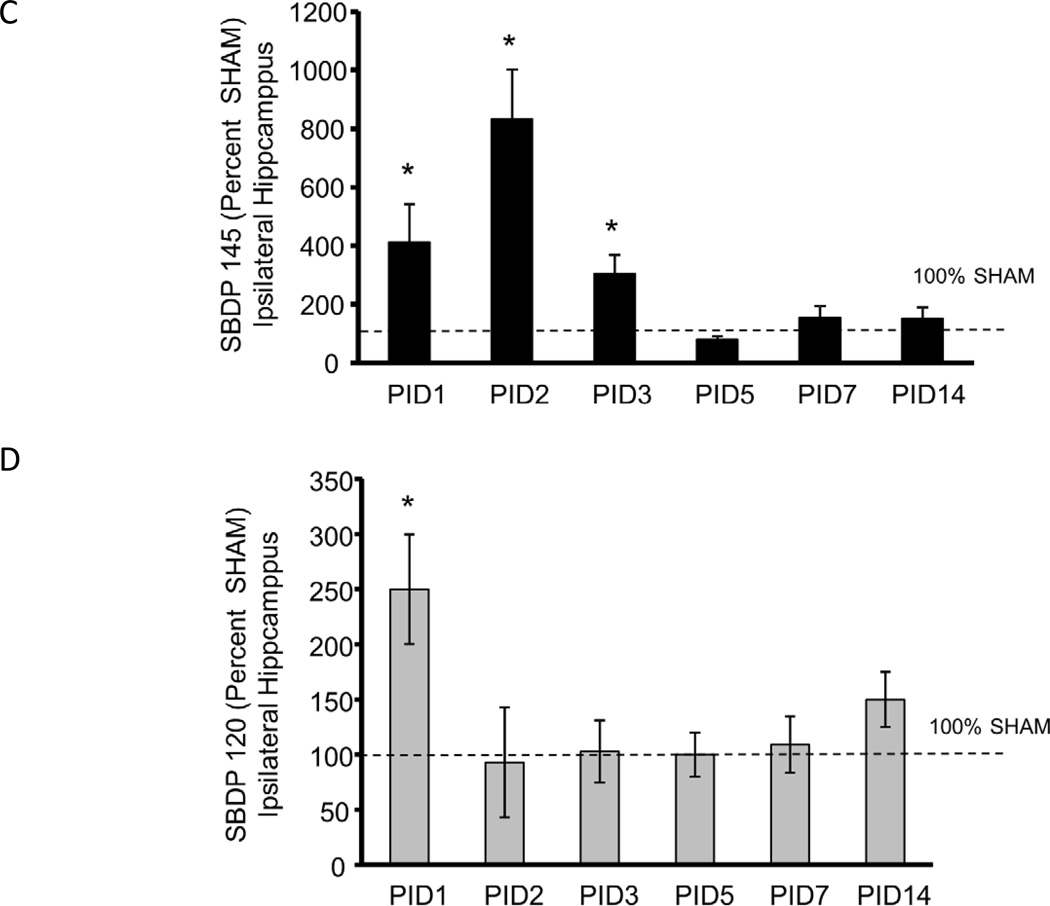
Figure 1A shows a representative blot from PID1 ipsilateral hippocampus in which the intact Alpha II Spectrin and the 150, 145, 120 SBDP and tubulin bands are shown. In the hippocampus ipsilateral to injury, as shown in Figures 1 B, C and D, CCI increased 150 SBDPs at PID2, 3 and 7 relative to SHAM (267 ± 33%, 151 ± 18%, and 129 ± 6% of SHAM, respectively, p<0.05). CCI markedly increased 145 SBDPs at PID 1–3 (412 ± 129%, 833 ± 167%, and 305 ± 63% of SHAM, respectively, p<0.05). CCI increased 120 hippocampal SBDPs only at PID1, to 250 ± 50% of SHAM (p<0.05). Values at subsequent time points were not different from SHAM.
Figure 1. Controlled cortical impact (CCI) increased Alpha II Spectrin Breakdown Products (SBDPs) in the immature rat hippocampus ipsilateral to injury.
A. Representative immunoblot of hippocampal tissue ipsilateral to injury from CCI (C) and SHAM (S) rat pups at day one after injury showing the intact Alpha II Spectrin band as well as the 150 kDa SBDP (150 SBDP), 145 kDa SBDP (145 SBDP), the 120 kDa SBDP (120 SBDP) and tubulin bands. B. Graphs B, C and D represent the immunoblot results for 150, 145 and 120 SBDPs, respectively. For all graphs, data for immature rat hippocampus ipsilateral to injury is expressed as a percent of SHAM values at post injury days (PID) 1, 2, 3, 5, 7 and 14 after CCI. The dotted line shows the value corresponding to 100% SHAM. N=6/group and * p<0.05. CCI increased 150 kDa SBDPs relative to SHAM values in the ipsilateral hippocampus at PID 2, 3 and 7. C. CCI increased 145 kDa SBDPs relative to SHAM values in the ipsilateral hippocampus at PID 1, 2 and 3. D. CCI increased 120 kDa SBDPs relative to SHAM values in the ipsilateral hippocampus at PID 1.
