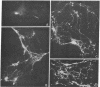
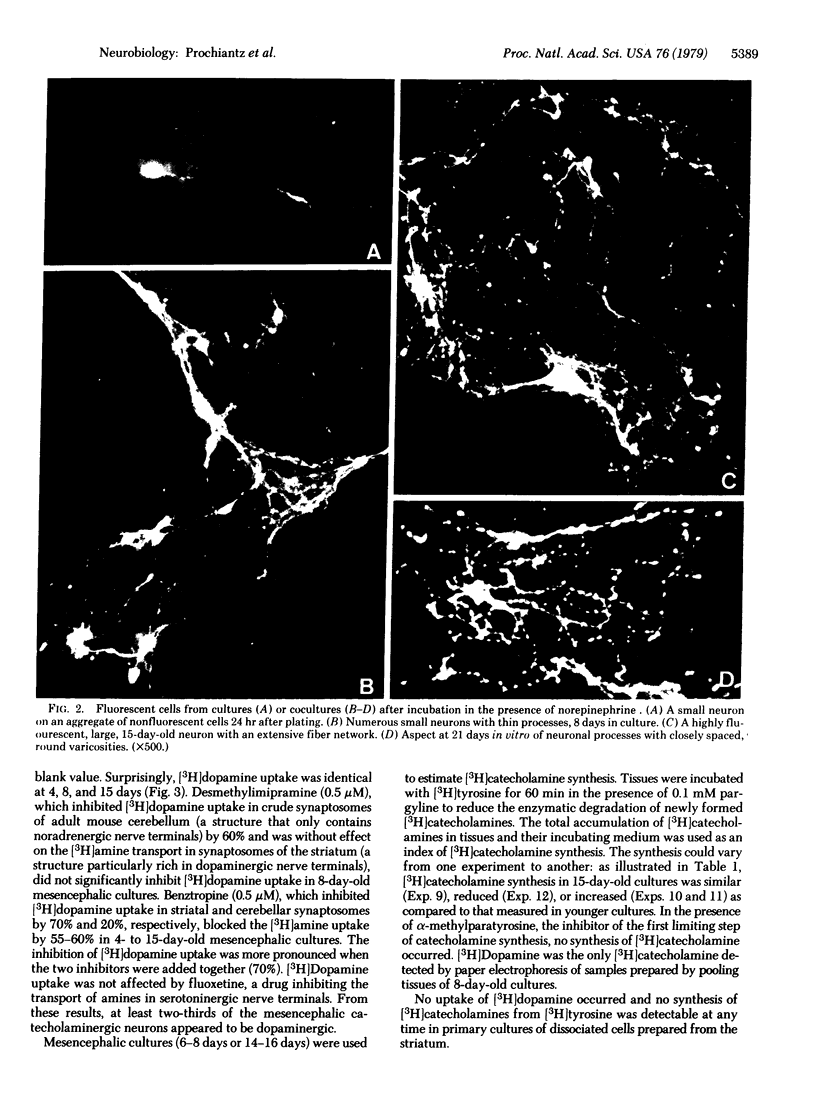
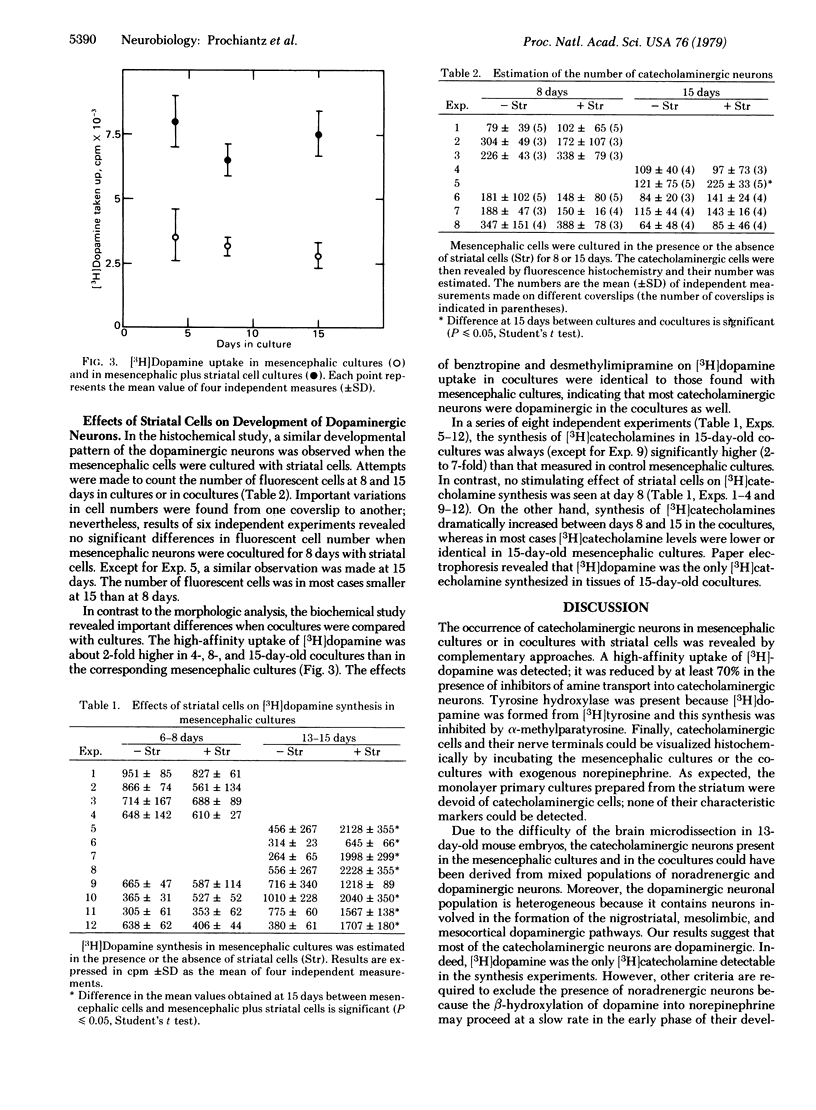
Abstract
Long-term survival of mesencephalic and striatal cells from mouse embryos in dissociated primary cultures is described. Catecholaminergic neurons in mesencephalic culutres were identified histochemically and by measuring [3H]dopamine uptake and synthesis from [3H]tyrosine. According to experiments using specific inhibitors of catecholamine uptake, at least two-thirds of the catecholaminergic neurons are dopaminergic. These neurons differentiated whether or not striatal target cells were present, but striatal cells stimulated the development of the dopaminergic neurons. [3H]Dopamine uptake was increased by at least 2-fold regardless of the age of the cocultures (4-15 days). Enhanced [3H]dopamine synthesis was also observed (at least 2-fold) at later times (12-15 days).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger B., Glowinski J. Dopamine uptake in serotoninergic terminals in vitro: a valuable tool for the histochemical differentiation of catecholaminergic and serotoninergic terminals in rat cerebral structures. Brain Res. 1978 May 19;147(1):29–45. doi: 10.1016/0006-8993(78)90770-9. [DOI] [PubMed] [Google Scholar]
- Chiba T., Hwang B. H., Williams T. H. A method for studying glyoxylic acid induced fluorescence and ultrastructure of monoamine neurons. Histochemistry. 1976 Oct 22;49(2):95–106. doi: 10.1007/BF00495673. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Campochiaro P. Ontogenesis of dopaminergic-cholinergic interactions in the rat striatum: a neurochemical study. J Neurochem. 