Abstract
Epidemiological studies have suggested that interleukin-17 (IL-17) polymorphisms are associated with cancer risk. However, the results of these studies are inconsistent. Therefore, we performed a meta-analysis to obtain a precise conclusion. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the association of the IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms with cancer risk. Publication bias and sensitivity analyses were performed to ensure the statistical power. Overall, 10 relevant case-control studies involving 4,516 cases and 5,645 controls were included. The pooled ORs with 95% CIs indicated that the IL-17A rs2275913G>A polymorphism was significantly associated with increased cancer risk (for A versus G: OR = 1.28, 95% CI: 1.16–1.41, P < 0.001, I 2 = 61.1%; for GA versus GG: OR = 1.12, 95% CI: 1.02–1.23, P = 0.015, I 2 = 27.8%; for AA versus GG: OR = 1.71, 95% CI: 1.38–2.41, P < 0.001, I 2 = 69.6%; for GA + AA versus GG: OR = 1.23, 95% CI: 1.13–1.34, P < 0.001, I 2 = 6.4%; for AA versus GG + GA: OR = 1.62, 95% CI: 1.27–2.07, P < 0.001, I 2 = 81.4%). Succeeding analysis of HWE and stratified analysis of gastric cancer and the Asian (and Chinese) population revealed similar results. The IL-17F rs763780T>C polymorphism was also significantly associated with gastric cancer development. Overall, the present meta-analysis suggests that IL-17 polymorphisms increase the risk of developing cancer, particularly gastric cancer, in the Asian (and Chinese) population.
1. Introduction
Cancer is one of the most common malignancies worldwide; it is the leading cause of death in economically developed countries and the second leading cause of death in developing countries [1]. Approximately 12.7 million new cases of cancer and 7.6 million cancer-related deaths were reported in 2008 [2]. Despite the efforts exerted by many researchers to elucidate the mechanism of carcinogenesis, this process remains unclear to date. Environmental factors, diet, lifestyle, and smoking and drinking habits have been implicated in the development of cancer [3, 4]. Various epidemiological studies have revealed that inflammation-associated factors, such as interleukin- (IL-) 1, IL-6, IL-10, and tumor necrosis factor-α, are associated with cancer tumorigenesis [5].
Interleukin-17 (IL-17) is a proinflammatory cytokine that serves important functions in inflammation, autoimmune disorders, and cancer [6]. The IL-17 cytokine family consists of six members (IL-17A to IL-17F) and five receptors (IL-17RA to IL-17RD and SEF) [7, 8]. These cytokines are primarily produced from a subset of CD4+ effector cells known as Th17 cells [9, 10]. Clinical studies have shown increased IL-17 expression in malignant tumors [11–14].
Single nucleotide polymorphisms (SNPs) can alter gene functions and protein expression, which influence cell proliferation and increase cancer risk. The IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms are the most common loci associated with IL-17 activity and cancer risk. In 2009, Shibata et al. [15] conducted the first study and reported a positive relationship between gastric cancer and the IL-17A rs2275913G>A polymorphism in a Japanese population. But no significant association was found between gastric cancer and polymorphisms of IL-17F rs763780 T>C. Many epidemiological studies have focused on the association of the IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms with cancer risk. However, the results of these studies are inconsistent. Therefore, we performed a meta-analysis to clarify the possible association of the IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms with cancer risk.
2. Materials and Methods
2.1. Search Strategy
The PubMed, Embase, and Chinese National Knowledge Infrastructure databases were searched using the terms “cancer,” “tumor,” “interleukin-17,” “IL-17,” and “polymorphism,” (last search was updated on January 20, 2014). The “Related Articles” option was also used in each research article to find potential relevant studies on the same topic. Only studies published in English or Chinese were included. The inclusion criteria in this meta-analysis were as follows: (a) researches that focused on population, (b) studies that evaluated the association of the IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms with cancer risk, (c) case-controls studies, and (d) studies that contain available genotype frequency to estimate the odds ratio (OR) and 95% confidence intervals (CIs). The largest or the most recent publication was selected when some data were the same or overlapped.
2.2. Data Extraction
Two investigators (Yu-Ming Niu and Hua Yuan) independently extracted the following information from each eligible publication: first author's name, publication year, country of origin, ethnicity of the individuals involved (categorized as Asian or Caucasian), source of controls, number of cases and controls, number of genotypes cases and controls, Hardy-Weinberg equilibrium (HWE) and minor allele frequency (MAF), and cancer category. Discrepancies were adjudicated by another author until consensus was achieved.