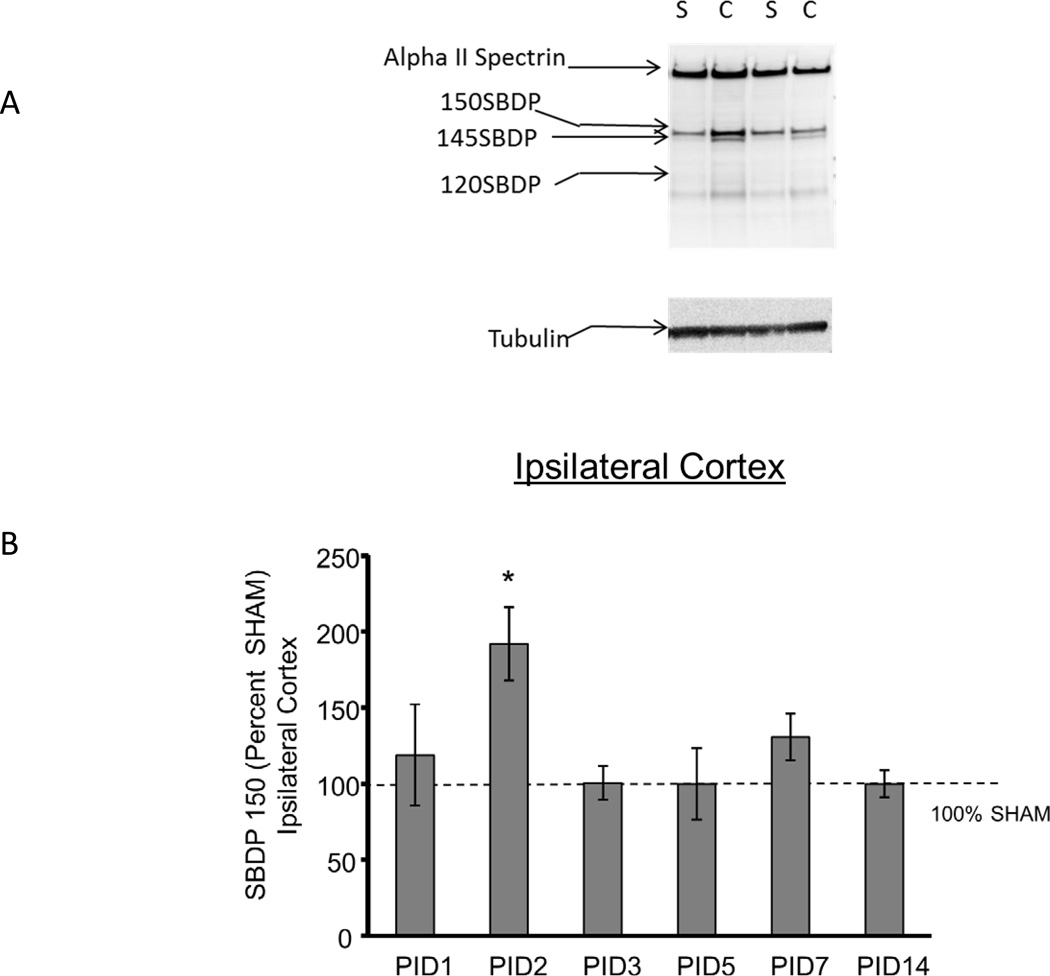
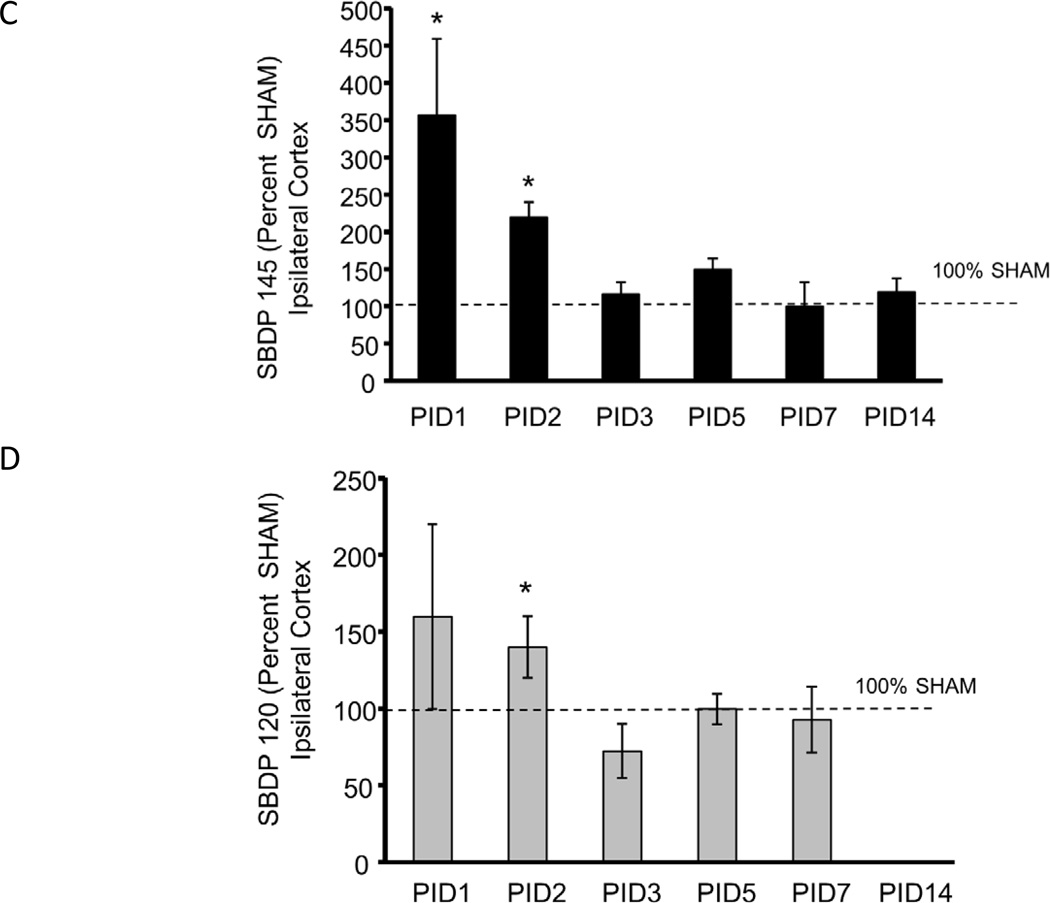
Figure 2A shows a representative blot from PID1 ipsilateral cortex in which the intact Alpha II Spectrin and the 150, 145, 120 SBDP and tubulin bands are shown. In the cortex ipsilateral to injury, as shown in Figure 2 B, C and D, CCI increased 150 SBDPs at PID2 relative to SHAM (192 ± 24% of SHAM, p<0.05). At PID7, 150 SBDP elevations approached statistical significance (131 ± 12% of SHAM, p=0.07). CCI increased 145 SBDPs at PID 1 and 2 (357 ± 103% and 220 ± 20% of SHAM, respectively, p<0.05). Finally, CCI increased 120 SBDPs only at PID2, to 140 ± 20% of SHAM (p<0.05). Values at subsequent time points were not different from SHAM.
Figure 2. Controlled cortical impact (CCI) increased Alpha II Spectrin Breakdown Products (SBDPs) in the immature rat cortex ipsilateral to injury.
A. Representative immunoblot of cortical tissue ipsilateral to injury from CCI (C) and SHAM (S) rat pups at day 1 after injury showing the intact Alpha II Spectrin band as well as the 150 kDa SBDP (150 SBDP), 145 kDa SBDP (145 SBDP), the 120 kDa SBDP (120 SBDP) and tubulin bands. B. Graphs B, C and D represent the immunoblot results for 150, 145 and 120 SBDPs, respectively. For all graphs, data for immature rat pup cortex ipsilateral to injury is expressed as a percent of SHAM values at post injury days (PID) 1, 2, 3, 5, 7 and 14 after CCI. The dotted line shows the value corresponding to 100% SHAM. N=6/group and * p<0.05. CCI increased 150 SBDPs relative to SHAM values in the ipsilateral cortex at PID 2. C. CCI increased 145 SBDPs relative to SHAM values in the ipsilateral cortex at PID 1 and 2. D. CCI increased 120 SBDPs relative to SHAM values in the ipsilateral cortex at PID 2.
We found no differences between CCI and SHAM in contralateral hippocampi at any of the studied time points. We found no differences in contralateral cortices except at PID2, when CCI increased 145 SBDPs in contralateral cortex (157 ± 11% of SHAM, p<0.05). In contralateral hippocampi at PID3, increased 145 SBDPs approached statistical significance (162 ± 29% SHAM, p=0.06).
