Abstract
Leukaemia inhibitory factor (LIF) plays an indispensible role in embryo implantation. Aberrant LIF production is linked to implantation failure. LIF regulates multiple processes prior to and during implantation such as uterine transformation into a receptive state, decidualization, blastocyst growth and development, embryo-endometrial interaction, trophoblast invasion, and immune modulation. Due to its critical role, LIF has been a target for a nonhormonal contraception. In this review, we summarize up-to-date information on the role of LIF in implantation and its role in contraception.
1. Introduction
Leukemia inhibitory factor (LIF), a pleiotropic cytokine from interleukin- (IL-) 6 family, regulates various cellular functions via binding to membrane-bound LIF receptor (LIFR) and gp130 [1]. Currently, three spliced variants of LIF have been identified which include membrane-associated, diffusible, and truncated forms acting as paracrine factors in embryo implantation [2]. Binding of LIF to LIFR recruits gp130 to form high affinity functional receptor complex leading to activation of downstream signal transduction pathway such as signal transducer and activator of transcription (STAT) [3]. In addition to the membrane-bound receptor, a number of soluble forms of LIF receptor have been identified which are involved in either potentiating or dampening LIF activities. The soluble forms of LIFR and gp130 can function as antagonists that compete with membrane-bound receptor for the binding to LIF [4]. Meanwhile, suppressor of cytokine signaling 3 (SOCS3) can also inhibit LIF signaling and can act as a negative regulator for LIF action [5]. Following binding of LIF to LIFR, SOCS3 inhibits LIF action via JAK1-STAT3 signaling pathway [6]. SOCS3 can also attenuate other signaling cascades which are induced upon LIF binding to LIFR and gp130 such as ERK-MAPK signaling pathway [7]. Few studies have demonstrated that LIF, gp130, and STAT are crucial for embryo implantation. Failure of blastocyst to implant has been reported in LIF gene knockout mice [8]. Meanwhile, mice with gp130 mutation and STAT-binding site deletion are also infertile indicating that gp130 and STAT are essential in regulating LIF action [3]. In species such as mice, uterine LIF displays biphasic expression pattern with the first peak appearing in the glands in preparation for uterine receptivity while the second peak appears in the stroma surrounding the implanting blastocyst at the time of attachment reaction [9]. In parallel, LIFR and gp130 are expressed in the luminal epithelia and stroma throughout the peri-implantation period [9] which further reinforce the critical role of LIF in embryo implantation.
Ovarian steroids are reported to play important role in regulating LIF, LIFR, and gp130 expressions in the uterus throughout the implantation window period. In mice, endometrial LIF secretion can be induced by nidatory estrogen at day 4 of pregnancy [10] while exogenous estrogen and progesterone administration to ovariectomised mice were able to increase gp130 expression in the uterine glands [11]. However, in humans, a report has indicated that luteal estrogen was not required to initiate the implantation process [12]. In hamsters, LIF secretion was induced by estrogen while the expression of LIFR and gp130 was induced by progesterone [12]. Currently, there is limited information with regard to regulations of LIF, LIFR, and gp130 expression in humans. An in vitro study using human endometrial stromal cell line indicated that concomitant administration of estrogen and progesterone was able to upregulate LIF receptor mRNA expression [13]. In humans, chorionic gonadotrophins (hCG) was also reported to upregulate LIF expression [14]. hCG and transforming growth factor- (TGF-) β increase LIF secretion by the cultured endometrial epithelial cells derived from follicular and secretory phases of the menstrual cycle [15]. Meanwhile, male seminal fluid was also found to stimulate LIF secretion by human endometrial epithelial cells in vitro [16].
Several strands of clinical evidences indicated important role of LIF during human embryo implantation. A moderate to high LIF expression was detected during the proliferative and secretory phases of the menstrual cycle in normal fertile women with low expression observed in infertile women with implantation failure. However, no differences in endometrial expression of gp130 were noted between fertile and infertile women [17]. Further assessment of uterine luminal fluid indicated that endometrium of infertile women secretes significantly lesser amount of LIF and gp130 than normal fertile women [18] between luteal days (LH) 6 to 13 which coincides with implantation window period [19].
Evidences have shown that LIF is involved in the following events during implantation which include (i) endometrial transformation into a receptive state [2], (ii) embryo-endometrial interaction [20], (iii) stromal decidualization [21], (iv) trophoblast invasion [22], (v) blastocyst growth and development [8], and (vi) uterine leukocyte infiltration [13]. LIF has also been found to play an important role in regulating synthesis of prostaglandins (PGs), an important mediator of implantation and decidualization [23]. This review summarizes the current knowledge on the role of LIF in embryo implantation which could be used to guide further research in this field. Additionally, potential application of LIF as a target for nonhormonal contraception was also discussed. Figure 1 summarizes the role of LIF in multiple steps during embryo implantation and placentation.
Figure 1.
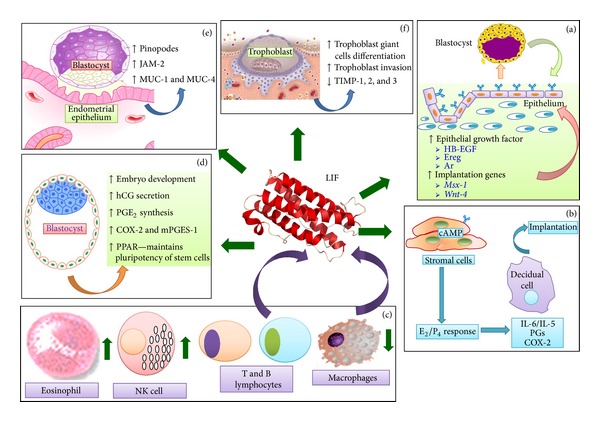
Summary of the known roles of LIF in embryo implantation. LIF increases the expression of EGF and implantation genes in receptive endometrium. LIF produced by endometrium and blastocyst regulates growth and development of the embryo. Meanwhile, LIF stimulates stromal decidualization by increasing the production of cytokines and prostaglandins. LIF is also involved in enhancing embryo-endometrial interaction through pinopodes and adhesion molecules. LIF stimulates trophoblast cells differentiation and increase trophoblast capability to invade the uterine stroma. Finally, LIF is involved in recruitment of specific cohort of leucocytes which participates in inflammatory response during implantation. LIF: leukemia inhibitory factor, HB-EGF: heparin binding-epidermal growth factor, Ereg: epiregulin, Ar: amphiregulin, E2: estrogen, P4: progesterone, IL: interleukins, PGs: prostaglandins, COX: cyclo-oxygenase, NK: natural killer, PPAR: peroxisome proliferator-activated receptor, PGE2: prostaglandins E2, hCG: human chorionic gonadotrophin, MUC: mucin, JAM: junctional adhesion molecules.