2.3. Statistical Analysis
The strength of the association of the IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms with cancer risk was assessed by calculating crude ORs with 95% CIs. Pooled ORs were conducted for minor allele versus major allele with five models. Stratified analyses were performed by ethnicity, study design, and cancer category. Heterogeneity was calculated based on the I 2 statistic with low, moderate, and high I 2 values of 25%, 50%, and 75%, respectively [16, 17]. When I 2 ≤ 50% (which indicated a lack of heterogeneity), the OR estimation of each model was calculated by using the fixed-effects model (Mantel-Haenszel method); otherwise, the random-effects model (DerSimonian and Laird method) was used. We generated forest plots sorted by publication year. Potential publication bias was estimated using Egger's linear regression test with funnel plot [18]. Sensitivity analyses were assessed by deleting each study to reflect the influence of individual datasets on the pooled ORs [19]. Statistical analysis was performed using STATA version 11.0 (Stata Corporation, College Station, TX, USA) with two-sided P values. P < 0.05 was considered significant.
3. Results
3.1. Study Characteristics
A flow chart showing the study selection is presented in Figure 1. A total of 185 relevant studies were found with the research words and manual research. After careful review, 10 published case-control studies involving 4,516 cases and 5,645 controls met our inclusion criteria [15, 20–28]. We found 10 and 7 eligible studies with adequate genotype and research subjects according to IL-17A rs2275913G>A and IL-17Frs763780T>C polymorphism. All characteristics of the selected studies are summarized in Table 1. Nine studies involved Asian populations (seven involved the Chinese population), and one study involved a Caucasian population. Diverse genotyping methods were used, including polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) [23, 27], TaqMan [21, 24, 28], polymerase chain reaction-sequence specific primers (PCR-SSCP) [15, 25], MassARRAY [20, 22], and SNaPshot SNP assay [26] methods in eligible publications. The genotypic distribution of controls in only two and three studies deviated from HWE in the IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms, respectively.
Figure 1.

Flow chart of study selection.
Table 1.
Characteristics of case-control studies on IL-17A rs2275913G>A and IL-17F rs763780 T>C polymorphisms and cancer risk included in the meta-analysis.
| First author | Year | Country | Ethnicity | Source of controls | Case | Control | Genotype distribution | P for HWEa | MAF | Genotyping method | Cancer category | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||||||||||
| G/G | G/A | A/A | G/G | G/A | A/A | |||||||||||
| Zhang [20] | 2014 | China | Asian | Population controls | 260 | 512 | 110 | 102 | 48 | 258 | 187 | 67 | <0.01 | 0.31 | MassARRAY | Gastric cancer |
| Zhou [21] | 2013 | China | Asian | Hospital controls | 301 | 446 | 79 | 154 | 68 | 164 | 204 | 78 | 0.29 | 0.40 | TaqMan | Bladder cancer |
| Zhu [22] | 2014 | China | Asian | Hospital controls | 293 | 550 | 126 | 122 | 45 | 273 | 216 | 61 | 0.07 | 0.31 | MassARRAY | Gastric cancer |