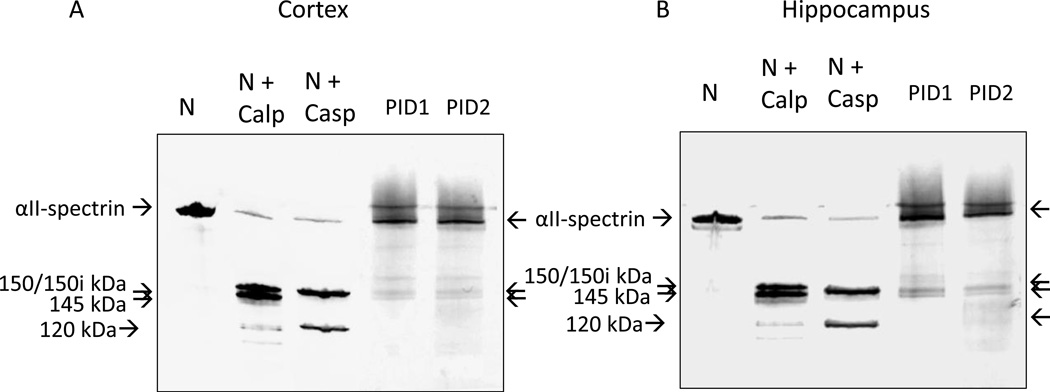
We assessed the specificity of anti-Fodrin immunoblotting by comparing the detection of 150,145 and 120 SBDPs in various samples on the same gel, as shown in Figure 3. Cortical samples (Figure 3A) included naïve (taken from 18 day old rat pups unexposed to any surgery or manipulation), treated naïve (naïve samples exposed to in vitro digestion with Calpain 1 or Caspase 3) and CCI (PID1 and 2). A parallel study using hippocampal samples is shown in Figure 3B. First, as expected, Calpain 1 digestion of the naïve, immature rat cortical and hippocampal lysates produced 150 kDa and 145 kDa SBDPs, while Caspase 3 digestion produced a 150i kDa SBDP (which is slightly smaller than 150 kDa SBDP150) and a characteristic 120 kDa SBDP (Liu et al., 2006). Additionally, we observed that indeed the major 150 kDa and 145 kDa SBDPs found in the rat pup ipsilateral cortices and hippocampi after CCI have apparent molecular weight identical to those generated by Calpain 1 digestion in vitro on immunoblots. Similarly, the 120 kDa SBDP in CCI hippocampus has an apparent molecular weight identical to the 120kDa SBDP counterpart produced by Caspase 3 in vitro. We also noted that overall SBDP intensity was stronger with in vitro protease (Calpain 1 and Caspase 3) digestion, as the proteolysis of intact Alpha II-Spectrin was more extensive. Taken together, these data demonstrate that the 150 kDa/145 kDa and 120 kDa SBDPs we observed in rat pup brain after CCI are most likely equivalent to those generated by endogenous Calpain 1 and Caspase 3-mediaed proteolysis, as described previously in adult rats and humans after TBI (Pineda et al., 2007; Ringger et al., 2004).
Figure 3. Comparison of apparent molecular weight of SBDPs detected in immature rat cortical (A) and hippocampal (B) lysates obtained from naïve (N, unexposed to surgery or any manipulation), naïve exposed to in vitro digestion with Calpain 1(N+ Calp) or Caspase 3 (N+ Casp), and from CCI rat cortex/hippocampus ipsilateral to injury at PID 1 and 2 (PID1 and PID2).
Arrows indicates intact Alpha II Spectrin, SBDP 150 kDa, 145 kDa by Calpain 1 and SBDPs of 150i kDa and 120 kDa by Caspase 3. Representative PID1 and PID2 ipsilateral cortical and hippocampal samples from CCI rats were used. SBDP of 150i kDa (by Caspase 3) is slightly smaller than SBDP of 150 kDa (by Calpain) (Liu et al., 2006).
3. DISCUSSION
As anticipated, CCI increased 145 SBDPs in the ipsilateral hippocampus and cortex of the 17 day old rat during the first three days after injury. Relative to SHAM, 145 SBDP elevations were greater and longer lasting in the hippocampus than the cortex of the immature rat. Contrary to expectations, CCI did not increase hippocampal or cortical levels of 120 SBDPs beyond the first two days after injury. Changes in contralateral tissues were limited to elevated 145 SBDPs in the cortex at PID2.
To our knowledge, published data on rat pup brain SBDPs after TBI are limited to one study (Aikman et al., 2006). In agreement with our findings, Aikman et al showed that CCI increased cortical 145 SBPDs in the 9 day old rat on day 1 and 2, but not at day 3, after injury. Further comparisons are not possible because they did not report hippocampal SBDP levels at any time point, nor any SBDP results after the first 72hrs (Aikman et al., 2006). In the adult rodent, cortical 145 SBDP elevations lasted at least three days and as long as five days after CCI (Pike et al., 1998; Pike et al., 2001). Other models of TBI, such as blast and weight drop, similarly show long-lasting elevations in 145 SBDPs in the injured cortex of the adult rodent (Kupina et al., 2003; Valiyaveettil et al., 2014). Time to resolution, then, appears to be the most striking difference between brain 145 SBDPs in the adult and the immature rat cortex after CCI.
145 SBDP elevations in our model persisted longer in the ipsilateral hippocampus than cortex (until day 3 and 2 after injury, respectively). 145 SBDPs also increased more markedly in the hippocampus than cortex, peaking at 833 ± 167% of SHAM compared to 357 ± 103% of SHAM. Regional 145 SBDP elevation differences may be attributed to the higher hippocampal density of n-methyl-d-aspartate receptors (NMDARs) (Geddes et al., 2003), whose activity is associated with calpain activation (Deng et al., 2007), and/or regional differences in brain tolerance to oxygen-glucose deprivation (Xu et al., 2001). In the adult rat after CCI, Pike et al reported that 145 SBDP levels relative to SHAM increased more dramatically, but normalized sooner, in the cortex than in the hippocampus (Pike et al., 1998). Developmental effects on NMDAR activity (Giza et al., 2006; Saransaari and Oja, 2003) could contribute to these maturational differences.