2. LIF Role in Uterine Transformation into a Receptive State
At the beginning of implantation window period in human, the expression of chicken ovalbumin upstream promoter transcription factor (COUP-TF) II, which is encoded by NR2F2 gene [24] was increased in uterine stroma under the influence of progesterone [25]. This increase will result in suppression of uterine luminal epithelial cell proliferation via inhibition on estrogen receptor- (ER-) α activity [26]. Meanwhile, another endometrial transcription factor, Hand2, which was upregulated by progesterone also inhibits fibroblast growth factor- (FGF-) induced epithelial cell proliferation via downregulating ER-α expression and ERK1/2 signaling pathway in uterine luminal epithelia [27]. The role of LIF in the inhibition of epithelial proliferation at the onset of uterine receptivity period remains elusive. During uterine, receptivity, several changes in protein expression have been reported to occur in the uterine luminal epithelia which include increased synthesis of epithelial growth factor (EGF), for example, heparin-binding epidermal growth factor (HB-EGF) and its receptors, ErbB1 and ErbB2 [28]. In addition, increased expression of cytokines [18, 29] and intercellular adhesion molecules such as ICAM and fibrinogen-γ (FGG) has also been documented during this period [30].
LIF prepares the endometrium for embryo implantation. Several reports have indicated that in mice, peak expression of LIF occurs in the glands at the time of ovulation and prior to the onset of implantation [31, 32]. Epithelial-derived LIF was reported to act as autocrine regulator in the preparation of endometrium for implantation [8]. Female mice lacking of LIF gene suffered from implantation failure [32]. Meanwhile in humans, LIF expression in the endometrium was restricted to the glands, which was the highest during midluteal phase of the cycle [33]. In fertile women, LIF was also detected in uterine luminal fluid during the luteal phase of the menstrual cycle [14] and at the expected time of implantation [34]. In parallel, expression of LIFR-β was reported to be the highest in the luminal epithelia during secretory phases of the menstrual cycle while expression of gpl30 was found both in the luminal and glandular epithelia throughout menstrual cycle phases [20].
During receptivity period, LIF either binds directly to LIFR which is expressed on the blastocyst [35] or endometrial surfaces, in which the latter participates in autoregulation of LIF secretion [4]. LIF affects synthesis of growth factors in the endometrial epithelia. In LIF-deficient female mice, EGF-like growth factors such as amphiregulin (Ar), heparin binding epidermal growth factor (HB-EGF), and epiregulin (Ereg) were not expressed at the site of blastocyst apposition [36], although expressions of EGF receptors were not affected [36]. The dependency of Ar on LIF was evident from lack of expression of this growth factor in uterine luminal epithelia following administration of inhibitor to LIF (hLIF-05) [37]. LIF was also required to induce expression of implantation genes including Msx-1 and Wnt-4 [38]. Despite of these effects, direct role of LIF in regulating expression of adhesion molecules such as L-selectins, E-cadherins [39] and tight junction proteins, for example, claudin and occludin [40] which are expressed in the receptive endometrium remains elusive.
3. LIF Role in Decidualization
During the luteal phase of menstrual cycle and diestrus stage of oestrous cycle, stromal cells proliferate and differentiate into decidual cells which then produced various factors that help to prepare endometrium for blastocyst adhesion and subsequently trophoblast invasion. CCAAT/enhancer-binding protein β (C/EBPβ) is a transcription factor that has been identified as a regulator of uterine stromal cell proliferation and differentiation in mice [41] and humans [21]. C/EBPβ controls proliferation of primary human endometrial stromal cells (HESCs) in vitro by regulating expression of several key cell cycle-regulatory factors [42]. C/EBPβ also increases the response of HESCs to estrogen, progesterone, and cyclic AMP (cAMP) and regulates interleukin- (IL-) 11 receptor and its downstream STAT3 transcription factor expression [21]. Female mice lacking C/EBPβ gene are infertile with their uteri irresponsive towards deciduogenic stimuli while proliferation and differentiation of stromal cells were also impaired [43].
LIF plays important role in decidualization. Failure of stromal cells to differentiate into primary decidual cells has been reported in LIF-deficient mice [44]. LIF also enhances estrogen and progesterone-induced decidualization in HESCs via STAT3 phosphorylation [13]. Meanwhile, LIF was also found to upregulate the secretion of IL-6 and IL-15 from decidualized HESCs in vitro [13]. During decidualization, SOCS3 protein is stimulated in response to cytokine-induced STAT3 phosphorylation which acts as a negative-feedback inhibitor to hinder LIFR activity [45]. LIF was reported to indirectly stimulate the synthesis of PGs which is an important mediator of decidualization via IL-1 [46] and is required for cyclooxygenase-2 (COX-2) expression, in which the latter is a rate-limiting enzyme in the PGs synthesis [36]. Female mice lacking LIF gene suffered from implantation failure due to impaired PGs synthesis [36].
4. LIF Role in Leukocyte Recruitment during Implantation
In early pregnancy, infiltration of immune cells such as dendritic cells (DC), macrophages, T and B lymphocytes, natural killer (NK) cells [47], and neutrophils and eosinophils [48] into the endometrium was initially stimulated by factors in the seminal fluid [49] and later by the implanting blastocyst [50]. DCs are involved in immune tolerance, tissue remodeling, angiogenesis, and development of T regulatory (Treg) cells [51]. In humans, primary unexplained infertility was found to be associated with reduced expression of Treg in the endometrial tissue [52]. Macrophages participate in the progression of inflammation, counteract nitric oxide synthesis, tissue remodeling, angiogenesis, and immune tolerance towards the implanting blastocyst [53]. Meanwhile, T cells produced type-1 and type-2 cytokines which are involved in proinflammatory and anti-inflammatory responses in which changes in their ratio would determine the success of implantation [54]. In the late secretory phase and in early pregnancy, percentage of endometrium/decidual NK cells increases rapidly reaching up to 70% of the total uterine leukocyte population [55]. However, following implantation, endometrial NK cells differentiate into decidual NK cells, which begin to secrete cytokines (TNF-α, IL-10, GM-CSF, IL-1β, TGF-β1, CSF-1, LIF, and IFN-γ), growth factors, angiogenic factors as well as being involved in tissue remodelling, trophoblast migration, and decidualization [56].
