Abstract
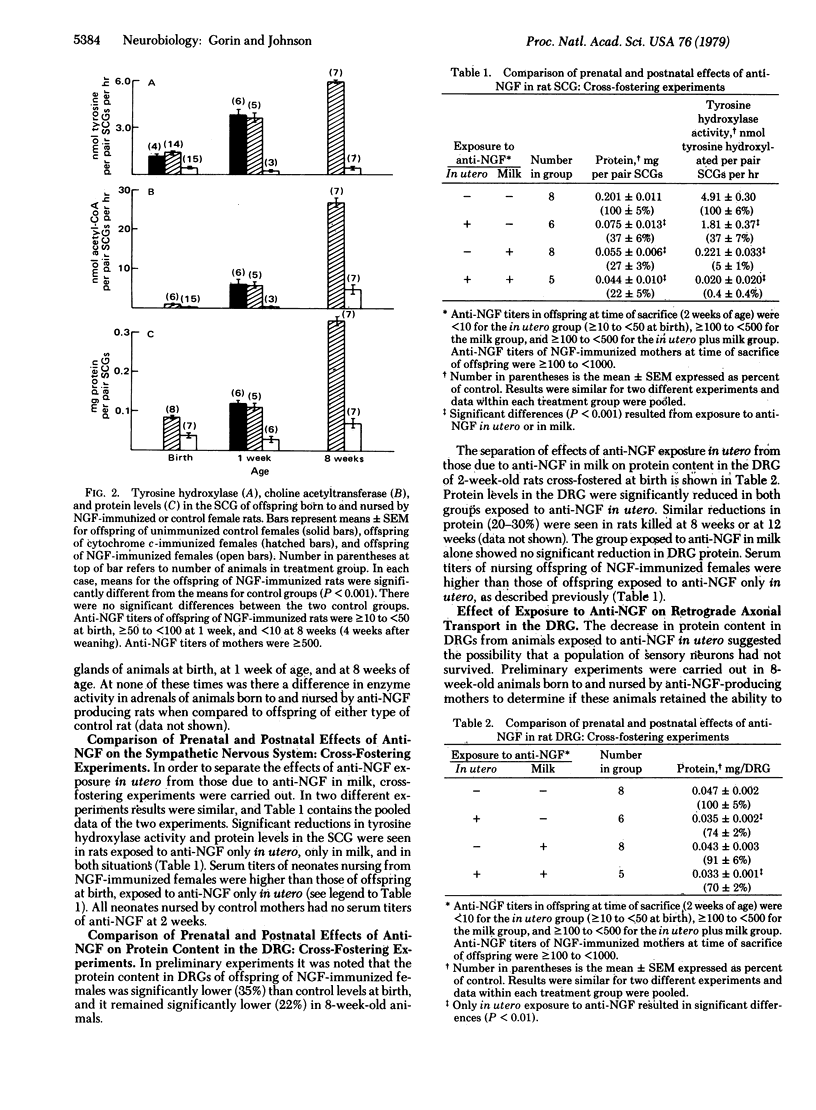
An experimental autoimmune model of nerve growth factor (NGF) deprivation has been used to assess the role of NGF in the development of various cell types in the nervous system. Adult rats immunized with 2.5S mouse NGF in complete Freund's adjuvant produced antibodies that crossreacted with their own NGF and that were transferred in utero to the fetus and in milk to the neonate. Cross-fostering experiments were carried out to separate the effects of exposure to anti-NGF in utero from those due to exposure through the milk. Anti-NGF transferred in utero and in milk resulted in the destruction of peripheral sympathetic neurons assessed by morphological methods (light microscopy) and biochemical methods (tyrosine hydroxylase activity, choline acetyltransferase activity, and protein content). No effects were observed on the adrenal medulla. Offspring of NGF-immunized females exposed to anti-NGF in utero had a decreased protein content in the dorsal root ganglia and were unable to transport 125I-labeled NGF injected in the forepaw to the dorsal root ganglia. These results suggest that a subpopulation of sensory neurons is dependent on NGF for survival during some period of fetal development. This model offers the potential for determining the degree and time of dependence of various cell types on NGF.