1976 Sep;27(3):673–678. doi: 10.1111/j.1471-4159.1976.tb10393.x. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F., Gershon M. D., Crain S. M. Innervation of hippocampal explants by central catecholaminergic neurons in co-cultured fetal mouse brain stem explants. Brain Res. 1979 Feb 9;161(3):431–445. doi: 10.1016/0006-8993(79)90673-5. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. G., Barker D. L., Herbert E., Kravitz E. A. Screening for neurotransmitters: a rapid radiochemical procedure. J Neurobiol. 1971;2(3):231–246. doi: 10.1002/neu.480020305. [DOI] [PubMed] [Google Scholar]
- Levitt P., Moore R. Y., Garber B. B. Selective cell association of catecholamine neurons in brain aggregates in vitro. Brain Res. 1976 Jul 30;111(2):311–320. doi: 10.1016/0006-8993(76)90776-9. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Cheramy A., Glowinski J. Release of dopamine evoked by electrical stimulation of the motor and visual areas of the cerebral cortex in both caudate nuclei and in the substantia nigra in the cat. Brain Res. 1978 Apr 21;145(1):69–83. doi: 10.1016/0006-8993(78)90797-7. [DOI] [PubMed] [Google Scholar]
- Panula P., Rechardt L., Hervonen H. Observations on the morphology and histochemistry of the rat neostriatum in tissue culture. Neuroscience. 1979;4(2):235–248. doi: 10.1016/0306-4522(79)90086-1. [DOI] [PubMed] [Google Scholar]
- Schlumpf M., Shoemaker W. J., Bloom F. E. Explant cultures of catecholamine-containing neurons from rat brain: biochemical, histofluorescence, and electron microscopic studies. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4471–4475. doi: 10.1073/pnas.74.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]