LIF was reported to play important role in the regulation of immune response in the uterus in early pregnancy. LIF affects uterine leukocyte subpopulation and recruits specific cohort of leucocytes to the site of implantation [57]. LIF mRNA is expressed in decidual leucocytes itself [58]. LIF-deficient mice were found to have increased number of uterine macrophages although the number of NK cells and eosinophils [48] was reduced. Macrophage-derived LIF facilitates development of implantation-receptive endometrium by modulating the surface glycan structure of epithelial cells [59] as well as regulating the expression of fucosyltransferase enzyme in the uterine epithelia which is involved in the synthesis of embryo adhesive fucosylated glycoconjugates during the period of inflammatory response towards insemination [60]. A recent study reported that intrauterine administration of peripheral blood mononuclear cells (PBMCs) in mice helped to improve endometrial receptivity as evidence from a high pregnancy rate associated with increased endometrial LIF and vascular endothelial growth factor (VEGF) expressions [61]. PBMCs were found to produce cytokines and angiogenic factors necessary for implantation [56]. A study in humans has indicated that intrauterine infusion of PBMCs could help to improve the clinical pregnancy rate in patients with repeated implantation failure during in vitro fertilization-embryo transfer (IVF-ET) procedure [62], indicating that leucocytes play important role in ensuring the success of human embryo implantation.
5. LIF Role in Blastocyst Growth and Development
Following fertilization, embryo divides from 2- to 4-cell stages and subsequently 8-cell stage, in which the latter formed a morula which then develops into blastocyst that hatches upon entering the uterine cavity [63]. Blastocyst is then brought closer to the uterine wall by a force generated from fluid reabsorption in the uterine glands [64]. During blastocyst development, pluripotent inner cells are prepared for specific differentiation while outer trophectoderm cells interact with uterine epithelium in preparation for trophoblast invasion [65]. Mouse blastocysts were reported to express LIF mRNA transcript [66] which helps to increase rate of preimplantation embryo development [67, 68]. Meanwhile, mouse [69], rabbit [70] and human [71] blastocysts expressed LIFR and gp130 where the latter promotes preimplantation human embryo development in culture [72]. A combined administration of insulin-like growth factor- (IGF-) I, β-fibroblast growth factor (FGF), transforming growth factor- (TGF-) β1, granulocyte-monocyte colony stimulating factor (GM-CSF), and LIF have been reported to accelerate blastocyst development in vitro, especially changes from expanded to hatched blastocyst stages [73].
Leptin, a hormone linked to fertility acted via LIF to cause increased proportion of hatched blastocysts while causing decreased rate of embryo cell apoptosis in vitro via STAT3 signaling pathway [74]. LIF was found to affect hCG secretion by the trophoblast cells in vitro [75]. LIF was also reported to induce prostaglandin E (PGE2) production by human trophoblast cell line via stimulating COX-2 and microsomal PGE synthase-1 (mPGES-1) enzymes expression that are involved in PGE synthesis [23]. Meanwhile, LIF maintains pluripotency of mouse embryonic stem cells in culture via stimulating peroxisome proliferator-activated receptors (PPARs), a nuclear receptor transcription factors that regulates LIF-induced growth and self-renewal via tyrosine kinase 2-STAT3 signaling pathway [76].
6. Role of LIF in Embryo-Endometrial Interaction
During apposition phase of implantation, blastocyst initiates loose physical contact with the receptive endometrium which occurs prior to firm adhesion onto the endometrial surface. Mucin-1 (MUC-1), a glycocalyx which is expressed at the apical membrane of luminal epithelia, prevents firm blastocyst attachment [39]. At the site of trophoblast invasion, MUC-1 expression was markedly reduced [77]. Lack or aberrant MUC-1 production was reported to be one of the reasons of ectopic pregnancy [78]. MUC-1 provides scaffold for L-selectin ligand, which binds L-selectin on the blastocyst surface, allowing loose physical contact between blastocyst and endometrium as well as facilitating blastocyst rolling over the endometrial surface [79]. Meanwhile, L-selectin ligand, which is expressed on the pinopode (uterodomes) surface [80], also helps in blastocyst rolling. Finally, increased expression of other adhesion molecules such as αvβ3 integrin [81], trophinins [82], junctional adhesion molecule (JAM) [83], and HB-EGF/errB4 complex [84] resulted in the blastocyst movement to come to a standstill which allows the blastocysts to firmly attach onto the endometrial surface prior to uterine invasion.
LIF plays indispensable role in initiating embryo-endometrial interaction. In mice lacking of LIF, absence of pinopodes was observed [44]. Meanwhile, human endometrium with uterodomes at different stages of development had both luminal and glandular epithelia expressing high levels of LIF and LIFR in the luteal days 6 through to 9 [85]. A study has shown that expression of LIFR and gp130 in the endometrium of fertile women positively correlated with pinopode formation, while the opposite was observed in women with unexplained infertility. A reduced level of LIF has been reported in hydrosalpinx [86] but not in recurrent pregnancy loss [87]. LIF, involving STAT3 phosphorylation, was reported to induce the expression of JAM-2 adhesion molecule which mediates the interaction between hatched blastocyst and receptive endometrium between days 3 and 4 of pregnancy in mice [83]. Although LIF effect on the expression of other adhesion molecules remains elusive, synchronous reduction in the levels of LIF, integrin β3, and MUC-1 was observed in the uterus of patients with hydrosalpinx [86], suggesting that the expression of these molecules was interdependent. Meanwhile, limited observation indicated that LIF affects expression of genes that encode antiadhesive mucins, MUC-1 and MUC-4 [60]. Other than these findings, LIF involvement in facilitating embryo-endometrial interactions remains to be fully elucidated.