| Rafiei [23] | 2013 | Iran | Caucasian | Healthy controls | 161 | 171 | 56 | 61 | 44 | 78 | 72 | 21 | 0.49 | 0.33 | PCR-RFLP | Gastric cancer |
| Quan [24] | 2012 | China | Asian | Hospital controls | 311 | 463 | 93 | 142 | 76 | 168 | 215 | 80 | 0.43 | 0.40 | TaqMan | Cervical cancer |
| Arisawa [25] | 2012 | Japan | Asian | Hospital controls | 333 | 583 | 112 | 137 | 84 | 218 | 293 | 72 | 0.08 | 0.37 | PCR-SSCP | Gastric cancer |
| Wang [26] | 2012 | China | Asian | Population controls | 491 | 501 | 165 | 234 | 92 | 198 | 245 | 58 | 0.17 | 0.36 | SNaPshot SNP assay | Breast cancer |
| Wu [27] | 2010 | China | Asian | Population controls | 945 | 768 | 210 | 485 | 250 | 193 | 371 | 204 | 0.35 | 0.51 | PCR-RFLP | Gastric cancer |
| Chen [28] | 2010 | China | Asian | Population controls | 1042 | 1090 | 300 | 522 | 220 | 325 | 541 | 224 | 0.97 | 0.45 | TaqMan | Gastric cancer |
| Shibata [15] | 2009 | Japan | Asian | Hospital controls | 287 | 523 | 94 | 124 | 69 | 175 | 299 | 49 | <0.01 | 0.38 | PCR-SSCP | Gastric cancer |
|
| ||||||||||||||||
| T/T | T/C | C/C | T/T | T/C | C/C | |||||||||||
|
| ||||||||||||||||
| Zhang [20] | 2014 | China | Asian | Population controls | 260 | 512 | 209 | 30 | 21 | 429 | 53 | 30 | <0.01 | 0.11 | MassARRAY | Gastric cancer |
| Zhou [21] | 2013 | China | Asian | Hospital controls | 301 | 446 | 240 | 57 | 4 | 317 | 124 | 5 | 0.06 | 0.15 | TaqMan | Bladder cancer |
| Zhu [22] | 2014 | China | Asian | Hospital controls | 293 | 550 | 241 | 35 | 17 | 463 | 58 | 29 | <0.01 | 0.11 | MassARRAY | Gastric cancer |
| Quan [24] | 2012 | China | Asian | Hospital controls | 311 | 462 | 222 | 85 | 4 | 332 | 126 | 5 | 0.06 | 0.15 | TaqMan | Cervical cancer |
| Wang [26] | 2012 | China | Asian | Population controls | 491 | 502 | 382 | 103 | 6 | 396 | 99 | 7 | 0.77 | 0.11 | SNaPshot SNP assay | Breast cancer |
| Wu [27] | 2010 | China | Asian | Population controls | 927 | 777 | 540 | 332 | 55 | 527 | 214 | 36 | 0.02 | 0.18 | PCR-RFLP | Gastric cancer |
| Shibata [15] | 2009 | Japan | Asian | Hospital controls | 280 | 523 | 221 | 55 | 4 | 419 | 100 | 4 | 0.46 | 0.10 | PCR-SSCP | Gastric cancer |
aHWE in control.
MAF: minor allele frequency in control group.
3.2. Quantitative Synthesis
Table 2 shows the results of this meta-analysis and the heterogeneity test. The IL-17A rs2275913G>A polymorphism showed significant associations with cancer risk in all populations (for A versus G: OR = 1.28, 95% CI: 1.16–1.41, P < 0.001, I 2 = 61.1%; for GA versus GG: OR = 1.12, 95% CI: 1.02–1.23, P = 0.015, I 2 = 27.8%; for AA versus GG: OR = 1.71, 95% CI: 1.38–2.41, P < 0.001, I 2 = 69.6%; for GA + AA versus GG: OR = 1.23, 95% CI: 1.13–1.34, P < 0.001, I 2 = 6.4% (Figure 2); and for AA versus GG + GA: OR = 1.62, 95% CI: 1.27–2.07, P < 0.001, I 2 = 81.4%). The succeeding stratified analysis according to HWE, ethnicity, and study design subgroup also presented that the IL-17A rs2275913G>A polymorphism may be a strong risk factor in the development of cancer, especially gastric cancer, in the Chinese population.
Table 2.
Summary of ORs and 95% CI of IL-17A rs2275913G>A and IL-17F rs763780T>C polymorphisms and cancer risk.