The pattern of 150 SBPD elevations in our study was very similar to that of 145 SBDPs, with the exception of PID7. Hippocampal 150, but not 145, SBDPs increased in CCI rats at PID7. Cortical 150 SBDP elevations in CCI rats approached significance, while 145 SBDPs were equal to 100% of SHAM values. We speculate that hippocampal 150 SBDP elevations at PID7 may be more indicative of caspase than calpain activation.
Hippocampal and cortical 120 SBDPs increased at day 1 and 2, respectively, followed by normalization. In the 9 day old rat, cortical 120 SBPDs increased at day 1, but not at day 2 after CCI (Aikman et al., 2006). The timing and duration of increased 120 SBDPs after adult experimental TBI are more variable than those of 145 SBDPs. CCI in the adult rat increased 120 SBDP levels only in the first few hours after injury in some studies (Pike et al., 1998; Pike et al., 2001), but in others CCI increased 120 SBDPs during the first 72 hours, limited to cortex (Beer et al., 2000; Hall et al., 2005).
Our results are consistent with studies showing developmental differences in activation of caspase, but not calpain, after brain injury. Apoptosis in the developing rat brain after TBI decreases in severity with increasing post natal age. The severity of trauma-triggered apoptosis was highest in 3- and 7-day old rat pups and decreased with age in rats up to 30 days old (Bittigau et al., 1999). Data in the immature mouse after experimental hypoxic ischemic brain injury suggest that developmental age has more impact on the caspase-specific SBDPs than on the calpain-specific fragments: brain caspase levels and activity dropped dramatically in the 21 and 60 day old mice relative to those in the 5 and 9 day old mice. In contrast, calpain levels and activity did not change with development (Zhu et al., 2005). Similar to the adult rat, calpainmediated cell death occurs acutely in the immature rat after TBI. After TBI in 17 and 28 day old rats, cortical calcium (a trigger of necrotic cell death) accumulated immediately (Osteen et al., 2001) and, in rat pups 11 and 17 days old, calpain was quickly activated in the contused cortex (Huh and Raghupathi, 2007). We speculate that caspase-dependent apopotosis is not the predominant death pathway after trauma in rat pups older than ten days, thus accounting for the observed relatively small effect of CCI on 120 SBDPs in the 17 day old rat brain. However, it is certainly possible that other apoptotic pathways, such as those via Apoptosis-Inducing Factor, could play an important role after TBI in the immature brain.
3.1 Limitations and Strengths
Apoptosis may be more prominent in regions remote from the impact, as shown in the newborn rat pup (Bayly et al., 2006). While we did not study all brain regions, our inclusion of hippocampi and cortices contralateral to the impact suggests that distance from the impact did not play a significant role in our 120 SBDP findings. A second limitation is the use of an antibody that does not distinguish between 150 SBDPs generated by activation of calpain versus caspase. We chose this antibody to facilitate comparisons with previously published results, since it is the same as the one used to study the 9 day old and adult rat after CCI (Aikman et al., 2006; Pike et al., 1998). Future studies could employ an ELISA specific for the two forms of the 150 SBDPs. Finally, direct comparisons between animals of different ages are limited by the importance of injury type and severity on SBDP elevations (Ringger et al., 2004; Saatman et al., 2010).
An important strength of our study is the use of SHAM rats matched for age and time post injury to control for developmental effects on Alpha II Spectrin levels. The approach towards protein level normalization further strengthens the results of this study, as described in the Methods.
3.2 Conclusions
145 SBDP elevations in the 17 day old rat hippocampus and cortex in the first few days after CCI are remarkably similar to those found previously in the adult rat. Unexpectedly, 120 SBDP elevations after CCI were short-lived in the 17 day old rat. While further study is needed, our findings suggest that calpain-dependent, but not caspase-dependent, processes constitute predominant cell death pathways after trauma in rat pups older than ten days.
The time course of elevated 145 SBDPs in cerebrospinal fluid (CSF) in the first seven days after adult human TBI is a strong predictor of outcome (Brophy et al., 2009; Cardali and Maugeri, 2006; Mondello et al., 2010). CSF SBDP levels after TBI in children are not known. Based on our findings, we speculate that a pediatric study could be modeled after adult trials and that pediatric CSF samples should be obtained within the first 24–48 hours after injury. CSF SBDP levels could help select those patients most likely to benefit from a particular agent, such as a calpain inhibitor, to improve neurologic outcome after pediatric TBI.
4. EXPERIMENTAL PROCEDURE
4.1 Animals
All experimental protocols were approved by the Animal Care and Use Committees at the University of Utah, in accordance with US NIH guidelines and carried out at the University of Utah. All surgical procedures were performed using aseptic technique.