7. Role of LIF in Trophoblast Invasion
Trophoblast giant cells, the first cell lineage derived from trophoblast stem cells [88], have the ability to invade into the decidua to initiate the implantation reaction [89]. As it moves towards the uterine compartment, trophoblast cells are confronted by various extracellular matrix (ECM) proteins and basement membranes such as collagen, fibronectin, laminin, vitronectin, trophin, and tastin which are able to bind to integrins on the trophoblast surface. These molecules help in controlling adhesion, migration, differentiation, and spreading of the trophoblast cells [90]. Invasion involves degradation of extracellular matrix (ECM) elements in the direction of migration which requires involvement of protease enzymes, such as matrix metalloproteinases (MMPs) 2, 9, and 14 [22] and is controlled by tissue inhibitor of metalloproteinases (TIMPs), for example, TIMPs 1, 2, and 3 [91].
LIF plays an important role in trophoblast invasion. LIF stimulates differentiation of trophoblast giant cells via JAK1-STAT3 pathway [6]. Meanwhile, soluble LIF provides extracellular signal that stimulates trophoblast invasion via STAT3 activation [92, 93]. LIF induces trophoblast cell proliferation via stimulating cell transition into G(2)/M phase of the cell cycle and activates both STAT3 and ERK1/2 signaling cascades [94]. Recently, LIF has been shown to increase invasiveness of human trophoblast cell line (HTR-8/SVneo cells) in vitro via STAT1 and STAT3 activation [95] as well as increase invasiveness of extravillous trophoblast cells via stimulating adhesion to the extracellular matrix elements including fibronectin, vitronectin, and laminin [96]. On the other hand, LIF was also reported to downregulate the expression of genes that encode TIMP1, TIMP2, and TIMP3 [95], therefore helping to reduce the expression of enzymes that are involved in potentiating trophoblast invasion. LIF was also reported to decrease the expression of integrin β 4 mRNA in the trophoblast cells which promotes trophoblast invasion [96].
8. LIF as a Target for Nonhormonal Contraception
A study in humans indicated that low concentration of LIF in the maternal plasma was associated with increased risks of early pregnancy loss during embryo transfer [97], which points towards critical role of LIF in ensuring the success of embryo implantation. However, despite of this report, administration of recombinant LIF during assisted reproductive techniques (ART) has revealed no improvement in implantation rates in women with recurrent unexplained implantation failure [98]. Meanwhile, LIF has been identified as a potential target in the development of nonhormonal birth control vaccine. A study in mice indicated that intraperitoneal injection of anti-LIF antibody inhibited embryo implantation [99] while immunization of female mice with LIF or LIFR peptide vaccines induced long-lasting antibody development which could block fertility [100]. A preliminary study in rhesus monkey indicated that administration of monoclonal anti-LIF antibody could prevent embryo implantation [101].
In mice, intraperitoneal administration of LIF antagonist (LA) alone or conjugated to polyethylene glycol (PEGLA) between days 2.5 and 3.5 of pregnancy resulted in implantation failure which demonstrates that this compound could effectively be used as a nonhormonal contraceptive agent that targets LIF signaling in the endometrium [102]. Recently, vaginal administration of PEGLA in mice has been proven to be effective in inhibiting embryo implantation [103] while systemic administration of this compound to cynomolgus monkey reduced endometrial STAT3 phosphorylation, inhibited LIF-induced expression of cochlin, insulin-like growth factor-binding protein- (IGF-BP-) 3, vascular endothelial growth factor- (VEGF-) A, and COX-2 enzyme which are essential for embryo implantation [104]. While most works related to the use of LIF antagonist and PEGLA as nonhormonal contraceptive agents were preliminary and were limited to animal studies, a study using human tissue has been recently performed in vitro by Lalitkumar et al. [105]. In this study, the effect on human embryo attachment rate, embryo quality, and blastocyst expression of cell survival factor (Akt) and caspase-3 following exposure to endometrial tissue collected at luteal day 4 (LH+4) treated with PEGLA were determined. The findings indicated that in tissues treated with PEGLA, embryo attachment rate was reduced with embryonic LIF triggered apoptosis being inhibited. Meanwhile, endometrial LIF expression was also downregulated which was associated with the reduction in blastocyst survival rate and the increase in caspase-3 expression in the blastocyst. Currently, no clinical trials have been conducted in humans to assess effectiveness of LA or PEGLA as nonhormonal contraceptive agents. Table 1 summarizes the studies performed using various models to investigate the effectiveness of LA or PEGLA as potential nonhormonal contraception agents.
Table 1.
Summary of the literatures that reported the use of LIF antagonist in preventing implantation in different models. So far, only one study has been performed on human uterine tissues in-vitro which investigated this effect.
| Authors | Antagonist | Route of administration | Detectable in uterine tissue | Model | Effects |
|---|---|---|---|---|---|
| Aschenbach et al. (2013) [104] | PEGLA | Intramuscular and subcutaneous | Yes luminal and glandular epithelia; endometrial lysates (intramuscular administration) |
Cynomolgus monkeys | Reduced endometrial STAT3 protein phosphorylation in vivo and in vitro Inhibited LIF induced expression of cochlin, IGF-BP 3, VEGF A, and COX-2 in endometrial explants in vitro |
| Vaginal | No | ||||
|
| |||||
| Menkhorst et al. (2011) [103] | PEGLA | Vaginal | Yes (no systemic side effects) |
Mice | Blocked implantation |
|
| |||||
| White et al. (2007) [102] | LIF antagonist (LA) | Intraperitoneal plus continuous administration via miniosmotic pump | Mice | Blocked implantation Reduced STAT3 phosphorylation in luminal epithelial cells |
|
| PEGLA | Intraperitoneal | Yes; uterine luminal epithelium (no systemic side effects) | Mice | Inhibited implantation Reduced STAT3 phosphorylation in luminal epithelial cells |
|
|
| |||||
| Lalitkumar et al. (2013) [105] | PEGLA | In-vitro study on timed human endometrial biopsy tissue | Yes | Human | Reduced embryo attachment rate to endometrium Decreased LIF mRNA and protein in endometrium Inhibition of embryonic LIF triggered endometrial cell apoptosis Downregulation in AKT activation and increase of caspase-3 activation in blastocysts |
|
| |||||
| Sengupta et al. (2006) [101] | Anti-LIF monoclonal Ab | Intrauterine | Yes | Rhesus Monkey | Significant decline in pregnancy outcome |
9. Perspective
LIF is undoubtedly important in embryo implantation in rodents, primates, and humans. LIF has been shown to mediate multiple processes of embryo implantation ranging from blastocyst growth and development, uterine preparation for implantation, decidualization, uterine inflammatory responses towards the implanting embryos, embryo-endometrial interaction, and trophoblast invasion. In view of these documented roles, LIF has been proposed as a potential target for nonhormonal contraception. While most information with regard to the mechanisms underlying LIF actions in uterus during implantation period was obtained mostly from studies involving rodents and endometrial cell lines, more works are needed in humans to elicit its role in blastocyst implantation.