| N ∗ | A versus G | GA versus GG | AA versus GG | GA + AA versus GG | AA versus GG + GA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | I 2 (%)a | OR | 95% CI | P | I 2 (%)a | OR | 95% CI | P | I 2 (%)a | OR | 95% CI | P | I 2 (%)a | OR | 95% CI | P | I 2 (%)a | ||
| Total | 10 | 1.28 | 1.16–1.41 | <0.001 | 61.1 | 1.12 | 1.02–1.23 | 0.015 | 27.8 | 1.71 | 1.38–2.14 | <0.001 | 69.6 | 1.23 | 1.13–1.34 | <0.001 | 6.4 | 1.62 | 1.27–2.07 | <0.001 | 81.4 |
| HWE | 8 | 1.26 | 1.13–1.41 | <0.001 | 66.4 | 1.15 | 1.04–1.27 | 0.008 | 0 | 1.63 | 1.29–2.08 | <0.001 | 70 | 1.23 | 1.12–1.36 | <0.001 | 9.7 | 1.51 | 1.18–1.93 | 0.001 | 78.4 |
| Ethnicity | |||||||||||||||||||||
| Asian | 9 | 1.25 | 1.14–1.37 | <0.001 | 56.5 | 1.12 | 1.02–1.23 | 0.019 | 35.6 | 1.65 | 1.32–2.05 | <0.001 | 68.6 | 1.22 | 1.12–1.33 | <0.001 | 4.4 | 1.55 | 1.21–1.99 | 0.001 | 81.6 |
| China | 7 | 1.21 | 1.10–1.34 | <0.001 | 56.2 | 1.19 | 1.07–1.32 | 0.001 | 0 | 1.46 | 1.20–1.79 | <0.001 | 54.3 | 1.24 | 1.13–1.37 | <0.001 | 14.9 | 1.30 | 1.09–1.56 | 0.004 | 56.6 |
| Design | |||||||||||||||||||||
| PB | 4 | 1.16 | 1.01–1.32 | 0.031 | 63.3 | 1.14 | 1.01–1.29 | 0.041 | 0 | 1.34 | 1.02–1.76 | 0.033 | 65 | 1.18 | 1.05–1.32 | 0.006 | 1.1 | 1.23 | 0.96–1.59 | 0.108 | 69.7 |
| HB | 5 | 1.35 | 1.23–1.48 | <0.001 | 0 | 1.10 | 0.87–1.39 | 0.447 | 63.1 | 1.97 | 1.64–2.37 | <0.001 | 0 | 1.28 | 1.12–1.46 | <0.001 | 11.8 | 1.87 | 1.38–2.52 | <0.001 | 69.2 |
| Location | |||||||||||||||||||||
| Gastric | 7 | 1.26 | 1.11–1.44 | <0.001 | 70 | 1.07 | 0.96–1.19 | 0.202 | 24.2 | 1.70 | 1.26–2.30 | 0.001 | 77.8 | 1.18 | 1.06–1.30 | 0.001 | 0 | 1.67 | 1.18–2.36 | 0.004 | 87 |
| Others | 3 | 1.33 | 1.19–1.49 | <0.001 | 0 | 1.27 | 1.05–1.53 | 0.013 | 6.6 | 1.81 | 1.43–2.29 | <0.001 | 0 | 1.39 | 1.18–1.65 | <0.001 | 0 | 1.56 | 1.27–1.91 | <0.001 | 0 |
|
| |||||||||||||||||||||
| C versus T | TC versus TT | CC versus TT | TC + CC versus TT | CC versus TT + TC | |||||||||||||||||
|
| |||||||||||||||||||||
| Total | 7 | 1.09 | 0.91–1.30 | 0.347 | 64.6 | 1.06 | 0.84–1.34 | 0.629 | 70 | 1.33 | 1.02–1.75 | 0.038 | 0 | 1.08 | 0.87–1.35 | 0.486 | 70.3 | 1.26 | 0.96–1.65 | 0.098 | 0 |
| HWE | 4 | 0.95 | 0.81–1.10 | 0.516 | 46.4 | 0.92 | 0.71–1.19 | 0.650 | 57.2 | 1.15 | 0.61–2.17 | 0.660 | 0 | 0.93 | 0.72–1.19 | 0.557 | 55.5 | 1.18 | 0.62–2.21 | 0.616 | 0 |
| Ethnicity | |||||||||||||||||||||
| China | 6 | 1.08 | 0.88–1.33 | 0.439 | 70.4 | 1.06 | 0.81–1.39 | 0.676 | 74.7 | 1.32 | 1.00–1.74 | 0.053 | 0 | 1.08 | 0.84–1.39 | 0.555 | 75.1 | 1.24 | 0.94–1.63 | 0.130 | 0 |
| Design | |||||||||||||||||||||
| PB | 3 | 1.29 | 1.13–1.41 | <0.001 | 32.4 | 1.34 | 1.14–1.57 | <0.001 | 43.4 | 1.40 | 1.01–1.96 | 0.045 | 0 | 1.34 | 1.05–1.57 | <0.001 | 45.8 | 1.29 | 0.92–1.79 | 0.136 | 0 |
| HB | 4 | 0.97 | 0.77–1.21 | 0.550 | 52.2 | 0.92 | 0.69–1.22 | 0.554 | 57.3 | 1.20 | 0.74–1.94 | 0.466 | 0 | 0.84 | 0.72–1.23 | 0.643 | 58.1 | 1.20 | 0.74–1.94 | 0.456 | 0 |
| Location | |||||||||||||||||||||
| Gastric | 4 | 1.29 | 1.14–1.46 | <0.001 | 0 | 1.33 | 1.13–1.55 | <0.001 | 21.6 | 1.40 | 1.04–1.88 | 0.026 | 0 | 1.34 | 1.16–1.55 | <0.001 | 16 | 1.30 | 0.97–1.74 | 0.082 | 0 |
| Others | 3 | 0.91 | 0.70–1.18 | 0.472 | 57.4 | 0.88 | 0.62–1.24 | 0.460 | 69.2 | 1.02 | 0.50–2.08 | 0.962 | 0 | 0.89 | 0.64–1.22 | 0.461 | 66.7 | 1.04 | 0.51–2.13 | 0.905 | 0 |
*Numbers of comparisons.