Briefly, male Sprague-Dawley rats were obtained from Charles Rivers Laboratories (Raleigh, NC) on post-natal day (P) 7–10. We studied only males to eliminate any potential confounding effects of gender. Rats were housed in litters of 10 with the lactating dam until weaning on P 21—23. After weaning, rats were housed 3—5 per cage and allowed free access to food and water. All cages were kept in a temperature- and light-controlled (12 h on/12 h off) environment.
Rats were randomized to experimental group at age P17, at which time rats underwent CCI or SHAM craniotomy. Randomization was distributed evenly within litters. We chose P17 because the maturation state of the rodent brain at this age is comparable to the human infant and young toddler (Dobbing and Sands, 1979; Rice and Barone, 2000), the population most commonly at risk for cognitive deficits after TBI (Anderson et al., 2005a; Bittigau et al., 2004). After weaning, rats were placed in cages without any segregation based on experimental group.
4. 2 CCI procedure
CCI was carried out as previously described. (Schober et al., 2010) On day 17 of life, rats undergoing CCI were anesthetized with 3% isoflurane for induction followed by 2—2.5% isoflurane for the duration of surgical preparation, using a VetEquip Bench Top Isoflurane Anesthesia System (Pleasanton, CA). Core temperature was monitored via a rectal probe and continuously controlled at 37±0.5 °C using a servo-controlled heating pad. Oxygenation, heart rate and respiratory rate were monitored using femoral probe pulse oximetry (Mousox ®, Starr Life Sciences, Oakmont, PA).
The rat was placed into a stereotaxic frame (David Kopf, Tujunga, CA). After shaving, prepping with povidone-iodine and incising the scalp, a craniotomy (6-mm×6-mm) was performed over the left parietal cortex (centered at the point 4-mm posterior and 4-mm lateral to bregma). Care was taken not to perforate the dura. Once the craniotomy was complete, anesthesia was reduced to 1% isoflurane for a 5-min equilibration period. CCI was then delivered (Pittsburgh Precision Instruments, Pittsburgh, PA) to the left parietal cortex using a 5-mm rounded tip to deliver a 2.0 mm deformation at 5 m/s velocity and 100 ms duration. Immediately after CCI, isoflurane was increased to 2—2.5%, and the bone flap was replaced and secured with dental cement (Patterson Dental, Salt Lake City, UT). The scalp incision was sutured, and triple antibiotic ointment and bupivacaine 0.5% were applied topically. Isoflurane was stopped, and rats were allowed to recover in a temperature-controlled chamber. Once fully awake, rats were returned to their dams and littermates. 17 day old SHAM rats underwent identical surgical craniotomy, equilibration, and closure procedures without CCI.
4.3 Tissues
Rats were anesthetized with intraperitoneal (IP) xylazine (8mg/kg) and ketamine (40mg/kg), and killed by swift decapitation (N= 6 per group). After brain removal, the entire hippocampus from each hemisphere, as well as the 6–7 mm contused cortex and corresponding contralateral cortex were quickly dissected on ice. The left (or ipsilateral to injury) brain region from CCI and SHAM rats was labeled either CCI ipsilateral or SHAM ipsilateral, respectively. The right (or contralateral to injury) brain region from CCI and SHAM rats was labeled either CCI contralateral or SHAM contralateral, respectively. Tissues were collected on the same post injury day for CCI and SHAM groups. Tissues were snap frozen in liquid nitrogen and stored at −80°C.
4.4 Immunoblot for Alpha II-Spectrin Breakdown Products
Frozen brain samples were pulverized over dry ice to a fine powder. The pulverized brain tissue was then lysed for 90 min at 4°C in lysis buffer (50 mM Tris (pH 7.4), 5 mM EDTA, 1% (v/v) Triton X-100, 1 mM DTT and 1× protease inhibitor cocktail (Roche Biochemicals)). The brain lysates were centrifuged at 8,000 g for 5 min at 4°C and the resulting supernatant was transferred to new tubes, snap-frozen and stored at −80C. Protein concentration was determined by the BCA method (Pierce Protein Research Products, Rockford, IL) and used to calculate volume for equal protein loading. Proteins were separated by SDS PAGE using Criterion XT Precast 3–8% Bis-Tris Gels (Bio-Rad, Hercules, CA) in MOPS buffer, followed by transfer to PVDF membranes. After the membranes were blocked with 5% milk in Tris-buffered saline and 0.1% Tween 20 (TBS-T) at room temperature for 1 h, bound proteins were exposed to anti-Fodrin (Alpha II-Spectrin) (BML-FG6090, ENZO Life Sciences, Inc., Farmingdale, NY) overnight at 4°C. After extensive washing in TBST, a 1:4,000 dilution of goat anti-mouse HRP secondary antibody (Cell Signaling Technology MA) was applied and incubated for 1 h at room temperature. Levels of α tubulin on the same blot were measured using mouse monoclonal primary antibody T6199 (Sigma Aldrich, St Louis, MO) at a dilution of 1:4000. Signals were detected with Western Lightning ECL (PerkinElmer Life Sciences) and quantified relative to α tubulin (mouse monoclonal 1:4000, T6199, Sigma Aldrich, St Louis, MO) by densitometry on a Kodak Image Station 2000R (Eastman Kodak/SIS, Rochester, NY).