Acknowledgment
Publication of this paper was supported by UMRG Grant (RG404/12HTM), University of Malaya, Kuala Lumpur, Malaysia.
Conflict of Interests
The authors have nothing to disclose.
References
- 1.Mathieu M-E, Saucourt C, Mournetas V, et al. LIF-dependent signaling: new pieces in the lego. Stem Cell Reviews and Reports. 2012;8(1):1–15. doi: 10.1007/s12015-011-9261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghajanova L. Leukemia inhibitory factor and human embryo implantation. Annals of the New York Academy of Sciences. 2004;1034(1):176–183. doi: 10.1196/annals.1335.020. [DOI] [PubMed] [Google Scholar]
- 3.Sun X, Bartos A, Whitsett JA, Dey SK. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Molecular Endocrinology. 2013;27(9):1492–1501. doi: 10.1210/me.2013-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metz S, Naeth G, Heinrich PC, Müller-Newen G. Novel inhibitors for murine and human leukemia inhibitory factor based on fused soluble receptors. Journal of Biological Chemistry. 2008;283(10):5985–5995. doi: 10.1074/jbc.M706610200. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO Journal. 2003;22(3):372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y, Takahashi M, Carpino N, et al. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Molecular Endocrinology. 2008;22(7):1673–1681. doi: 10.1210/me.2008-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle K, Robb L. The role of SOCS3 in modulating leukaemia inhibitory factor signalling during murine placental development. Journal of Reproductive Immunology. 2008;77(1):1–6. doi: 10.1016/j.jri.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart CL. Leukaemia inhibitory factor and the regulation of pre-implantation development of the mammalian embryo. Molecular Reproduction and Development. 1994;39(2):233–238. doi: 10.1002/mrd.1080390217. [DOI] [PubMed] [Google Scholar]
- 9.Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131(2):341–349. doi: 10.1530/rep.1.00956. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin JRA, Freeman TC, Stephens RJ, et al. Identification of genes regulated by leukemia-inhibitory factor in the mouse uterus at the time of implantation. Molecular Endocrinology. 2004;18(9):2185–2195. doi: 10.1210/me.2004-0110. [DOI] [PubMed] [Google Scholar]
- 11.Ni H, Ding N, Harper MJK, Yang Z. Expression of leukemia inhibitory factor receptor and gp130 in mouse uterus during early pregnancy. Molecular Reproduction and Development. 2002;63(2):143–150. doi: 10.1002/mrd.10168. [DOI] [PubMed] [Google Scholar]
- 12.Ding T, Song H, Wang X, Khatua A, Paria BC. Leukemia inhibitory factor ligand-receptor signaling is important for uterine receptivity and implantation in golden hamsters (Mesocricetus auratus) Reproduction. 2008;135(1):41–53. doi: 10.1530/REP-07-0013. [DOI] [PubMed] [Google Scholar]
- 13.Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025288.e25288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licht P, Lösch A, Dittrich R, Neuwinger J, Siebzehnrübl E, Wildt L. Novel insights into human endometrial paracrinology and embryo-maternal communication by intrauterine microdialysis. Human Reproduction Update. 1998;4(5):532–538. doi: 10.1093/humupd/4.5.532. [DOI] [PubMed] [Google Scholar]
- 15.d'Hauterive SP, Charlet-Renard C, Berndt S, et al. Human chorionic gonadotropin and growth factors at the embryonic-endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Human Reproduction. 2004;19(11):2633–2643. doi: 10.1093/humrep/deh450. [DOI] [PubMed] [Google Scholar]
- 16.Gutsche S, von Wolff M, Strowitzki T, Thaler CJ. Seminal plasma induces mRNA expression of IL-1β, IL-6 and LIF in endometrial epithelial cells in vitro. Molecular Human Reproduction. 2003;9(12):785–791. doi: 10.1093/molehr/gag095. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Yin Y, Zhao M, Hu L, Chen Q. The low expression of leukemia inhibitory factor in endometrium: possible relevant to unexplained infertility with multiple implantation failures. Cytokine. 2013;62(2):334–339. doi: 10.1016/j.cyto.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Tawfeek MA, Eid MA, Hasan AM, Mostafa M, El-Serogy HA. Assessment of leukemia inhibitory factor and glycoprotein 130 expression in endometrium and uterine flushing: a possible diagnostic tool for impaired fertility. BMC Women’s Health. 2012;12, article 10 doi: 10.1186/1472-6874-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwin JRA, Smith SK, Wilson A, Sharkey AM. Soluble gp130 is up-regulated in the implantation window and shows altered secretion in patients with primary unexplained infertility. Journal of Clinical Endocrinology and Metabolism. 2002;87(8):3953–3960. doi: 10.1210/jcem.87.8.8766. [DOI] [PubMed] [Google Scholar]
- 20.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(7):3115–3120. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPβ involves cyclin E-cdk2 and STAT3. Molecular Endocrinology. 2012;26(12):2016–2030. doi: 10.1210/me.2012-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reproductive Biology and Endocrinology. 2005;3(1, article 56) doi: 10.1186/1477-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR-8/SVneo. Human Reproduction. 2007;22(7):1801–1809. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- 24.Qiu Y, Krishnan V, Zeng Z, et al. Isolation, characterization, and chromosomal localization of mouse and human COUP-TF I and II genes. Genomics. 1995;29(1):240–246. doi: 10.1006/geno.1995.1237. [DOI] [PubMed] [Google Scholar]
- 25.Kurihara I, Lee D, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS genetics. 2007;3(6, article e102) doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D, Kurihara I, Jeong J, et al. Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Molecular Endocrinology. 2010;24(5):930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by hand2. Science. 2011;331(6019):912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Wang H, Matsumoto H, Roy SK, Das SK, Paria BC. Dual source and target of heparin-binding EGF-like growth factor during the onset of implantation in the hamster. Development. 2002;129(17):4125–4134. doi: 10.1242/dev.129.17.4125. [DOI] [PubMed] [Google Scholar]
- 29.Noda N, Minoura H, Nishiura R, et al. Expression of Tenascin-C in stromal cells of the murine uterus during early pregnancy: Induction by interleukin-1α, prostaglandin E2, and prostaglandin F(2α) Biology of Reproduction. 2000;63(6):1713–1720. doi: 10.1095/biolreprod63.6.1713. [DOI] [PubMed] [Google Scholar]