aTest for heterogeneity.
Figure 2.

OR and 95% CIs for the association between IL-17A rs2275913G>A polymorphism with cancer risk for the GA + AA versus GG model.
Statistical analysis also indicated that the IL-17F rs763780T>C polymorphisms were significantly associated with cancer risk, particularly gastric cancer (for C versus T: OR = 1.29, 95% CI: 1.14–1.46, P < 0.001, I 2 = 0%; for TC versus TT: OR = 1.33, 95% CI: 1.13–1.55, P < 0.001, I 2 = 21.6%; for CC versus TT: OR = 1.40, 95% CI: 1.04–1.88, P = 0.026, I 2 = 0%; for TC + CC versus TT: OR = 1.34, 95% CI: 1.16–1.55, P < 0.001, I 2 = 16% (Figure 3)).
Figure 3.

OR and 95% CIs for the association between IL-17F rs763780T>C polymorphisms and cancer risk in TC + CC versus TT model.
3.3. Sensitivity Analysis
A single study involved in the meta-analysis was deleted each time to reflect the influence of the individual data set on the pooled ORs, and the corresponding pooled ORs were not qualitatively altered. This indicated that the results about the association between IL-17 gene polymorphisms and cancer risk were statistically robust (Figures 4 and 5).
Figure 4.
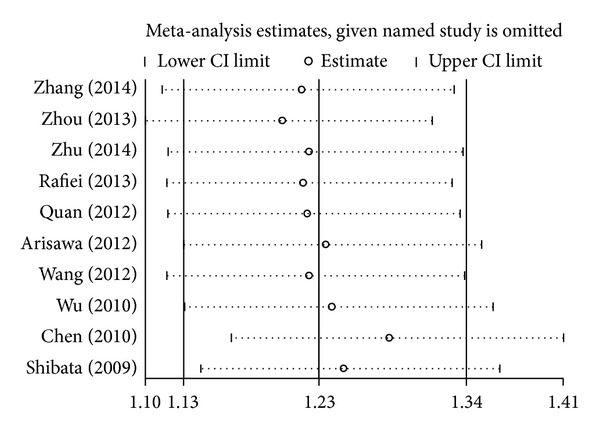
Sensitivity analysis through deleting each study to reflect the influence of the individual dataset on the pooled ORs in GA + AA versus GG model of IL-17A rs2275913G>A polymorphism.
Figure 5.
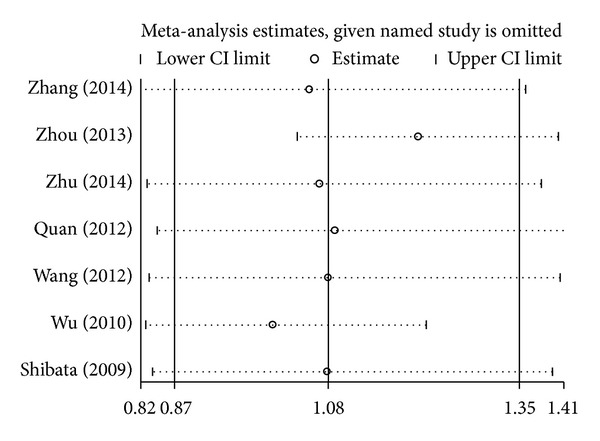
Sensitivity analysis through deleting each study to reflect the influence of the individual dataset on the pooled ORs in TC + CC versus TT model of IL-17F rs763780T>C polymorphism.