We used the BCA method to load equal protein amounts and thus minimize confounding by proteolysis. Extensive proteolysis after experimental TBI is a well-known phenomenon (Lo et al., 2002), affecting even those proteins commonly used to control for differences in protein loading. For example, TBI affected both actin and tubulin protein levels in the mouse brain (Rhinn et al., 2008). In the 17 day old rat pup after CCI, protein levels of actin and tubulin (Jenkins et al., 2002) differed from SHAM at 24hrs, but not at two weeks, after CCI (Kochanek et al., 2006). Therefore, we used alpha tubulin to normalize SBDP results only when we found that tubulin levels did not differ between CCI and SHAM at any time point. Normalizing to the intact Alpha II Spectrin would be less desirable since the decrease in Spectrin is far less than the increase in SBDPs (Kobeissy et al., 2006; Siman and Noszek, 1988).
4.5 Calpain 1 and Caspase 3 digestion of cortical and hippocampal lysates from naïve rat
After euthanasia described in 4.3, Naïve (unexposed to any surgery or manipulation) 18 day old rat pup cortex and hippocampus was dissected from the brain and snap frozen, then lysed for 90 minutes at 4°C with lysis buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 5 mM EGTA, 1% Triton X-100, and 1 mM DTT and debris cleared by centrifugation (3,000 × g, 5 min). In vitro digestion of cortex or hippocampus lysate (100 ug) was performed with human erythrocyte Calpain 1 (Calbiochem/EMD Bioscience) at 1:200 and human recombinant Caspase 3 (BD Pharmingen) at 1:50 protease/substrate ratio, in a buffer containing 100 mM Tris-HCl (pH 7.4), 20 mM DTT, with or without 1 mM CaCl2, at room temperature for 30 min (Calpain 1) or 12 h (Caspase 3) (Liu et al., 2006). Protease reactions were stopped by the addition of 8× sample loading buffer containing 0.25 M Tris (pH 6.8), 0.2 M DTT, 8% SDS, 0.02% bromophenol blue, and 20% glycerol in distilled water. Twenty (20) ug of protein per lane was loaded and resolved by 4–20%SDS-PAGE and separated proteins were laterally transferred to polyvinylidene fluoride (PVDF) membranes. The following primary antibody was used: anti-Fodrin (Alpha II-Spectrin) (1:1000, BML-FG6090, ENZO Life Sciences, Inc., Farmingdale, NY).
4.6 Statistics
Data were analyzed using 1-way ANOVA treating each of the two groups, CCI and SHAM, as an independent group. Comparisons between the two groups were limited to samples from the same hemisphere. Thus, comparisons were made between ipsilateral hemispheres or between contralateral hemispheres from CCI and SHAM rats. We used Statview® software for all analyses. Post Hoc testing was done using Fisher’s Protected Least Significant Difference and we defined statistical significance as a p value less than 0.05.
HIGHLIGHTS.
Alpha II spectrin breakdown product (SBDP) data after developmental TBI are lacking
Rat pup SBDPs may identify developmental TBI death pathways and inform CSF studies
TBI elevated rat pup brain 145 SBDPs on day 1–3 and 120 SBDPs on day 1 post injury
TBI increased calpain more than caspase-mediated cell death in immature rat brain
ACKNOWLEDGEMENTS
Statement of Financial Support: Funding for this study was provided by the PCMC Foundation Integrative Grant PCMCF-ISA-KS01-2011-01, the CHRCDA (NIH K12HD001410) and from the Divisions of Neonatology and Pediatric Critical Care Medicine, Department of Pediatrics, University of Utah, Salt Lake City UT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No competing financial interests exist.