- 30.Lecce L, Kaneko Y, Madawala RJ, Murphy CR. ICAM1 and fibrinogen-γ are increased in uterine epithelial cells at the time of implantation in rats. Molecular Reproduction and Development. 2011;78(5):318–327. doi: 10.1002/mrd.21307. [DOI] [PubMed] [Google Scholar]
- 31.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(24):11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 33.Chen D-B, Hilsenrath R, Yang Z, et al. Leukaemia inhibitory factor in human endometrium during the menstrual cycle: cellular origin and action on production of glandular epithelial cell prostaglandin in vitro. Human Reproduction. 1995;10(4):911–918. doi: 10.1093/oxfordjournals.humrep.a136060. [DOI] [PubMed] [Google Scholar]
- 34.Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Human Reproduction. 1997;12(3):569–574. doi: 10.1093/humrep/12.3.569. [DOI] [PubMed] [Google Scholar]
- 35.Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. Journal of Reproduction and Fertility. 1994;101(2):421–426. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- 36.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Molecular Endocrinology. 2000;14(8):1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 37.Mohamet L, Heath JK, Kimber SJ. Determining the LIF-sensitive period for implantation using a LIF-receptor antagonist. Reproduction. 2009;138(5):827–836. doi: 10.1530/REP-09-0113. [DOI] [PubMed] [Google Scholar]
- 38.Daikoku T, Song H, Guo Y, et al. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Molecular Endocrinology. 2004;18(5):1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A, Kumar P. Understanding implantation window, a crucial phenomenon. Journal of Human Reproductive Sciences. 2012;5(1):2–6. doi: 10.4103/0974-1208.97777. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Satterfield MC, Dunlap KA, Hayashi K, Burghardt RC, Spencer TE, Bazer FW. Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone. Endocrinology. 2007;148(8):3922–3931. doi: 10.1210/en.2007-0321. [DOI] [PubMed] [Google Scholar]
- 41.Mantena SR, Kannan A, Cheon Y, et al. C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(6):1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Li Q, Bagchi IC, Bagchi MK. The CCAAT/enhancer binding protein β is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology. 2010;151(8):3929–3940. doi: 10.1210/en.2009-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagchi MK, Mantena SR, Kannan A, Bagchi IC. Control of uterine cell proliferation and differentiation by C/EBPβ: functional implications for establishment of early pregnancy. Cell Cycle. 2006;5(9):922–925. doi: 10.4161/cc.5.9.2712. [DOI] [PubMed] [Google Scholar]
- 44.Fouladi-Nashta AA, Jones CJP, Nijjar N, et al. Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Developmental Biology. 2005;281(1):1–21. doi: 10.1016/j.ydbio.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Dimitriadis E, Stoikos C, Tan Y, Salamonsen LA. Interleukin 11 signaling components signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3) regulate human endometrial stromal cell differentiation. Endocrinology. 2006;147(8):3809–3817. doi: 10.1210/en.2006-0264. [DOI] [PubMed] [Google Scholar]
- 46.Fouladi-Nashta AA, Mohamet L, Heath JJ, Kimber SS. Interleukin 1 signaling is regulated by leukemia inhibitory factor (LIF) and is aberrant in Lif-/- mouse uterus. Biology of Reproduction. 2008;79(1):142–153. doi: 10.1095/biolreprod.107.065219. [DOI] [PubMed] [Google Scholar]
- 47.Lee JY, Lee M, Lee SK. Role of endometrial immune cells in implantation. Clinical and Experimental Reproductive Medicine. 2011;38(3):119–125. doi: 10.5653/cerm.2011.38.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schofield G, Kimber SJ. Leukocyte subpopulations in the uteri of leukemia inhibitory factor knockout mice during early pregnancy. Biology of Reproduction. 2005;72(4):872–878. doi: 10.1095/biolreprod.104.034876. [DOI] [PubMed] [Google Scholar]
- 49.Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. Journal of Animal Science. 2007;85(13) supplement:E36–E44. doi: 10.2527/jas.2006-578. [DOI] [PubMed] [Google Scholar]
- 50.Grümmer R, Winterhager E. Blastocyst-mediated induction of endometrial connexins: an inflammatory response? Journal of Reproductive Immunology. 2011;90(1):9–13. doi: 10.1016/j.jri.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Plaks V, Birnberg T, Berkutzki T, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. The Journal of Clinical Investigation. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Molecular Human Reproduction. 2006;12(5):301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- 53.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies . American Journal of Reproductive Immunology. 2010;63(6):460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 54.Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1:Th2 dichotomy of pregnancy and preterm labour. Mediators of Inflammation. 2012;2012:12 pages. doi: 10.1155/2012/967629.967629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: Key regulators of placental development (a review) Journal of Reproductive Immunology. 2002;57(1-2):151–168. doi: 10.1016/s0165-0378(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 56.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nature Medicine. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 57.Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation:Endocrine and paracrine interactions. Seminars in Reproductive Medicine. 2009;27(1):62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharkey AM, King A, Clark DE, et al. Localization of leukemia inhibitory factor and its receptor in human placenta throughout pregnancy. Biology of Reproduction. 1999;60(2):355–364. doi: 10.1095/biolreprod60.2.355. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura H, Jasper MJ, Hull ML, Aplin JD, Robertson SA. Macrophages regulate expression of α1,2-fucosyltransferase genes in human endometrial epithelial cells. Molecular Human Reproduction. 2012;18(4):204–215. doi: 10.1093/molehr/gar070.gar070 [DOI] [PubMed] [Google Scholar]