3.4. Publication Bias
Funnel plot and Egger's test were performed to estimate the publication bias of literature. Publication bias was detected in the meta-analyses on the allele contrast, homozygote (AA versus GG), dominant, and recessive models of the IL-17A rs2275913G>A polymorphism (Figure 6 for GA + AA versus GG model), except for the GA versus GG model (P = 0.652). Stratified analyses were conducted only with the HWE and Chinese population, but the results were not substantially different. For the IL-17F rs763780T>C polymorphism, the funnel plots did not show any asymmetrical evidence in all genetic models (Figure 7). The result was further supported by analysis using Egger's tests (P = 0.102 for C versus T; P = 0.185 for TC versus TT; P = 0.382 for CC versus TT; P = 0.114 for TC + CC versus TT (Figure 7); P = 0.792 for CC versus TT + TC).
Figure 6.
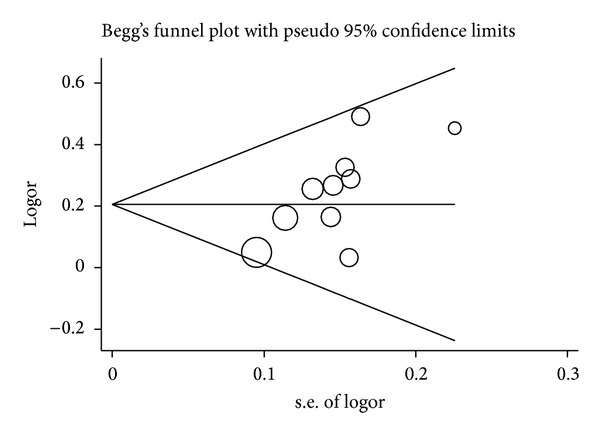
Funnel plot analysis to detect publication bias for GA + AA versus GG model of IL-17A rs2275913G>A polymorphism. Each point represents a separate study.
Figure 7.
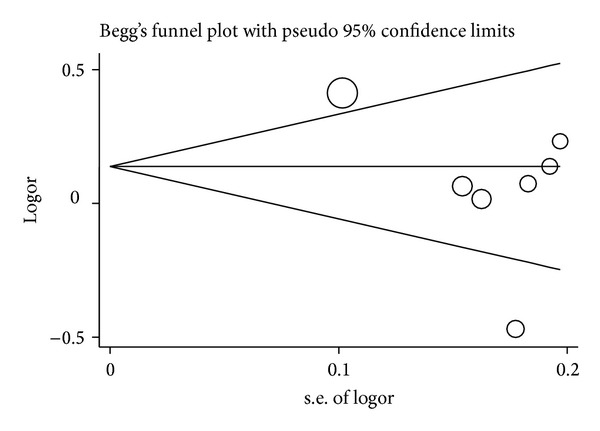
Funnel plot analysis to detect publication bias for TC + CC versus TT model of IL-17F rs763780T>C polymorphism. Each point represents a separate study.
4. Discussion
Carcinogenesis is a multistep process that involves numerous factors, such as smoking, drinking, xenobiotics infections, nutrition deficiency, and host genetic factor. Cancer-related inflammation factors have been recently confirmed to increase the risk of developing malignant tumors. IL-17 is a relatively novel cytokine family that is connected with adaptive and innate immune systems. IL-17A and IL-17F are members of the IL-17 cytokine family that are responsible for the pathogenic activity of IL-17 cells, the lineage of CD4+ effector cells, and multiple proinflammatory mediators [29].
Genetic polymorphisms of the IL-17A and IL-17F cytokines could change the function and expression of cytokines, which influence the activity of ILs [12, 30, 31]. Several studies have revealed that IL-17A and IL-17F polymorphisms are associated with gastric cancer, breast cancer, and so on. However, the results of these studies are inconsistent.
In 2009, Shibata et al. [15] were the first to report that the AA homozygote was significantly correlated with the development of gastric cancer compared with the common homozygous genotype (GG) in a Japanese population (OR = 3.02, 95% CI: 1.86–4.91). One year later, Chen et al. [28] also found a positive relationship between gastric cancer and the IL-17A rs2275913G>A polymorphism in a Chinese Han population with a drinking habit (for A versus G: OR = 1.37, 95% CI: 1.07–1.76). Similar results were reported by Arisawa et al. [25], Rafiei et al. [23], Zhang et al. [20], and Zhuet al. [22] in gastric cancer. Furthermore, the mutation of IL-17A rs2275913G>A locus was also demonstrated as tumorigenic for bladder cancer [21], breast cancer [26], and cervical cancer [24]. However, another article detected no significant association between the IL-17A rs2275913G>A polymorphism and gastric cancer risk [27].