Keywords: immunosympathectomy, neuronal development, trophic factor
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Peyronnard J. M., Terry L. C., Romine J. S., Bray G. M. Neonatal neuronal loss in rat superior cervical ganglia: retrograde effects on developing preganglionic axons and Schwann cells. J Neurocytol. 1976 Apr;5(2):137–155. doi: 10.1007/BF01181653. [DOI] [PubMed] [Google Scholar]
- Aloe L., Levi-Montalcini R. Nerve growth factor-induced transformation of immature chromaffin cells in vivo into sympathetic neurons: effect of antiserum to nerve growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1246–1250. doi: 10.1073/pnas.76.3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. The role of post-synaptic neurones in the biochemical maturation of presynaptic cholinergic nerve terminals in a mouse sympathetic ganglion. J Physiol. 1972 Feb;221(1):149–159. doi: 10.1113/jphysiol.1972.sp009745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Black I. B. Regulation of autonomic development. Annu Rev Neurosci. 1978;1:183–214. doi: 10.1146/annurev.ne.01.030178.001151. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham P., Raiborn C., Varon S. Replacement of nerve-growth factor by ganglionic non-neuronal cells for the survival in vitro of dissociated ganglionic neurons. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3556–3560. doi: 10.1073/pnas.69.12.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin M. D., Boyer D. M., Black I. B. Embryologic development of a mouse sympathetic ganglion in vivo and in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3438–3442. doi: 10.1073/pnas.74.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton E. L. Tissue culture assay of nerve growth factor and of the specific antiserum. Exp Cell Res. 1970 Mar;59(3):383–392. doi: 10.1016/0014-4827(70)90645-2. [DOI] [PubMed] [Google Scholar]
- Harper G. P., Pearce F. L., Vernon C. A. Production of nerve growth factor by the mouse adrenal medulla. Nature. 1976 May 20;261(5557):251–253. doi: 10.1038/261251a0. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta-nerve growth factor receptor in development. J Cell Biol. 1975 Oct;67(1):118–125. doi: 10.1083/jcb.67.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingman G. I. In utero immunosympathectomy of mice. Int J Neuropharmacol. 1966 Mar;5(2):163–170. doi: 10.1016/0028-3908(66)90019-0. [DOI] [PubMed] [Google Scholar]
- Klingman G. I., Klingman J. D. Catecholamines in peripheral tissues of mice and cell counts of sympathetic ganglia after the prenatal and postnatal administration of the nerve growth factor antiserum. Int J Neuropharmacol. 1967 Nov;6(6):501–508. doi: 10.1016/0028-3908(67)90050-0. [DOI] [PubMed] [Google Scholar]
- Konkol R. J., Mailman R. B., Bendeich E. G., Garrison A. M., Mueller R. A., Breese G. R. Evaluation of the effects of nerve growth factor and anti-nerve growth factor on the development of central catecholamine-containing neurons. Brain Res. 1978 Apr 14;144(2):277–285. doi: 10.1016/0006-8993(78)90154-3. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., ANGELETTI P. U. Growth control of the sympathetic system by a specific protein factor. Q Rev Biol. 1961 Jun;36:99–108. doi: 10.1086/403331. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., COHEN S. Effects of the extract of the mouse submaxillary salivary glands on the sympathetic system of mammals. Ann N Y Acad Sci. 1960 Mar 29;85:324–341. doi: 10.1111/j.1749-6632.1960.tb49963.x. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., MEYER H., HAMBURGER V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 1954 Jan;14(1):49–57. [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. EXCESSIVE GROWTH OF THE SYMPATHETIC GANGLIA EVOKED BY A PROTEIN ISOLATED FROM MOUSE SALIVARY GLANDS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):373–384. doi: 10.1073/pnas.46.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley W. C., Server A. C., Ishii D. N., Riopelle R. J., Shooter E. M. Nerve growth factor (first of three parts). N Engl J Med. 1977 Nov 17;297(20):1096–1104. doi: 10.1056/NEJM197711172972005. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Server A. C., Ishii D. N., Riopelle R. J., Shooter E. M. Nerve growth factor (second of three parts). N Engl J Med. 1977 Nov 24;297(21):1149–1158. doi: 10.1056/NEJM197711242972105. [DOI] [PubMed] [Google Scholar]
- Phillipson O. T., Sandler M. The effect of hydrocortisone and adrenocorticotrophic hormone on monoamine oxidase and tyrosine hydroxylase in explant cultures of embryonic chick sympathetic ganglia. Brain Res. 1975 Jun 13;90(2):283–296. doi: 10.1016/0006-8993(75)90308-x. [DOI] [PubMed] [Google Scholar]
- Schrier B. K., Shuster L. A simplified radiochemical assay for choline acetyltransferase. J Neurochem. 1967 Oct;14(10):977–985. doi: 10.1111/j.1471-4159.1967.tb09509.x. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975 May 16;89(1):1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Kettler R., Saner A. Time course of the development of enzymes involved in the synthesis of norepinephrine in the superior cervical ganglion of the rat from birth to adult life. Brain Res. 1972 May 26;40(2):459–468. doi: 10.1016/0006-8993(72)90146-1. [DOI] [PubMed] [Google Scholar]
- Varon S., Raiborn C., Tyszka E. In vitro studies of dissociated cells from newborn mouse dorsal root ganglia. Brain Res. 1973 May 17;54:51–63. doi: 10.1016/0006-8993(73)90033-4. [DOI] [PubMed] [Google Scholar]