- 60.Jasper MJ, Care AS, Sullivan B, Ingman WV, Aplin JD, Robertson SA. Macrophage-derived LIF and IL1B regulate alpha(1,2)fucosyltransferase 2 (Fut2) expression in mouse uterine epithelial cells during early pregnancy. Biology of Reproduction. 2011;84(1):179–188. doi: 10.1095/biolreprod.110.085399. [DOI] [PubMed] [Google Scholar]
- 61.Yu N, Yang J, Guo Y, et al. Intrauterine administration of peripheral blood mononuclear cells (PBMCs) improves endometrial receptivity in mice with embryonic implantation dysfunction. American Journal of Reproductive Immunology. 2014;71(1):24–33. doi: 10.1111/aji.12150. [DOI] [PubMed] [Google Scholar]
- 62.Okitsu O, Kiyokawa M, Oda T, Miyake K, Sato Y, Fujiwara H. Intrauterine administration of autologous peripheral blood mononuclear cells increases clinical pregnancy rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. Journal of Reproductive Immunology. 2011;92(1-2):82–87. doi: 10.1016/j.jri.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Niakan KK, Han J, Pedersen RA, Simon C, Pera RAR. Human pre-implantation embryo development. Development. 2012;139(5):829–841. doi: 10.1242/dev.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naftalin RJ, Thiagarajah JR, Pedley KC, Pocock VJ, Milligan SR. Progesterone stimulation of fluid absorption by the rat uterine gland. Reproduction. 2002;123(5):633–638. doi: 10.1530/rep.0.1230633. [DOI] [PubMed] [Google Scholar]
- 65.Xenopoulos P, Kang M, Hadjantonakis A-K. Cell lineage allocation within the inner cell mass of the mouse blastocyst. In: Kubiak JZ, editor. Mouse Development. Berlin, Germany: Springer; 2012. pp. 185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Movaghar B, Askarian S. Expression of e-cadherin, leukemia inhibitory factor and progesterone receptor in mouse blastocysts after ovarian stimulation. Cell Journal. 2012;14(3):225–230. [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai H, Chang C, Hsieh Y, Hsu L, Chang S, Lo H. Effect of different concentrations of recombinant leukemia inhibitory factor on different development stage of mouse embryo in vitro. Journal of Assisted Reproduction and Genetics. 2000;17(6):352–355. doi: 10.1023/A:1009413329977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung L, Leung H, Bongso A. Effect of supplementation of leukemia inhibitory factor and epidermal growth factor on murine embryonic development in vitro, implantation, and outcome of offspring. Fertility and Sterility. 2003;80(2):727–735. doi: 10.1016/s0015-0282(03)00772-6. [DOI] [PubMed] [Google Scholar]
- 69.Chen H-F, Shew J, Ho H, Hsu W, Yang Y. Expression of leukemia inhibitory factor and its receptor in preimplantation embryos. Fertility and Sterility. 1999;72(4):713–719. doi: 10.1016/s0015-0282(99)00306-4. [DOI] [PubMed] [Google Scholar]
- 70.Lei T, Yang ZQ, Xia T, et al. Stage-specific expression of leukaemia inhibitory factor and its receptor in rabbit pre-implantation embryo and uterine epithelium during early pregnancy. Reproduction in Domestic Animals. 2004;39(1):13–18. doi: 10.1046/j.1439-0531.2003.00469.x. [DOI] [PubMed] [Google Scholar]
- 71.van Eijk MJT, Mandelbaum J, Salat-Baroux J, et al. Expression of leukaemia inhibitory factor receptor subunits LIFRβ and gp130 in human oocytes and preimplantation embryos. Molecular Human Reproduction. 1996;2(5):355–360. doi: 10.1093/molehr/2.5.355. [DOI] [PubMed] [Google Scholar]
- 72.Hambiliki F, Hanrieder J, Bergquist J, Hreinsson J, Stavreus-Evers A, Wånggren K. Glycoprotein 130 promotes human blastocyst development in vitro. Fertility and Sterility. 2013;99(6):1592–1599.e3. doi: 10.1016/j.fertnstert.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 73.Neira JA, Tainturier D, Peña MA, Martal J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-β1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology. 2010;73(5):595–604. doi: 10.1016/j.theriogenology.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 74.Fedorcsák P, Storeng R. Effects of leptin and leukemia inhibitory factor on preimplantation development and STAT3 signaling of mouse embryos in vitro . Biology of Reproduction. 2003;69(5):1531–1538. doi: 10.1095/biolreprod.103.019034. [DOI] [PubMed] [Google Scholar]
- 75.Leduc K, Bourassa V, Asselin É, Leclerc P, Lafond J, Reyes-Moreno C. Leukemia inhibitory factor regulates differentiation of trophoblastlike BeWo cells through the activation of JAK/STAT and MAPK3/1 MAP kinase-signaling pathways. Biology of Reproduction. 2012;86(2, article 54) doi: 10.1095/biolreprod.111.094334. [DOI] [PubMed] [Google Scholar]
- 76.Mo C, Chearwae W, Bright JJ. PPARγ regulates LIF-induced growth and self-renewal of mouse ES cells through Tyk2-Stat3 pathway. Cellular Signalling. 2010;22(3):495–500. doi: 10.1016/j.cellsig.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Meseguer M, Aplin JD, Caballero-Campo P, et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biology of Reproduction. 2001;64(2):590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 78.Refaat B, Simpson H, Britton E, et al. Why does the fallopian tube fail in ectopic pregnancy? The role of activins, inducible nitric oxide synthase, and MUC1 in ectopic implantation. Fertility and Sterility. 2012;97(5):1115–1123. doi: 10.1016/j.fertnstert.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 79.Carson DD, Julian J, Lessey BA, Prakobphol A, Fisher SJ. MUC1 is a scaffold for selectin ligands in the human uterus. Frontiers in Bioscience. 2006;11(3):2903–2908. doi: 10.2741/2018. [DOI] [PubMed] [Google Scholar]