Regarding the IL-17F rs763780T>C polymorphism, Wu et al. [27] found that the CT and CC genotypes are associated with an increased risk of gastric cancer compared with the TT genotype (OR = 1.51, 95% CI: 1.22–1.87 for CT; OR = 1.61, 95% CI: 1.03–2.51 for CC). By contrast, Zhou et al. [21] showed that bladder cancer patients have significantly higher frequencies of T allele than controls. This result indicates that this T allele is significantly associated with bladder cancer (OR = 1.46, 95% CI: 1.07–2.00). Furthermore, other researches did not find any significant association between the IL-17F rs763780T>C polymorphism and cancer risk [15, 20, 22, 24, 26].
To the best of our knowledge, this meta-analysis is the first to determine the association of IL-17 polymorphisms with cancer risk. This study focused on two common IL-17 polymorphisms, namely, IL-17A rs2275913G>A (10 studies with 4,516 cases and 5,645 controls) and IL-17F rs763780T>C (7 studies with 2,863 cases and 3,773 controls). Significant associations were found between the IL-17A rs2275913G>A polymorphism and cancer risk in all five genotype models of total populations. Besides, we also detected some association between the IL-17F rs763780T>C polymorphism and the risk of Asians (Chinese), population-based and hospital-based controls, and gastric cancer or other cancers in the subgroup analysis by HWE publications, ethnicity, control design, and cancer category. Interestingly, nine researches focused on Asian population; the results of our meta-analysis demonstrated that the IL-17A rs2275913G>A polymorphism may be a stranger canner risk for Asian ethnicity (including Chinese). Moreover, the category of control design did not influence the results; not only the population-based but also the hospital-based controls all showed that significant association existed between IL-17A rs2275913G>A polymorphism and cancer risk. In all selected publications, seven studies focused on gastric cancer and the results also indicated that IL-17A rs2275913G>A polymorphism plays an important role during the development of gastric cancer. For IL-17F rs763780T>C polymorphism, all selected studies were conducted in Asian population and significant association only was found in codominant model (CC versus TT) in total population. Furthermore, subgroup analyses revealed a significantly increased risk of gastric cancer with IL-17F rs763780T>C polymorphism in four models. Another stratified analysis of population-based control also drew a consistent conclusion.
This meta-analysis has several limitations in result interpretation. First, each gene only has a moderate effect on cancer development. A combination of relative genotypes may be a higher risk factor than a single locus genotype. Linkage disequilibrium and haplotype analyses of the two polymorphisms were not conducted because of the lack of original data on the individual genotypes from the included studies. Second, certain publication biases existed until subgroup analyses were conducted. These deviations would influence the correctness and reliability of the results. Third, these results were based on unadjusted estimates, and the evaluations were limited without the effects of gene-gene and gene-environment interactions. Finally, most of the included studies had been conducted on Asians but not on Caucasians and Africans, and the association between ethnicity variation and cancer risk could not be explored deeply.
In conclusion, despite these limitations, the present meta-analysis demonstrates that the IL-17A rs2275913G>A polymorphism is associated with cancer development. Furthermore, the IL-17F rs763780T>C polymorphism may be a potential risk factor in the development of gastric cancer. In the future, large-scale, case-control, and well-designed studies must be conducted to validate the findings of our meta-analysis and to comprehensively understand the potential gene-gene and gene-environment interactions between IL-17 polymorphisms and cancer risk.
Acknowledgments
The authors gratefully acknowledge the support of the subjects who participated in this study. This study was partly supported by the Foundation of Ministry of Education of Hubei Province (D20142102) and Foundation of Hubei University of Medicine (2013GPY07) and Taihe Hospital (EBM2013006 and EBM2013031).
Conflict of Interests
The authors declare that there is no conflict of interests.