REFERENCES
- Aikman J, et al. Alpha-II-spectrin after controlled cortical impact in the immature rat brain. Dev Neurosci. 2006;28:457–465. doi: 10.1159/000094171. [DOI] [PubMed] [Google Scholar]
- Aitken ME, et al. Family burden after traumatic brain injury in children. Pediatrics. 2009;123:199–206. doi: 10.1542/peds.2008-0607. [DOI] [PubMed] [Google Scholar]
- Anderson V, et al. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005a;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Anderson V, et al. 10 years outcome from childhood traumatic brain injury. Int J Dev Neurosci. 2012;30:217–224. doi: 10.1016/j.ijdevneu.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Anderson VA, et al. Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. J Neurol Neurosurg Psychiatry. 2005b;76:401–408. doi: 10.1136/jnnp.2003.019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly PV, et al. Spatiotemporal evolution of apoptotic neurodegeneration following traumatic injury to the developing rat brain. Brain Res. 2006;1107:70–81. doi: 10.1016/j.brainres.2006.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer R, et al. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J Neurochem. 2000;75:1264–1273. doi: 10.1046/j.1471-4159.2000.0751264.x. [DOI] [PubMed] [Google Scholar]
- Bittigau P, et al. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann Neurol. 1999;45:724–735. doi: 10.1002/1531-8249(199906)45:6<724::aid-ana6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bittigau P, et al. Neuropathological and biochemical features of traumatic injury in the developing brain. Neurotox Res. 2003;5:475–490. doi: 10.1007/BF03033158. [DOI] [PubMed] [Google Scholar]
- Bittigau P, et al. Apoptotic neurodegeneration in the context of traumatic injury to the developing brain. Exp Toxicol Pathol. 2004;56:83–89. doi: 10.1016/j.etp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Brophy GM, et al. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J Neurotrauma. 2009;26:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardali S, Maugeri R. Detection of alphaII-spectrin and breakdown products in humans after severe traumatic brain injury. J Neurosurg Sci. 2006;50:25–31. [PubMed] [Google Scholar]
- Chen SF, et al. Salidroside improves behavioral and histological outcomes and reduces apoptosis via PI3K/Akt signaling after experimental traumatic brain injury. PLoS One. 2012;7:e45763. doi: 10.1371/journal.pone.0045763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17:1104–1114. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- Deng Y, et al. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Faden AI, et al. Neuroprotective effects of novel small peptides in vitro and after brain injury. Neuropharmacology. 2005;49:410–424. doi: 10.1016/j.neuropharm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Geddes DM, LaPlaca MC, Cargill RS., 2nd Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol. 2003;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- Giza CC, Maria NS, Hovda DA. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006;23:950–961. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbel GT, et al. Diffuse alterations in synaptic protein expression following focal traumatic brain injury in the immature rat. Childs Nerv Syst. 2007;23:1171–1179. doi: 10.1007/s00381-007-0345-2. [DOI] [PubMed] [Google Scholar]
- Hall ED, et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hinzman JM, et al. Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J Neurotrauma. 2012;29:1197–1208. doi: 10.1089/neu.2011.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Raghupathi R. Chronic cognitive deficits and long-term histopathological alterations following contusive brain injury in the immature rat. J Neurotrauma. 2007;24:1460–1474. doi: 10.1089/neu.2006.3787. [DOI] [PubMed] [Google Scholar]
- Jenkins LW, et al. Conventional and functional proteomics using large format two-dimensional gel electrophoresis 24 hours after controlled cortical impact in postnatal day 17 rats. J Neurotrauma. 2002;19:715–740. doi: 10.1089/08977150260139101. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, et al. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics. 2006;5:1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- Kochanek AR, et al. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev Neurosci. 2006;28:410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupina NC, et al. Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp Neurol. 2003;180:55–73. doi: 10.1016/s0014-4886(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Liu MC, et al. Comparing calpain- and caspase-3-mediated degradation patterns in traumatic brain injury by differential proteome analysis. Biochem J. 2006;394:715–725. doi: 10.1042/BJ20050905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- McGinn MJ, et al. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J Neuropathol Exp Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, et al. alphaII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CA, et al. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol. 2007;205:145–153. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Osteen CL, et al. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Pike BR, et al. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. Neuroreport. 1998;9:2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- Pike BR, et al. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- Pineda JA, et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Polster BM, et al. Postnatal brain development and neural cell differentiation modulate mitochondrial Bax and BH3 peptide-induced cytochrome c release. Cell Death Differ. 2003;10:365–370. doi: 10.1038/sj.cdd.4401158. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J Neurotrauma. 2000;17:927–938. doi: 10.1089/neu.2000.17.927. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Huh JW. Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. J Neurotrauma. 2007;24:1596–1608. doi: 10.1089/neu.2007.3790. [DOI] [PubMed] [Google Scholar]
- Rhinn H, et al. Housekeeping while brain's storming Validation of normalizing factors for gene expression studies in a murine model of traumatic brain injury. BMC Mol Biol. 2008;9:62. doi: 10.1186/1471-2199-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringger NC, et al. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J Neurotrauma. 2004;21:1443–1456. doi: 10.1089/neu.2004.21.1443. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Characterization of N-methyl-D-aspartate-evoked taurine release in the developing and adult mouse hippocampus. Amino Acids. 2003;24:213–221. doi: 10.1007/s00726-002-0310-z. [DOI] [PubMed] [Google Scholar]
- Schober ME, et al. Early and sustained increase in the expression of hippocampal IGF-1, but not EPO, in a developmental rodent model of traumatic brain injury. J Neurotrauma. 2010;27:2011–2020. doi: 10.1089/neu.2009.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Valiyaveettil M, et al. Cytoskeletal protein alpha-II spectrin degradation in the brain of repeated blast exposed mice. Brain Res. 2014;1549:32–41. doi: 10.1016/j.brainres.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol. 2001;169:416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- Yan XX, Jeromin A, Jeromin A. Spectrin Breakdown Products (SBDPs) as Potential Biomarkers for Neurodegenerative Diseases. Curr Transl Geriatr Exp Gerontol Rep. 2012;1:85–93. doi: 10.1007/s13670-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, et al. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16:514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- Yeates KO, et al. Long-term attention problems in children with traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44:574–584. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]
- Zhu C, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–176. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]