- 80.Nejatbakhsh R, Kabir-Salmani M, Dimitriadis E, et al. Subcellular localization of L-selectin ligand in the endometrium implies a novel function for pinopodes in endometrial receptivity. Reproductive Biology and Endocrinology. 2012;10, article 46 doi: 10.1186/1477-7827-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao M, Chang C, Liu Z, Chen LM, Chen Q. Treatment with low-dose aspirin increased the level LIF and integrin β3 expression in mice during the implantation window. Placenta. 2010;31(12):1101–1105. doi: 10.1016/j.placenta.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Fukuda MN, Sugihara K. An integrated view of L-selectin and trophinin function in human embryo implantation. Journal of Obstetrics and Gynaecology Research. 2008;34(2):129–136. doi: 10.1111/j.1447-0756.2008.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su R-W, Jia B, Ni H, et al. Junctional adhesion molecule 2 mediates the interaction between hatched blastocyst and luminal epithelium: induction by progesterone and LIF. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034325.e34325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122(2):637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 85.Aghajanova L, Stavreus-Evers A, Nikas Y, Hovatta O, Landgren B. Coexpression of pinopodes and leukemia inhibitory factor, as well as its receptor, in human endometrium. Fertility and Sterility. 2003;79(supplement 1):808–814. doi: 10.1016/s0015-0282(02)04830-6. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Xu B, Chen Q, Sun X. Effects of hydrosalpinx on pinopodes, leukaemia inhibitory factor, integrin β3 and MUC1 expression in the peri-implantation endometrium. European Journal of Obstetrics Gynecology and Reproductive Biology. 2010;151(2):171–175. doi: 10.1016/j.ejogrb.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 87.Xu B, Sun X, Li L, Wu L, Zhang A, Feng Y. Pinopodes, leukemia inhibitory factor, integrin-β3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertility and Sterility. 2012;98(2):389–395. doi: 10.1016/j.fertnstert.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 88.Kent LN, Konno T, Soares MJ. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Developmental Biology. 2010;10, article 97 doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hemberger M. IFPA Award in Placentology Lecture. Characteristics and significance of trophoblast giant cells. Placenta. 2008;29:4–9. doi: 10.1016/j.placenta.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 90.Bischof P, Campana A. Molecular mediators of implantation. Bailliere’s Best Practice and Research in Clinical Obstetrics and Gynaecology. 2000;14(5):801–814. doi: 10.1053/beog.2000.0120. [DOI] [PubMed] [Google Scholar]
- 91.Bai SX, Wang Y, Qin L, Xiao ZJ, Herva R, Piao Y. Dynamic expression of matrix metalloproteinases (MMP-2, -9 and -14) and the tissue inhibitors of MMPs (TIMP-1, -2 and -3) at the implantation site during tubal pregnancy. Reproduction. 2005;129(1):103–113. doi: 10.1530/rep.1.00283. [DOI] [PubMed] [Google Scholar]
- 92.Poehlmann TG, Fitzgerald JS, Meissner A, et al. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005;26:S37–S41. doi: 10.1016/j.placenta.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Corvinus FM, Fitzgerald JS, Friedrich K, Markert UR. Evidence for a correlation between trophoblast invasiveness and STAT3 activity. American Journal of Reproductive Immunology. 2003;50(4):316–321. doi: 10.1034/j.1600-0897.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 94.Prakash GJ, Suman P, Prieto DMM, Markert UR, Gupta SK. Leukaemia inhibitory factor mediated proliferation of HTR-8/SVneo trophoblast cells is dependent on activation of extracellular signal-regulated kinase 1/2. Reproduction, Fertility and Development. 2011;23(5):714–724. doi: 10.1071/RD10315. [DOI] [PubMed] [Google Scholar]
- 95.Suman P, Shembekar N, Gupta SK. Leukemia inhibitory factor increases the invasiveness of trophoblastic cells through integrated increase in the expression of adhesion molecules and pappalysin 1 with a concomitant decrease in the expression of tissue inhibitor of matrix metalloproteinases. Fertility and Sterility. 2013;99(2):533.e2–542.e2. doi: 10.1016/j.fertnstert.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Tapia A, Salamonsen LA, Manuelpillai U, Dimitriadis E. Leukemia inhibitory factor promotes human first trimester extravillous trophoblast adhesion to extracellular matrix and secretion of tissue inhibitor of metalloproteinases-1 and -2. Human Reproduction. 2008;23(8):1724–1732. doi: 10.1093/humrep/den121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gremlich S, Chanson A, Urner F, et al. LIF and sIL-2R plasma concentrations in IVF patients on the day of embryo transfer: predictive markers of IVF outcome. Journal of Reproductive Immunology. 2012;94(2):175–182. doi: 10.1016/j.jri.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 98.Brinsden PR, Alam V, de Moustier B, Engrand P. Recombinant human leukemia inhibitory factor does not improve implantation and pregnancy outcomes after assisted reproductive techniques in women with recurrent unexplained implantation failure. Fertility and Sterility. 2009;91(4):1445–1447. doi: 10.1016/j.fertnstert.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 99.Terakawa J, Wakitani S, Sugiyama M, et al. Embryo implantation is blocked by intraperitoneal injection with anti-LIF antibody in mice. Journal of Reproduction and Development. 2011;57(6):700–707. doi: 10.1262/jrd.11-048h. [DOI] [PubMed] [Google Scholar]
- 100.Lemons AR, Naz RK. Birth control vaccine targeting leukemia inhibitory factor. Molecular Reproduction and Development. 2012;79(2):97–106. doi: 10.1002/mrd.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sengupta J, Lalitkumar PGL, Najwa AR, Ghosh D. Monoclonal anti-leukemia inhibitory factor antibody inhibits blastocyst implantation in the rhesus monkey. Contraception. 2006;74(5):419–425. doi: 10.1016/j.contraception.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 102.White CA, Zhang J, Salamonsen LA, et al. Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: a nonhormonal contraceptive strategy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19357–19362. doi: 10.1073/pnas.0710110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menkhorst E, Zhang J, Sims NA, et al. Vaginally administered PEGylated LIF Antagonist blocked embryo implantation and eliminated non-target effects on bone in mice. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019665.e19665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aschenbach LC, Hester KE, McCann NC, Zhang J, Dimitriadis E, Duffy DM. The LIF receptor antagonist PEGLA is effectively delivered to the uterine endometrium and blocks LIF activity in cynomolgus monkeys. Contraception. 2013;87(6):813–823. doi: 10.1016/j.contraception.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 105.Lalitkumar S, Boggavarapu NR, Menezes J, et al. Polyethylene glycated leukemia inhibitory factor antagonist inhibits human blastocyst implantation and triggers apoptosis by down-regulating embryonic AKT. Fertility and Sterility. 2013;100(4):1160.e2–1169.e2. doi: 10.1016/j.fertnstert.2013.06.023. [DOI] [PubMed] [Google Scholar]