Authors' Contribution
Yu-Ming Niu and Hua Yuan contributed equally to this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Doll R. The epidemiology of cancer. Cancer. 1980;45(10):2475–2485. doi: 10.1002/1097-0142(19800515)45:10<2475::aid-cncr2820451004>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Marron M, Boffetta P, Zhang Z, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. International Journal of Epidemiology. 2010;39(1):182–196. doi: 10.1093/ije/dyp291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 6.Song X, Qian Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cellular Signalling. 2013;25(12):2335–2347. doi: 10.1016/j.cellsig.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang S. IL-17 cytokine family. Journal of Allergy and Clinical Immunology. 2004;114(6):1265–1273. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine and Growth Factor Reviews. 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 11.Faghih Z, Rezaeifard S, Safaei A, Ghaderi A, Erfani N. IL-17 and IL-4 producing CD8+ T cells in tumor draining lymph nodes of breast cancer patients: positive association with tumor progression. Iranian Journal of Immunology. 2013;10(4):193–204. [PubMed] [Google Scholar]
- 12.Straus DS. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Molecular Cancer. 2013;12(1) article 78 doi: 10.1186/1476-4598-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan C, Huang X, Lin S, et al. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell and Tissue Research. 2013;352(2):351–359. doi: 10.1007/s00441-013-1567-0. [DOI] [PubMed] [Google Scholar]
- 14.Doroudchi M, Saidi M, Malekzadeh M, Golmoghaddam H, Khezri A, Ghaderi A. Elevated IL-17A levels in early stages of bladder cancer regardless of smoking status. Future Oncology. 2013;9(2):295–304. doi: 10.2217/fon.12.180. [DOI] [PubMed] [Google Scholar]
- 15.Shibata T, Tahara T, Hirata I, Arisawa T. Genetic polymorphism of interleukin-17A and -17F genes in gastric carcinogenesis. Human Immunology. 2009;70(7):547–551. doi: 10.1016/j.humimm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos-Corpas D, Santiago JC. Single large study or meta-analysis parameters: choosing the most appropriate tool for down syndrome screening in the first trimester. Prenatal Diagnosis. 2006;26(12):1124–1130. doi: 10.1002/pd.1568. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zheng L, Sun Y, Zhang X. Analysis of the association of interleukin-17 gene polymorphisms with gastric cancer risk and interaction with Helicobacter pylori infection in a Chinese population. Tumor Biology. 2014;35(2):1575–1580. doi: 10.1007/s13277-013-1217-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou B, Zhang P, Wang Y, et al. Interleukin-17 gene polymorphisms are associated with bladder cancer in a Chinese Han population. Molecular Carcinogenesis. 2013;52(11):871–878. doi: 10.1002/mc.21928. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Q, Wang Y, Chen Y, Zhang X, Zhang X. Effect of interleukin-17A and interleukin-17F gene polymorphisms on the risk of gastric cancer in a Chinese population. Gene. 2014;537(2):328–332. doi: 10.1016/j.gene.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Rafiei A, Hosseini V, Janbabai G, et al. Polymorphism in the interleukin-17A promoter contributes to gastric cancer. World Journal of Gastroenterology. 2013;19(34):5693–5699. doi: 10.3748/wjg.v19.i34.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan Y, Zhou B, Wang Y, et al. Association between IL17 polymorphisms and risk of cervical cancer in Chinese women. Clinical and Developmental Immunology. 2012;2012:6 pages. doi: 10.1155/2012/258293.258293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arisawa T, Tahara T, Shiroeda H, et al. Genetic polymorphisms of IL17A and pri-microRNA-938, targeting IL17A 3'-UTR, influence susceptibility to gastric cancer. Human Immunology. 2012;73(7):747–752. doi: 10.1016/j.humimm.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Jiang Y, Zhang Y, et al. Association analysis of IL-17A and IL-17F polymorphisms in Chinese han women with breast cancer. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0034400.e34400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Zeng Z, Chen B, et al. Association between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancer. International Journal of Cancer. 2010;127(1):86–92. doi: 10.1002/ijc.25027. [DOI] [PubMed] [Google Scholar]
- 28.Chen J. Association study of polymorphisms in IL23R and IL17A genes with the susceptibility of gastric cancer [M.S. thesis] Nanjing, China: Nanjing Medical University; 2010. [Google Scholar]
- 29.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. The Journal of Immunology. 2005;175(6):3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 30.Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. The Journal of Experimental Medicine. 2013;210(13):2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraiva AM, Alves e Silva MRM, Correia Silva JDF, et al. Evaluation of IL17A expression and of IL17A, IL17F and IL23R gene polymorphisms in Brazilian individuals with periodontitis. Human Immunology. 2013;74(2):207–214. doi: 10.1016/j.humimm.2012.10.026. [DOI] [PubMed] [Google Scholar]


